Device for Controlled Production of Hydrogen
Abstract
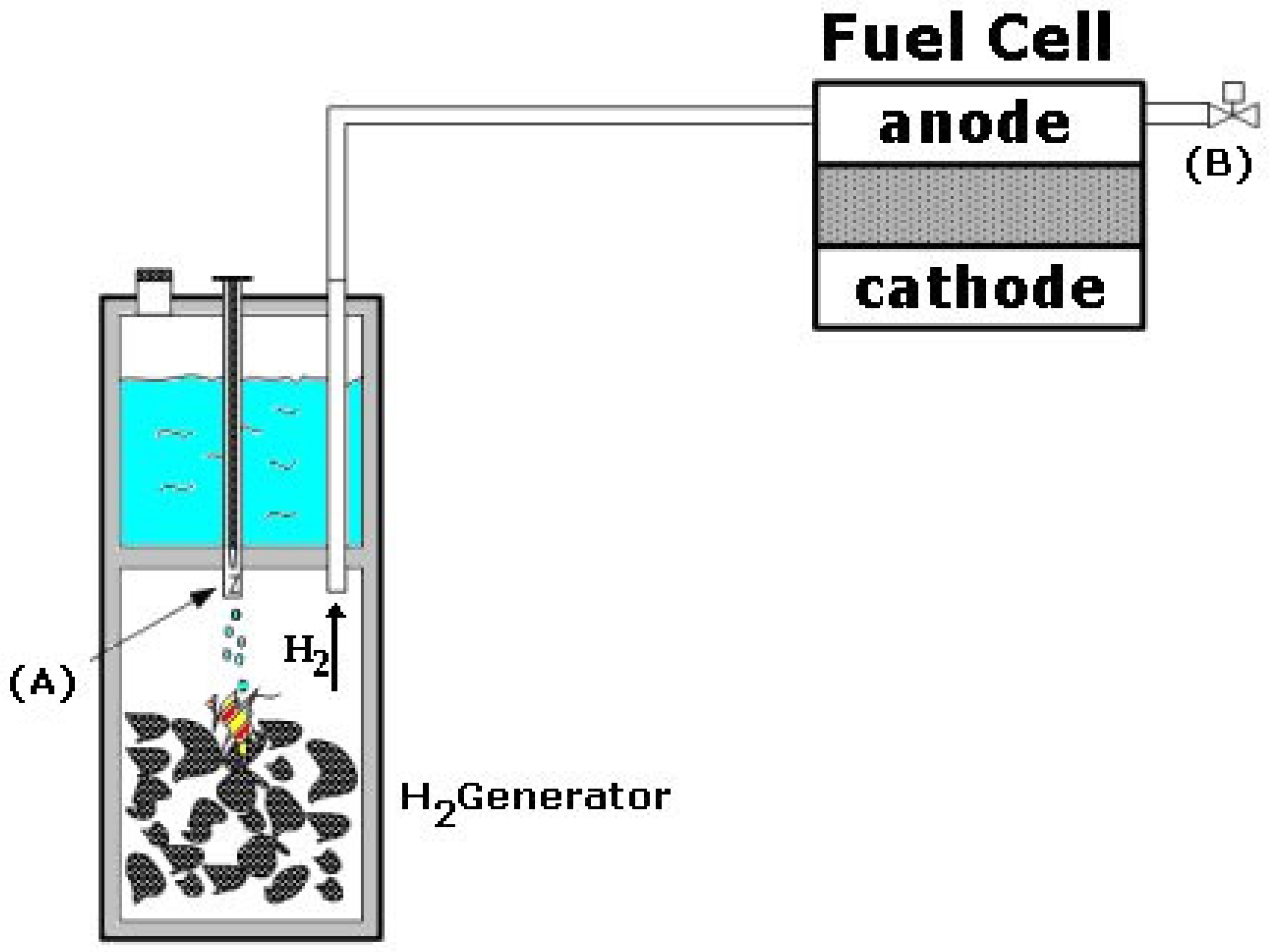
1. Introduction
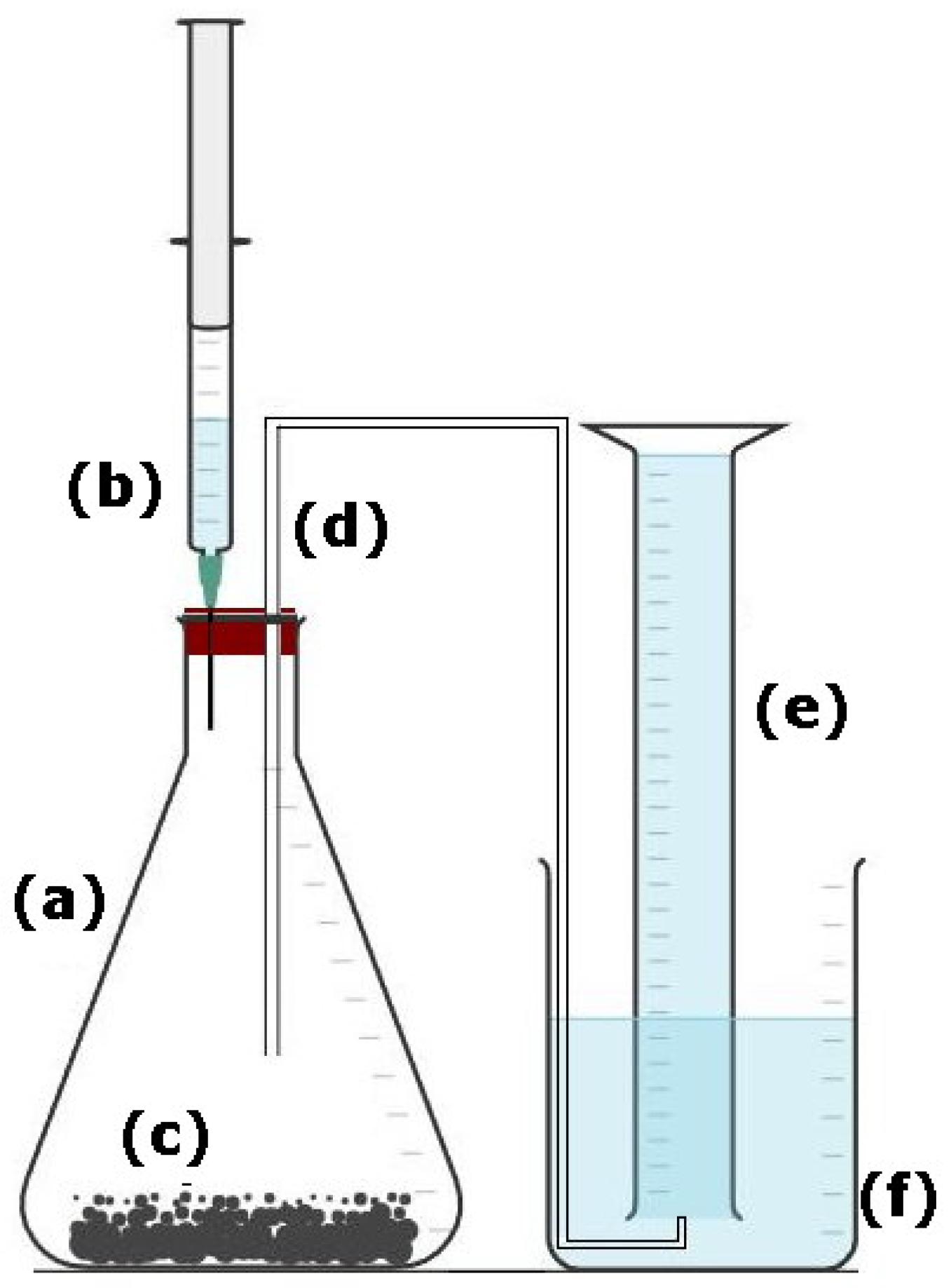
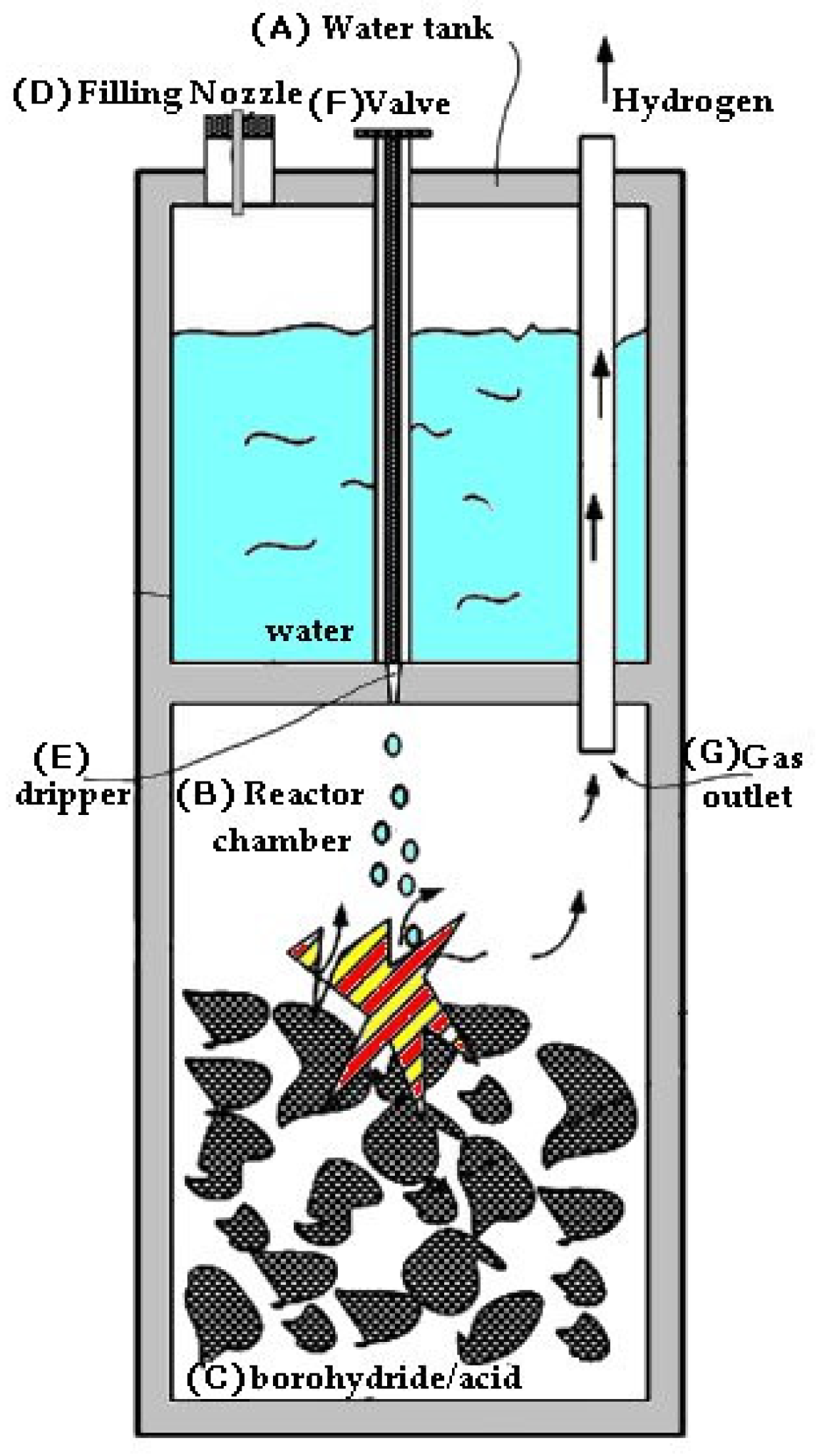
2. Materials and Methods
Static and Kinetic Test
3. Results
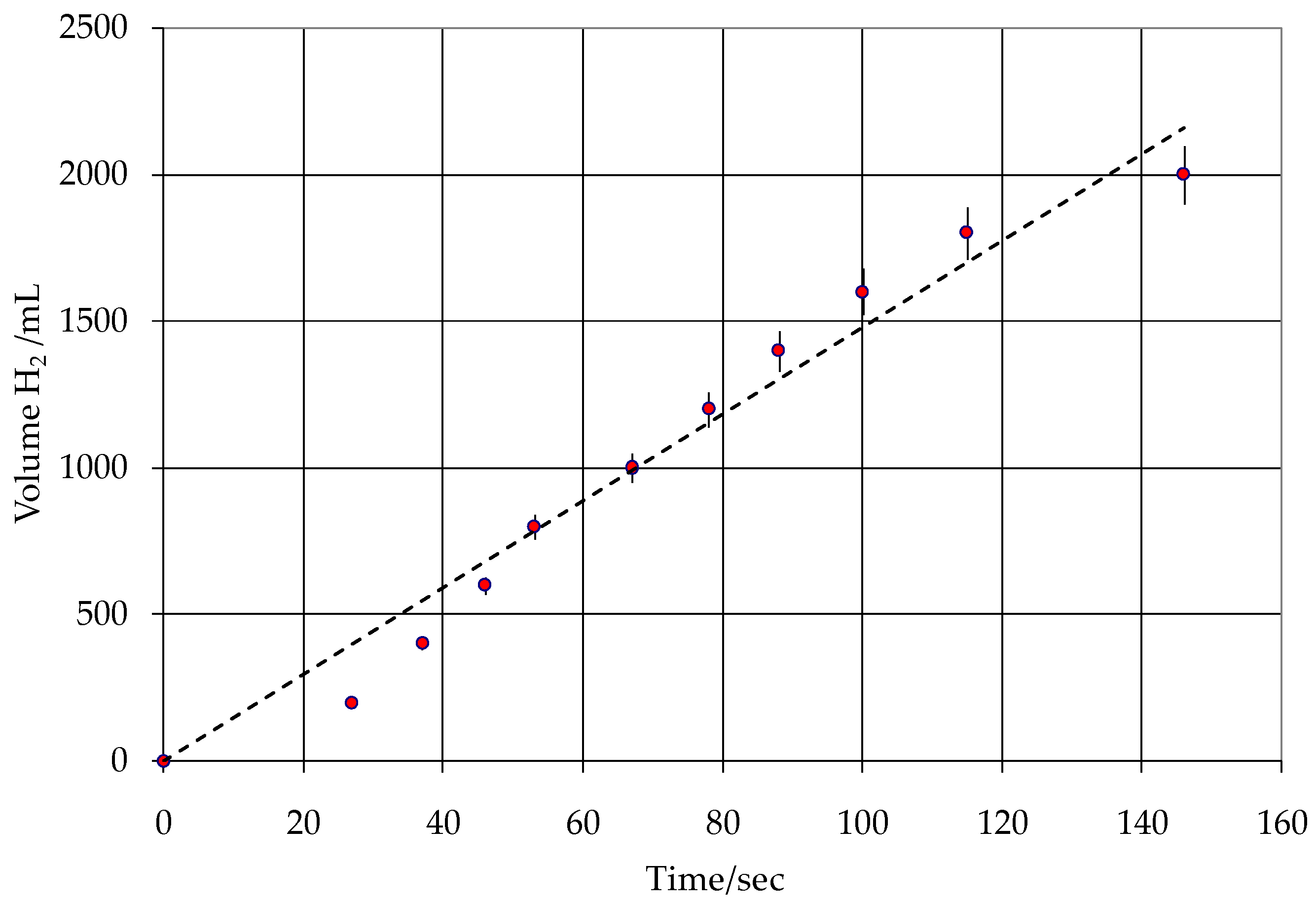
3.1. Static Tests
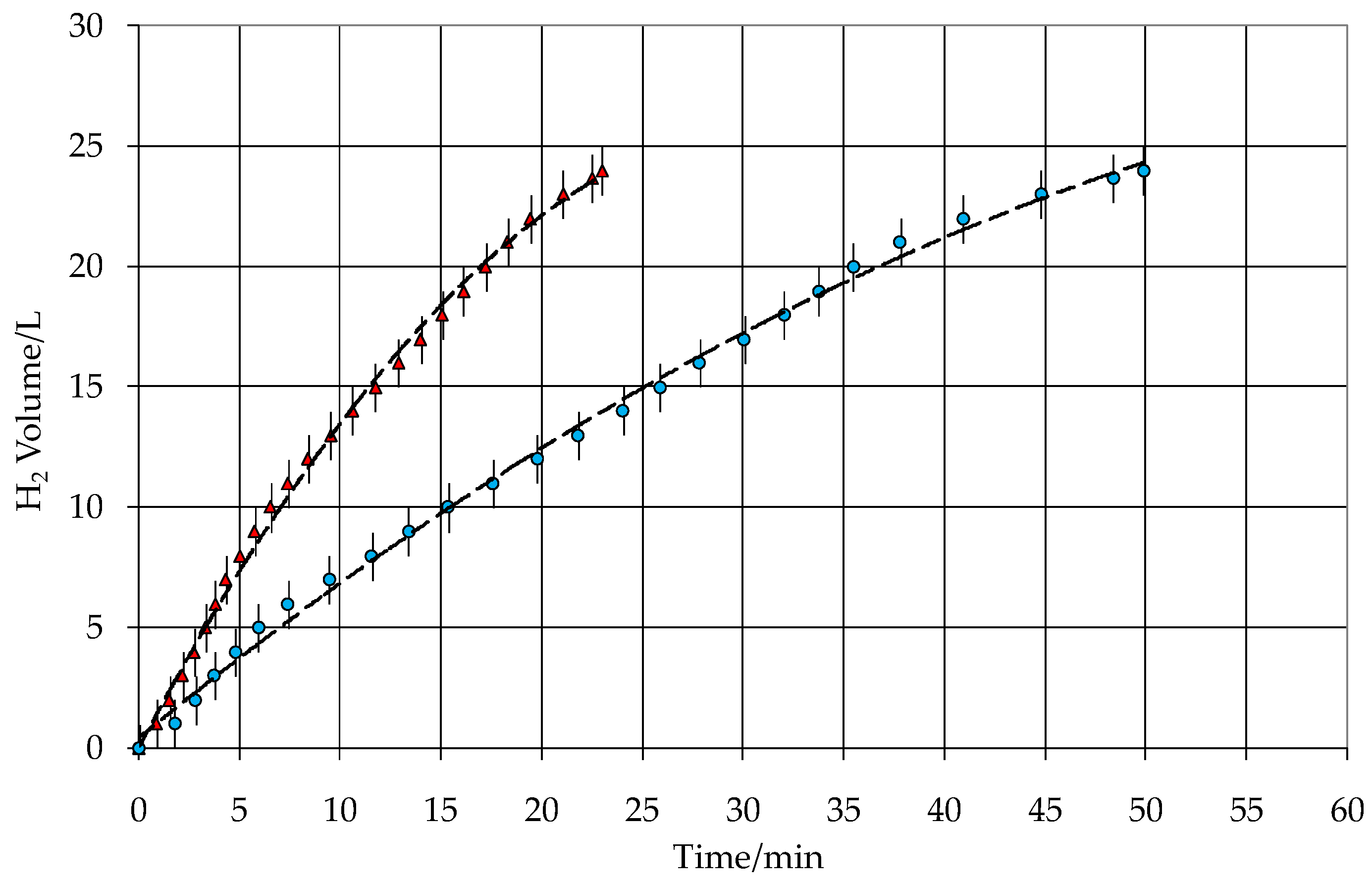
3.2. Kinetic Test
4. Conclusions
- a high chemical stability of the solid mixture used, resulting in the safe storage and transport for mobile production systems;
- reaction products that have a neutral pH and can be disposed of without any particular problems or chemical and biological hazards;
- reagents that have minimal or reduced toxicity;
- extremely low water usage and a minimal footprint of the production;
- a reaction conversion close to 100%;
- practical absence of catalysts;
- use of water of low quality and purity, avoiding the need to carry water refills (possible use of wastewater);
- a passive production system or one with a very limited energy consumption, self-regulating in terms of pressure and capable to produce hydrogen on demand.
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdalla, A.M.; Hossain, S.; Nisfindy, O.B.; Azad, A.T.; Dawood, M.; Azad, A.K. Hydrogen production, storage, transportation and key challenges with applications: A review. Energy Convers. Manag. 2018, 165, 602–627. [Google Scholar] [CrossRef]
- Niaz, S.; Manzoor, T.; Pandith, A.H. Hydrogen storage: Materials, methods and perspectives. Renew. Sustain. Energy Rev. 2015, 50, 457–469. [Google Scholar] [CrossRef]
- Bostic, E.; Sifer, N.; Bolton, C.; Ritter, U.; Dubois, T. The US Army Foreign Comparative Test fuel cell program. J. Power Sources 2004, 137, 76–79. [Google Scholar] [CrossRef]
- Patil, A.S.; Dubois, T.; Sifer, N.; Bostic, E.; Gardner, K.; Quah, M.; Bolton, C. Portable fuel cell systems for America’s army: Technology transition to the field. J. Power Sources 2004, 136, 220–225. [Google Scholar] [CrossRef]
- McConnell, V.P. High-temperature PEM fuel cells: Hotter, simpler, cheaper. Fuel Cells Bullet. 2009, 6, 12–16. [Google Scholar] [CrossRef]
- Oh, T.H.; Gang, B.G.; Kim, H.; Kwon, S. Sodium borohydride hydrogen generator using Co-P/Ni foam catalysts for 200W proton exchange membrane fuel cell system. Energy 2015, 90, 1163–1170. [Google Scholar] [CrossRef]
- Kim, T. Fully-integrated micro PEM fuel cell system with NaBH4 hydrogen generator. Int. J. Hydrogen Energy 2012, 37, 2440–2446. [Google Scholar] [CrossRef]
- Nunes, H.X.; Ferreira, M.J.F.; Rangel, C.M.; Pinto, A.M.F.R. Hydrogen generation and storage by aqueous sodium borohydride (NaBH4) hydrolysis for small portable fuel cells (H2—PEMFC). Int. J. Hydrogen Energy 2016, 41, 15426–15432. [Google Scholar] [CrossRef]
- Zhou, L. Progress and problems in hydrogen storage methods. Renew. Sustain. Energy Rev. 2005, 9, 395–408. [Google Scholar] [CrossRef]
- Sifer, N.; Gardner, K. An analysis of hydrogen production from ammonia hydride hydrogen generators. J. Power Sources 2004, 132, 135–138. [Google Scholar] [CrossRef]
- Zacharia, R.; Rather, S.U. Review of solid state hydrogen storage methods adopting different kinds of novel materials. J. Nanomater. 2015, 2015, 914845. [Google Scholar] [CrossRef]
- Ley, M.B.; Jepsen, L.H.; Lee, Y.S.; Cho, Y.W.; Von Colbe, J.M.B.; Dornheim, M.; Rokni, M.; Jensen, J.O.; Sloth, M.; Filinchuk, Y.; et al. Complex hydrides for hydrogen storage—New perspectives. Mater. Today 2014, 17, 122–128. [Google Scholar] [CrossRef]
- Kong, V.C.Y.; Kirk, D.W.; Foulkes, F.R.; Hinatsu, J.T. Development of hydrogen storage for fuel cell generators II: Utilization of calcium hydride and lithium hydride. Int. J. Hydrogen Energy 2003, 28, 205–214. [Google Scholar] [CrossRef]
- Dedrick, D.E.; Kanouff, M.P.; Replogle, B.C.; Gross, K.J. Thermal properties characterization of sodium alanates. J. Alloys Compd. 2005, 389, 299–305. [Google Scholar] [CrossRef]
- Zaluska, A.; Zaluski, L.; Strom-Olsen, J.O. Sodium alanates for reversible hydrogen storage. J. Alloys Compd. 2000, 298, 125–134. [Google Scholar] [CrossRef]
- Skolnik, E.G. Analysis of the sodium hydride-based hydrogen storage system being developed by PowerBall Technologies LLC, Energetics, Incorporated. In Proceedings of the DOE 2000 Hydrogen Program Annual Review, Livermore, CA, USA, 9–11 May 2020. [Google Scholar]
- Aakko-Saksa, P.T.; Cook, C.; Kiviaho, J.; Repo, T. Liquid organic hydrogen carriers for transportation and storing of renewable energy—Review and discussion. J. Power Sources 2018, 396, 802–823. [Google Scholar] [CrossRef]
- Puszkiel, J.; Garroni, S.; Milanese, C.; Gennari, F.; Klassen, T.; Dornheim, M.; Pistidda, C. Tetrahydroborates: Development and potential as hydrogen storage medium. Inorganics 2017, 5, 74. [Google Scholar] [CrossRef]
- Li, H.W.; Yan, Y.; Orimo, S.I.; Zuettel, A.; Jensen, C.M. Recent progress in metal borohydrides for hydrogen storage. Energies 2011, 4, 185–214. [Google Scholar] [CrossRef]
- Simagina, V.I.; Ozerova, A.M.; Komova, O.V.; Netskina, O.V. Recent Advances in Applications of Co-B Catalysts in NaBH4-Based Portable Hydrogen Generators. Catalysts 2021, 11, 268. [Google Scholar] [CrossRef]
- Rivarolo, M.; Improta, O.; Magistri, L.; Panizza, M.; Barbucci, A. Thermo-economic analysis of a hydrogen production system by sodium borohydride (NaBH4). Int. J. Hydrogen Energy 2018, 43, 1606–1614. [Google Scholar] [CrossRef]
- Hirscher, M.; Yartys, V.A.; Baricco, M.; Von Colbe, J.B.; Blanchard, D.; Bowman, R.C.; Broom, D.P.; Buckley, C.E.; Chang, F.; Chen, P. Materials for hydrogen-based energy storage—Past, recent progress and future outlook. J. Alloys Compd. 2020, 827, 153548. [Google Scholar] [CrossRef]
- Jung, E.S.; Kim, H.; Kwon, S.; Oh, T.H. Fuel cell system with sodium borohydride hydrogen generator for small unmanned aerial vehicles. Int. J. Green Energy 2018, 15, 385–392. [Google Scholar] [CrossRef]
- Okumus, E.; San, F.G.B.; Okur, O.; Turk, B.E.; Cengelci, E.; Kilic, M.; Karadag, C.; Cavdar, M.; Turkmen, A.; Yazici, M.S. Development of boron-based hydrogen and fuel cell system for small unmanned aerial vehicle. Int. J. Hydrogen Energy 2017, 42, 2691–2697. [Google Scholar] [CrossRef]
- Mochalov, K.N.; Khain, V.S.; Gilmanshin, G.G. Kinetic Study of the Intermediate Hydrolysis Steps of BH4−. Kinet. Catal. 1965, 6, 469–541. [Google Scholar]
- Amendola, S.C.; Sharp-Goldman, S.L.; Janjua, M.S.; Spencer, N.C.; Kelly, M.T.; Petillo, P.J.; Binder, M. A safe, portable, hydrogen gas generator using aqueous borohydride solution and Ru catalyst. Int. J. Hydrogen Energy 2000, 25, 969–975. [Google Scholar] [CrossRef]
- Amendola, S.C.; Binder, M.; Sharp-Goldman, S.L.; Kelly, M.T.; Petillo, P. System for Hydrogen Generation. U.S. Patent No. 6,534,033, 18 March 2003. [Google Scholar]
- Kojima, Y.; Suzuki, K.; Fukumoto, K.; Sasaki, M.; Yamamoto, T.; Kawai, Y.; Hayashi, H. Hydrogen generation using sodium borohydride solution and metal catalyst coated on metal oxide. Int. J. Hydrogen Energy 2002, 27, 1029–1034. [Google Scholar] [CrossRef]
- James, B.D.; Wallbridge, M.G.H. Metal Tetrahydroborates. Progr. Inorg. Chem. 1970, 11, 99–231. [Google Scholar]
- Pozio, A.; De Francesco, M.; Monteleone, G.; Oronzio, R.; Galli, S.; D’Angelo, C.; Marrucci, M. Apparatus for the production of hydrogen from sodiumborohydride in alkalinesolution. Int. J. Hydrogen Energy 2008, 33, 51–56. [Google Scholar] [CrossRef]
- Cento, C.; Gislon, P.; Prosini, P.P. Hydrogen generation by hydrolysis of NaBH4. Int. J. Hydrogen Energy 2009, 34, 4551–4554. [Google Scholar] [CrossRef]
- Gislon, P.; Granati, M.; Pozio, A.; Prosini, P.P.; Santomassimo, S. Dispositivo Portatile Erogatore di Idrogeno, in Particolare per uso in Celle a Combustibile. Italian Patent No. ITRM2005/0312, 18 March 2005. [Google Scholar]
- Zhang, Q.; Mohring, R.M.; Wu, Y. Systems and Methods for Hydrogen Generation from Solid Hydrides. U.S. Patent No. 2005/0238573, 14 April 2005. [Google Scholar]
- Akdim, O.; Demirci, U.; Miele, P. Acetic acid, a relatively green single-use catalyst for hydrogen generation from sodium borohydride. Int. J. Hydrogen Energy 2009, 34, 7231–7238. [Google Scholar] [CrossRef]
- Kim, H.J.; Shin, K.J.; Kim, H.J.; Han, M.; Kim, H.; Shul, Y.G.; Jung, K.T. Hydrogen generation from aqueous acid-catalyzed hydrolysis of sodium borohydride. Int. J. Hydrogen Energy 2010, 35, 12239–12245. [Google Scholar] [CrossRef]
- Kwon, S.M.; Kim, M.J.; Kang, S.; Kim, T. Development of a high-storage-density hydrogen generator using solid-state NaBH4 as a hydrogen source for unmanned aerial vehicles. Appl. Energy 2019, 251, 113331. [Google Scholar] [CrossRef]
- Yadav, A.A.; Hunge, Y.M.; Dhodamani, A.G.; Kang, S.W. Hydrothermally Synthesized Ag@MoS2 Composite for Enhanced Photocatalytic Hydrogen Production. Catalysts 2023, 13, 716. [Google Scholar] [CrossRef]
| Test N° | Reagents | Weight |
|---|---|---|
| S-1 | NaBH4 | 0.20 |
| C2H2O2 × 2H2O | 0.32 | |
| H2O | 1.00 | |
| S-2 | NaBH4 | 0.20 |
| C2H2O2 × 2H2O | 0.36 | |
| H2O | 0.80 | |
| S-3 | NaBH4 | 0.20 |
| C6H8O7 × H2O | 0.36 | |
| H2O | 1.00 |
| K-2 | K-3 | |
|---|---|---|
| NabH4 (g) | 10 | 10 |
| Citric acid (g) | 18 | 18 |
| Loaded volume of H2O (mL) | 100 | 100 |
| Theoretical volume of H2O (mL) | 40 | 40 |
| Residual volume of H2O (mL) | 55 | 40 |
| Initial temperature (°C) | 25 | 25 |
| Flow of H2O (mL min−1) | 1.4 | 1.9 |
| Volume of H2 produced (L) | 24 | 24 |
| Flow of H2 produced (L min−1) | 0.54 | 1.16 |
| Final pH | 5.5 | 5.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pozio, A. Device for Controlled Production of Hydrogen. Hydrogen 2023, 4, 434-443. https://doi.org/10.3390/hydrogen4030029
Pozio A. Device for Controlled Production of Hydrogen. Hydrogen. 2023; 4(3):434-443. https://doi.org/10.3390/hydrogen4030029
Chicago/Turabian StylePozio, Alfonso. 2023. "Device for Controlled Production of Hydrogen" Hydrogen 4, no. 3: 434-443. https://doi.org/10.3390/hydrogen4030029
APA StylePozio, A. (2023). Device for Controlled Production of Hydrogen. Hydrogen, 4(3), 434-443. https://doi.org/10.3390/hydrogen4030029







