A Techno-Economic Assessment of Steam Methane Reforming and Alkaline Water Electrolysis for Hydrogen Production
Abstract
1. Introduction
2. Materials and Methods
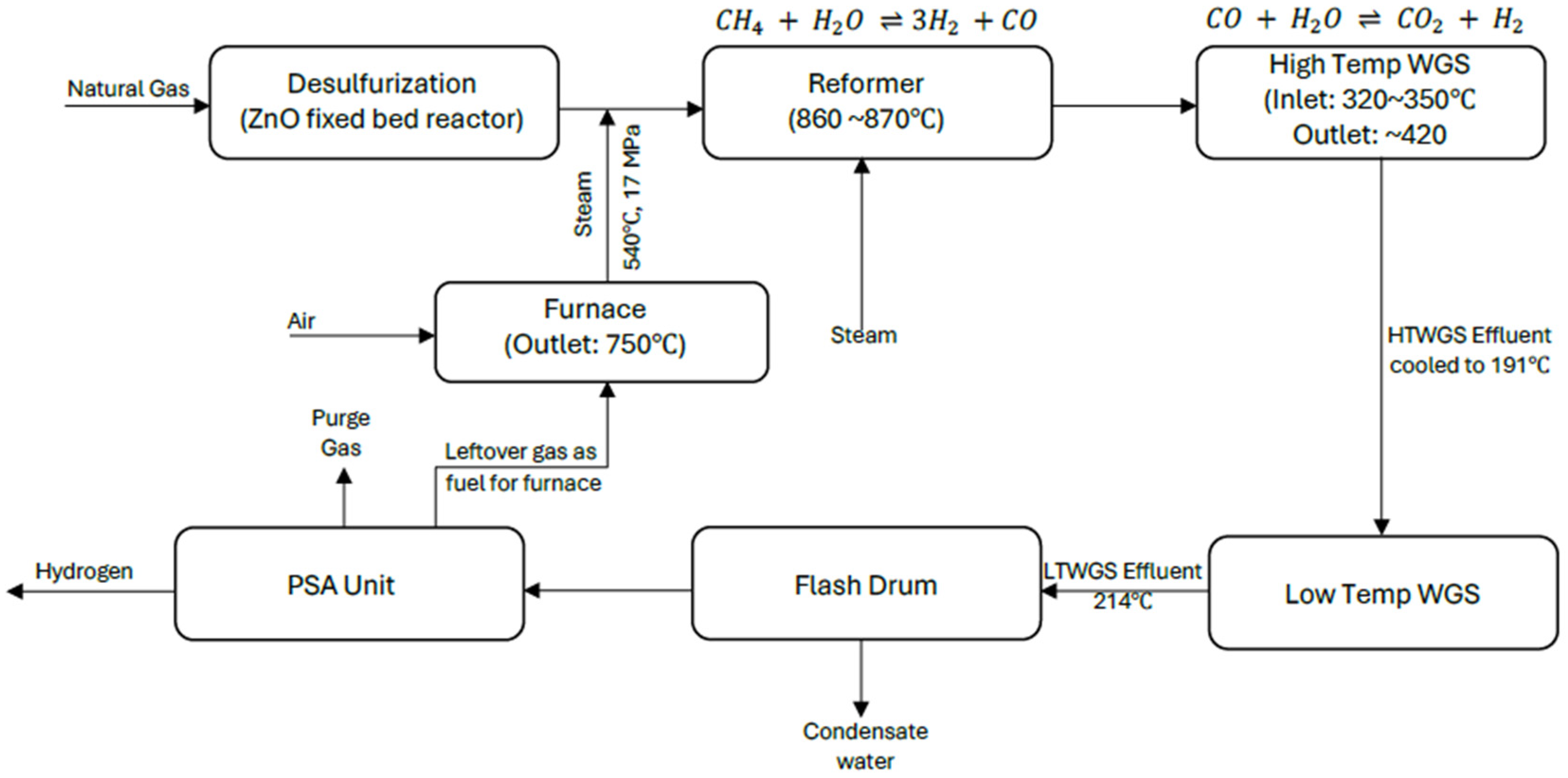
2.1. Overview of Steam Methane Reforming
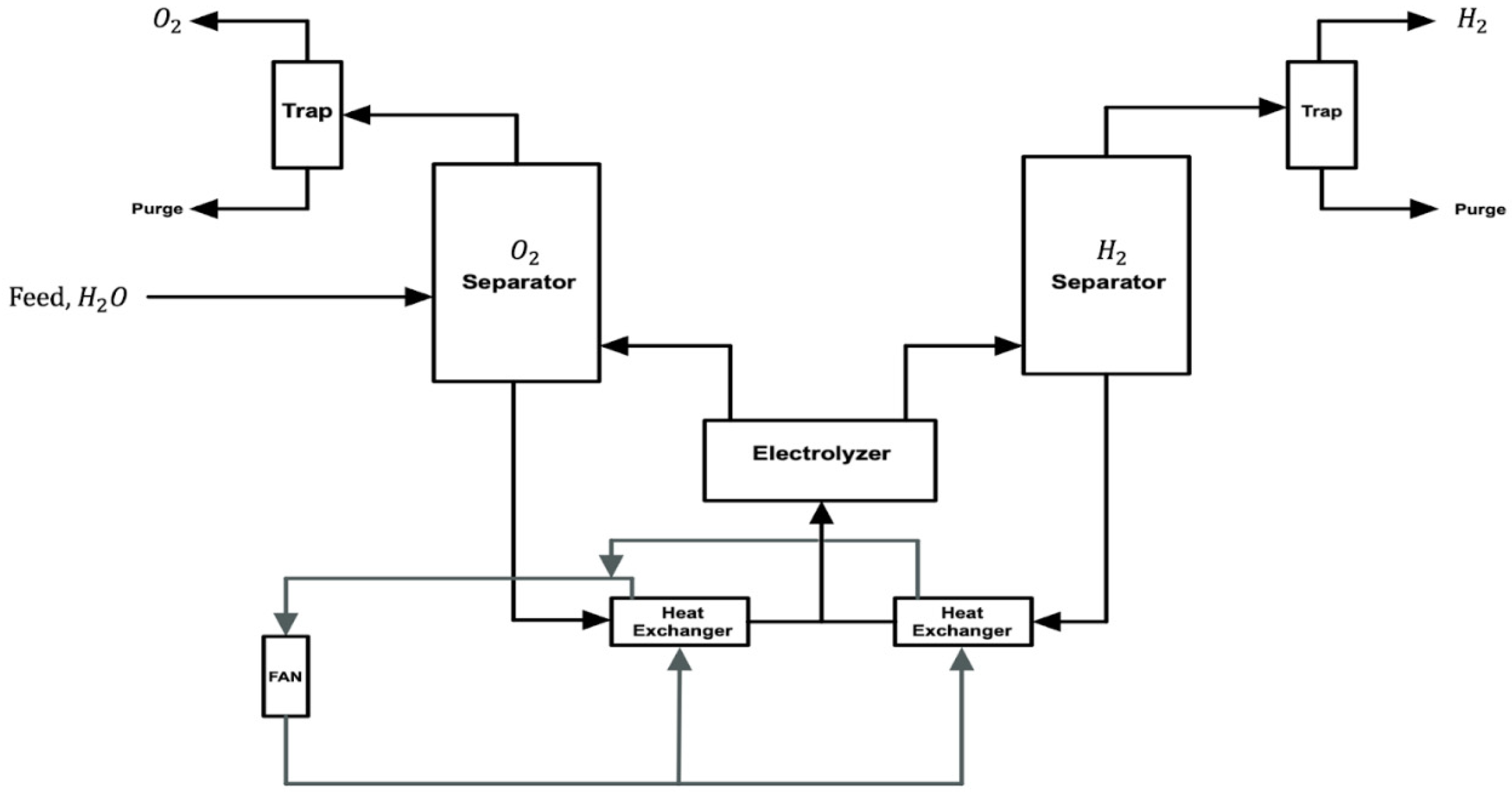
2.2. Overview of Alkaline Water Electrolysis
2.3. Mass and Energy Balance
2.3.1. Steam Methane Reforming
2.3.2. Alkaline Water Electrolysis
2.4. Techno-Economic Analysis
2.4.1. Net Present Value
2.4.2. Life Cycle Cost
Selling Price
Capital Cost
Operating Cost
Maintenance Cost
Replacement Cost
Feedstock Cost
2.5. Sensitivity Analysis
2.6. Environmental Impact Analysis
3. Results and Discussion
3.1. Data Requirement
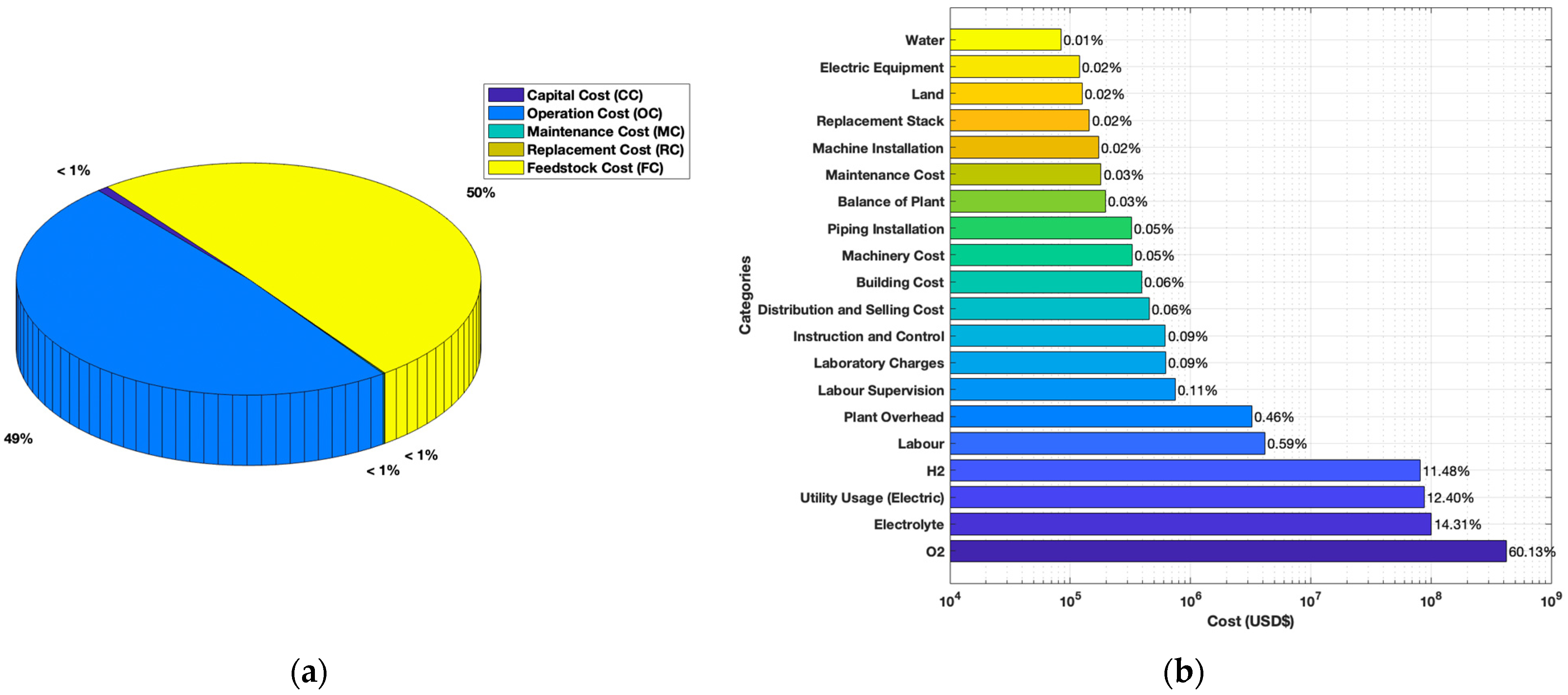
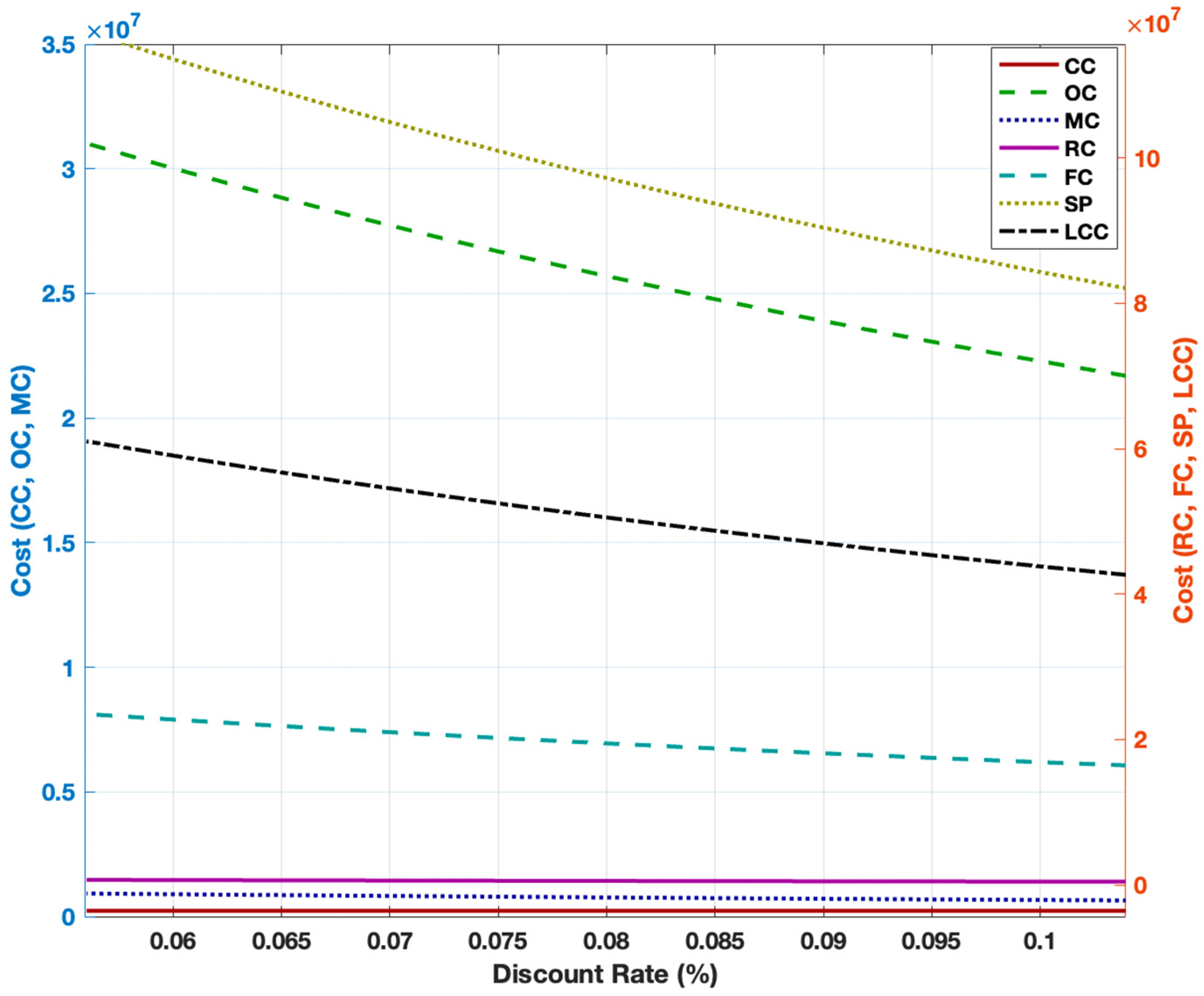
3.2. Cost Breakdown of Steam Methane Reforming
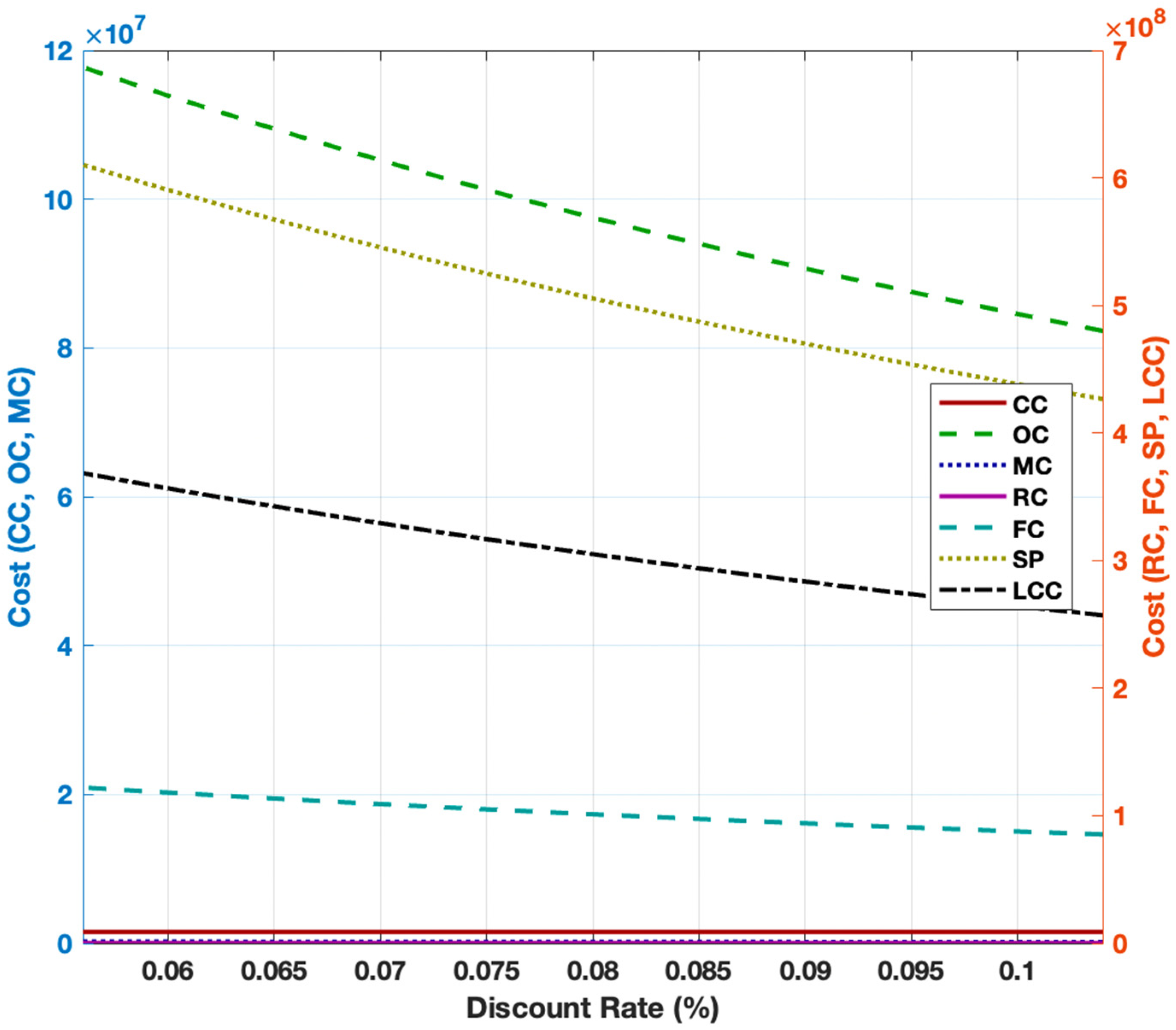
3.3. Cost Breakdown of Alkaline Water Electrolysis
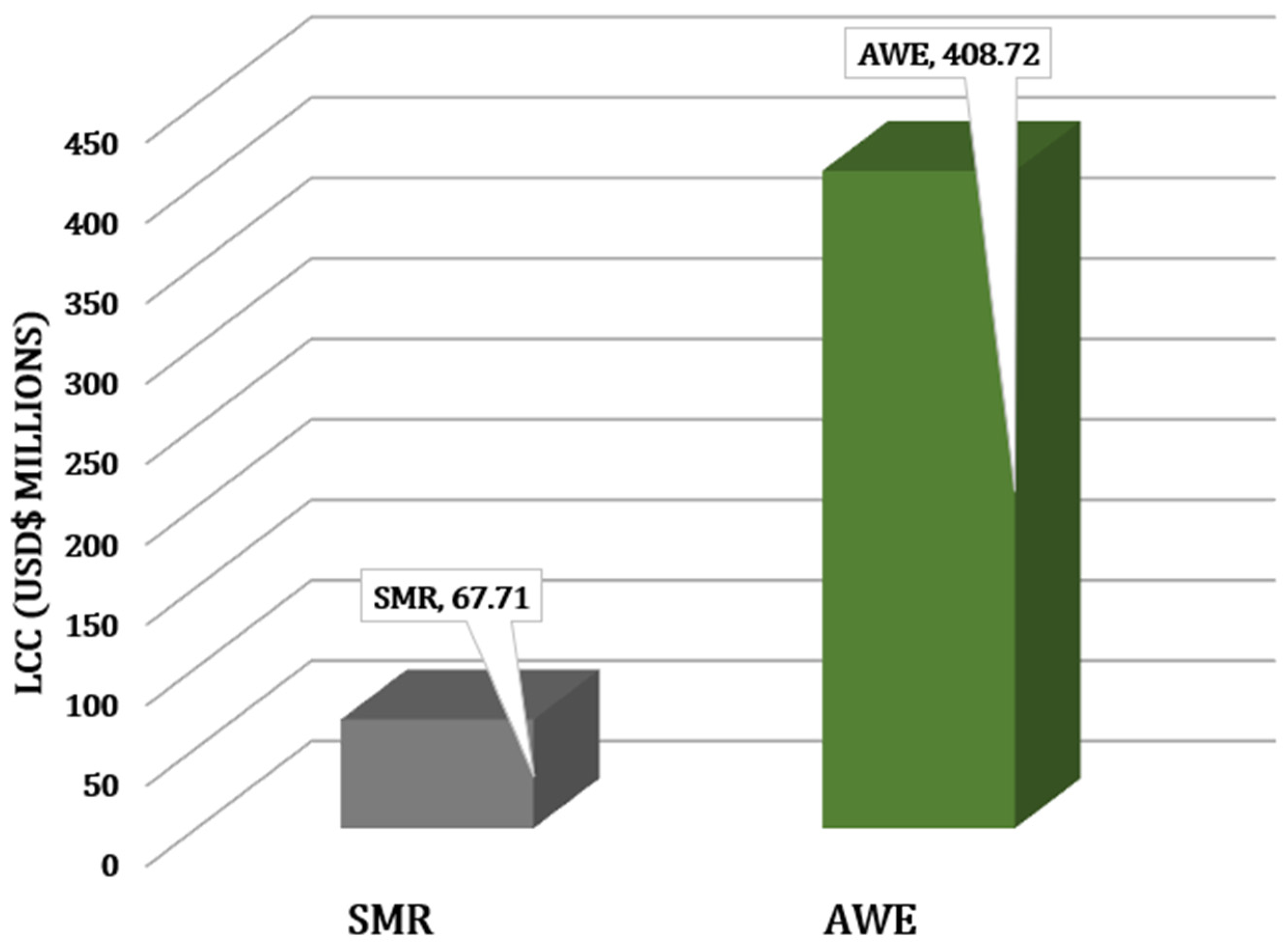
3.4. Life Cycle Cost Distribution
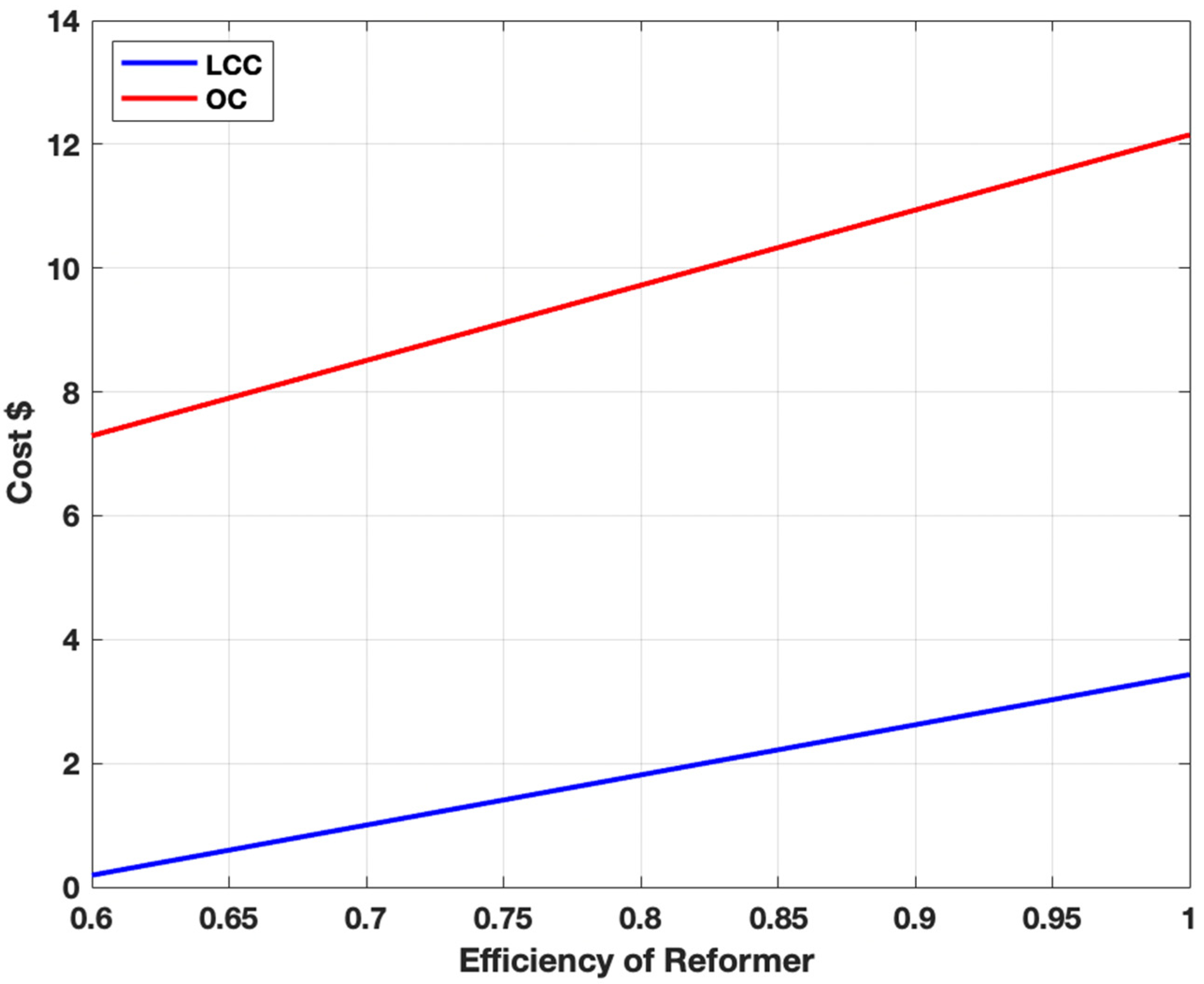
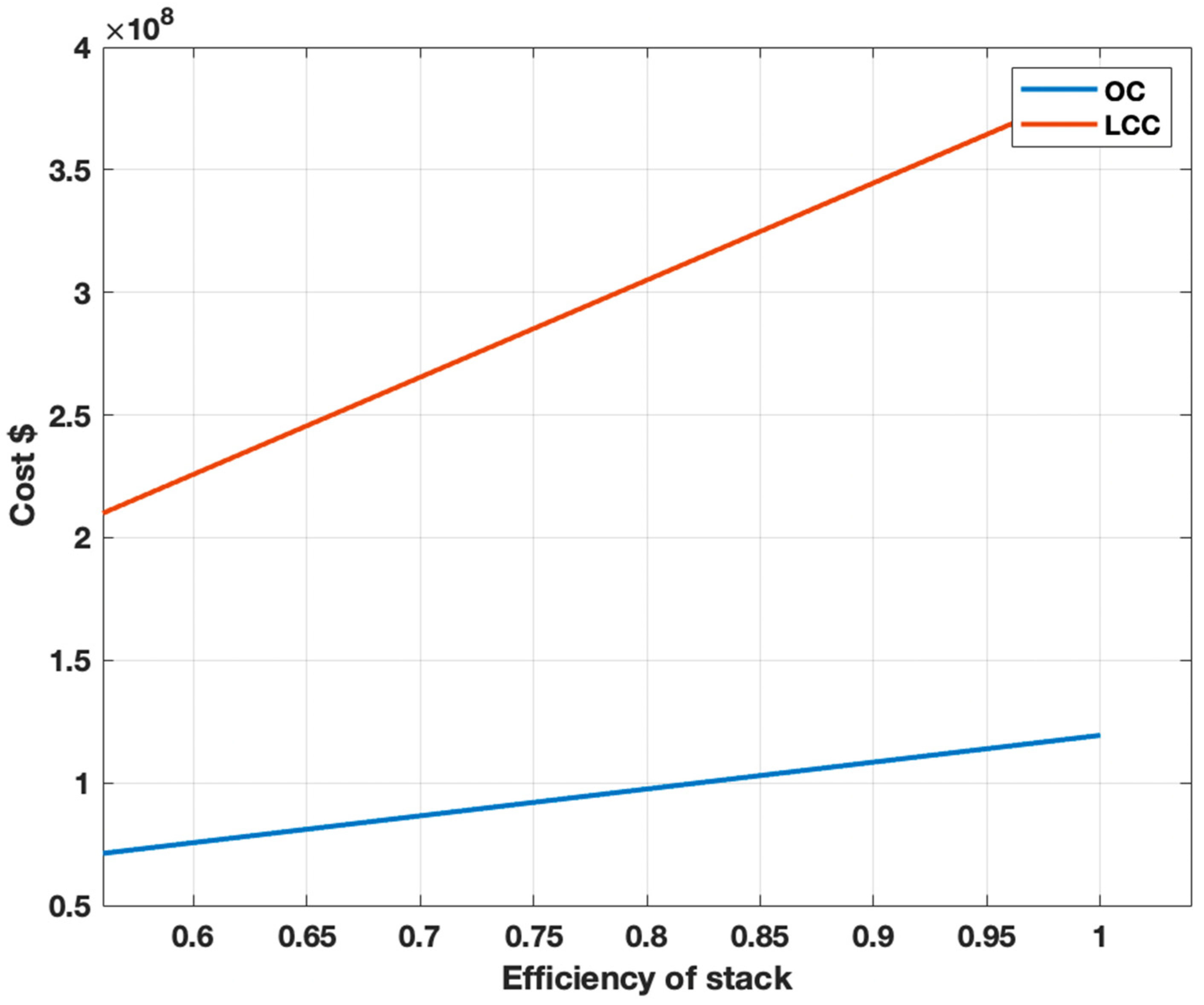
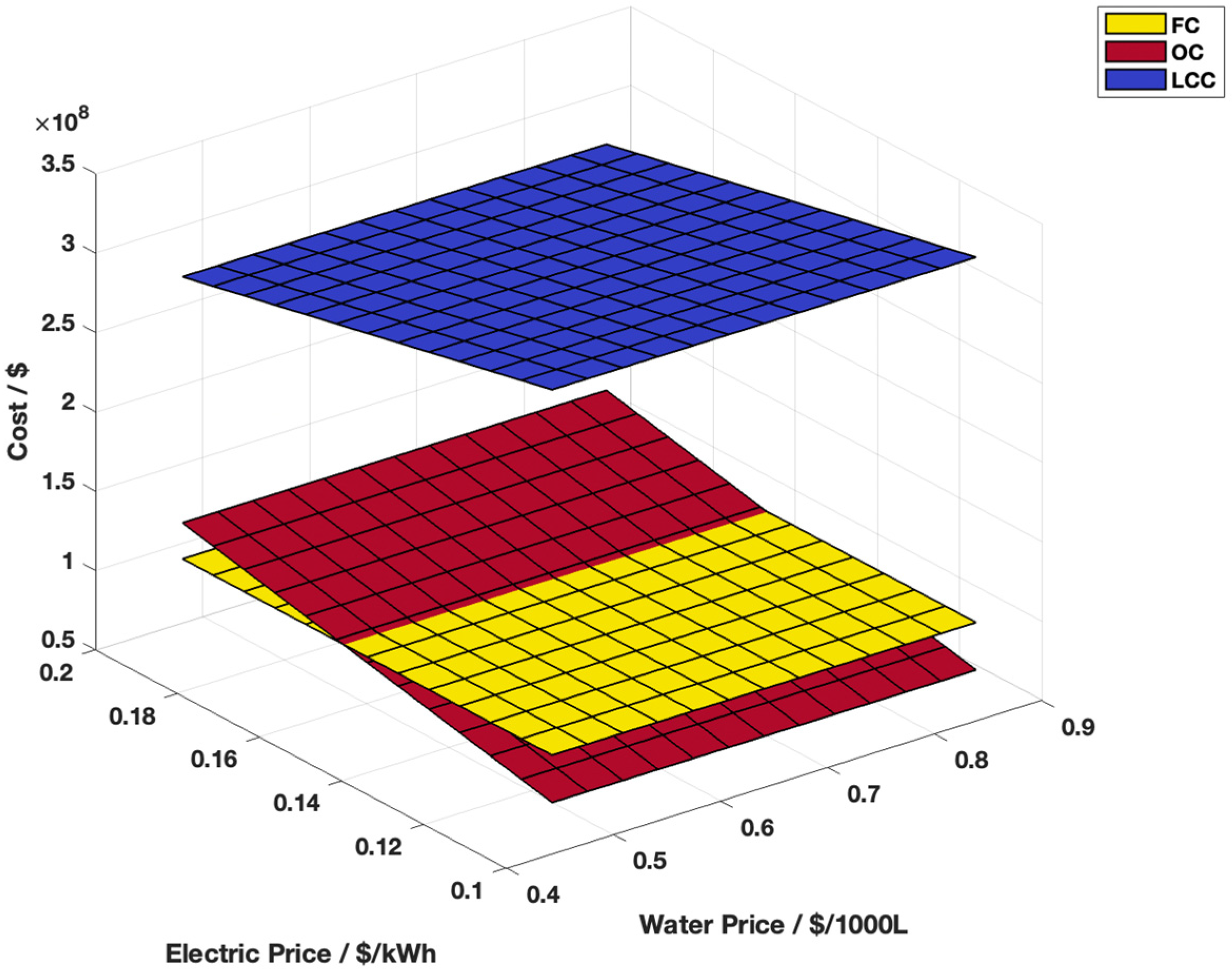
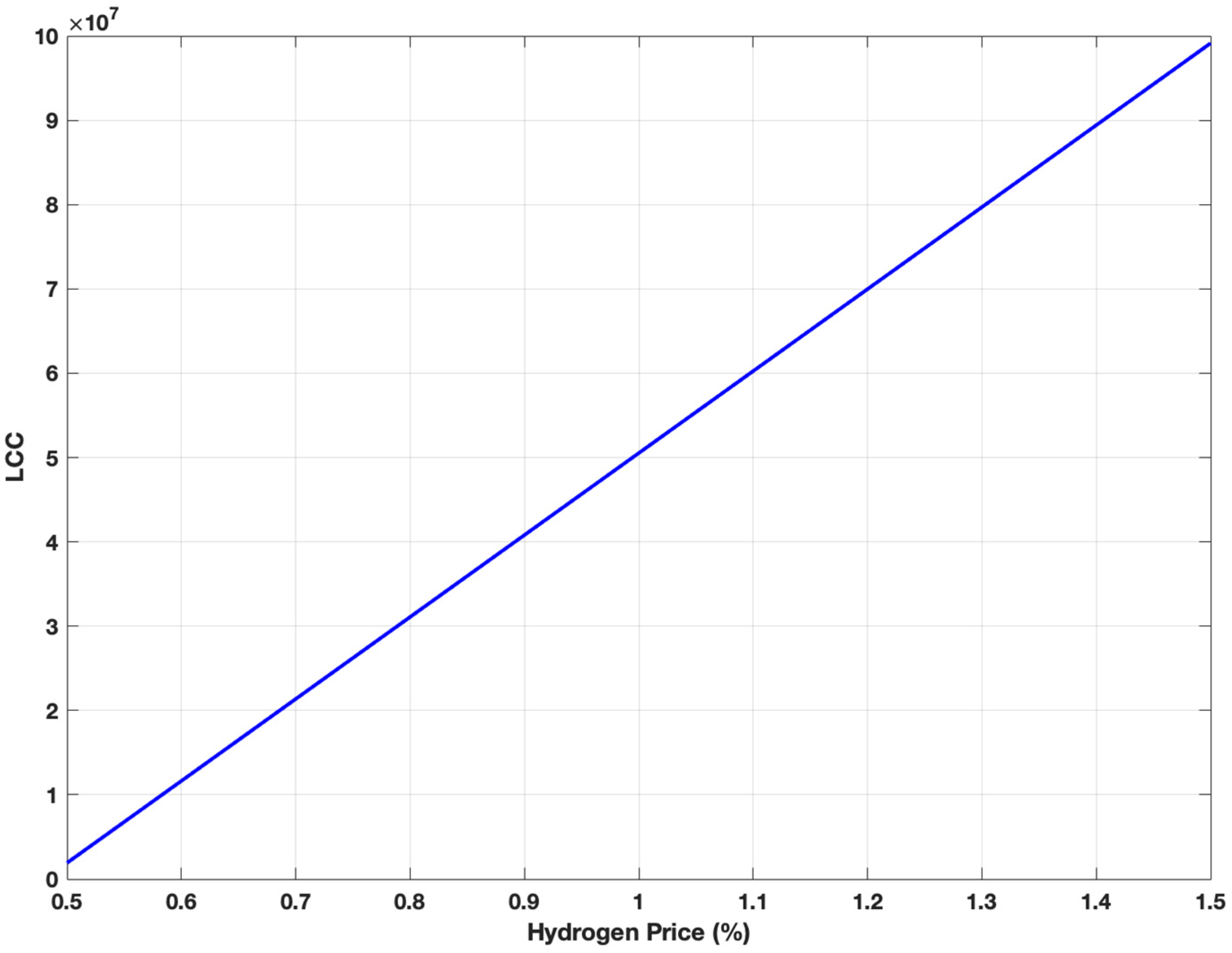
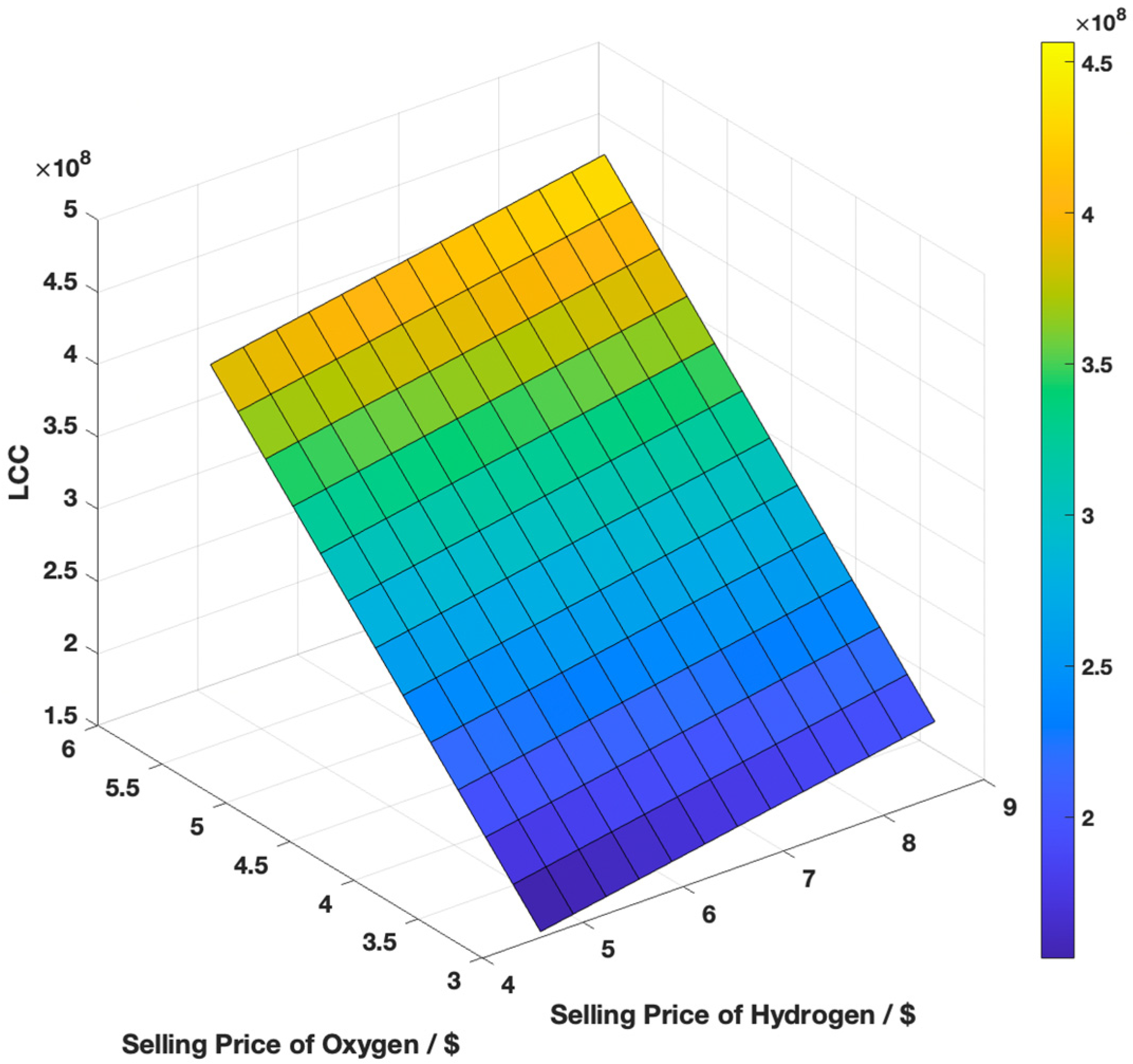
3.5. Sensitivity Analysis for Steam Methane Reforming and Alkaline Water Electrolysis
3.6. Environmental Impact Analysis
4. Opportunities and Challenges
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Mahlia, T.M.I.; Lim, J.Y.; Aditya, L.; Riayatsyah, T.M.I.; Abas, A.E.P.; Nasruddin. Methodology for implementing power plant efficiency standards for power generation: Potential emission reduction. Clean. Technol. Environ. Policy 2018, 20, 309–327. [Google Scholar] [CrossRef]
- Mahlia, T.M.I.; Syaheed, H.; Abas, A.E.P.; Kusumo, F.; Shamsuddin, A.H.; Ong, H.C.; Bilad, M.R. Organic Rankine Cycle (ORC) System Applications for Solar Energy: Recent Technological Advances. Energies 2019, 12, 2930. [Google Scholar] [CrossRef]
- Malik, A.Q. Assessment of the potential of renewables for Brunei Darussalam. Renew. Sustain. Energy Rev. 2011, 15, 427–437. [Google Scholar] [CrossRef]
- Manzella, A. Geothermal energy. EPJ Web Conf. 2017, 148, 12. [Google Scholar] [CrossRef]
- Mahlia, T.M.I.; Syazmi, Z.; Mofijur, M.; Abas, A.P.; Bilad, M.; Ong, H.C.; Silitonga, A. Patent landscape review on biodiesel production: Technology updates. Renew. Sustain. Energy Rev. 2020, 118, 109526. [Google Scholar] [CrossRef]
- Iqbal, M.; Khan, F.U.; Mehdi, M.; Cheok, Q.; Abas, E.; Nauman, M.M. Power harvesting footwear based on piezo-electromagnetic hybrid generator for sustainable wearable microelectronics. J. King Saud Univ.—Eng. Sci. 2022, 34, 329–338. [Google Scholar] [CrossRef]
- Aravindan, M.; Kumar, P. Hydrogen towards sustainable transition: A review of production, economic, environmental impact and scaling factors. Results Eng. 2023, 20, 101456. [Google Scholar] [CrossRef]
- Incer-Valverde, J.; Korayem, A.; Tsatsaronis, G.; Morosuk, T. “Colors” of hydrogen: Definitions and carbon intensity. Energy Convers. Manag. 2023, 291, 117294. [Google Scholar] [CrossRef]
- Li, F.; Wu, Q.; Yuan, W.; Chen, Z. Ruthenium-based single atom catalysts: Synthesis and application in the electrocatalytic hydrogen evolution reaction. Dalton Trans. 2024, 53, 12022–12033. [Google Scholar] [CrossRef]
- Li, X.; Yao, Y.; Tian, Y.; Jia, J.; Ma, W.; Yan, X.; Liang, J. Recent advances in key components of proton exchange membrane water electrolysers. Mater. Chem. Front. 2024, 8, 2493–2510. [Google Scholar] [CrossRef]
- Kwon, J.; Ko, S.; Kim, H.; Park, H.J.; Lee, C.; Yeo, J. Recent advances in vacuum- and laser-based fabrication processes for solar water-splitting cells. Mater. Chem. Front. 2024, 8, 2322–2340. [Google Scholar] [CrossRef]
- Bauer, C.; Treyer, K.; Antonini, C.; Bergerson, J.; Gazzani, M.; Gencer, E.; Gibbins, J.; Mazzotti, M.; McCoy, S.T.; McKenna, R.; et al. On the climate impacts of blue hydrogen production. Sustain. Energy Fuels 2022, 6, 66–75. [Google Scholar] [CrossRef]
- Howarth, R.W.; Jacobson, M.Z. How green is blue hydrogen? Energy Sci. Eng. 2021, 9, 1676–1687. [Google Scholar] [CrossRef]
- Zun, M.T.; McLellan, B.C. Cost Projection of Global Green Hydrogen Production Scenarios. Hydrogen 2023, 4, 932–960. [Google Scholar] [CrossRef]
- Reichartz, T.; Jacobs, G.; Rathmes, T.; Blickwedel, L.; Schelenz, R. Optimal position and distribution mode for on-site hydrogen electrolyzers in onshore wind farms for a minimal levelized cost of hydrogen (LCoH). Wind Energy Sci. 2024, 9, 281–295. [Google Scholar] [CrossRef]
- Jaszczur, M.; Hassan, Q.; Sameen, A.Z.; Salman, H.M.; Olapade, O.T.; Wieteska, S. Massive Green Hydrogen Production Using Solar and Wind Energy: Comparison between Europe and the Middle East. Energies 2023, 16, 5445. [Google Scholar] [CrossRef]
- Ingale, G.U.; Kwon, H.-M.; Jeong, S.; Park, D.; Kim, W.; Bang, B.; Lim, Y.-I.; Kim, S.W.; Kang, Y.-B.; Mun, J.; et al. Assessment of Greenhouse Gas Emissions from Hydrogen Production Processes: Turquoise Hydrogen vs. Steam Methane Reforming. Energies 2022, 15, 8679. [Google Scholar] [CrossRef]
- Sánchez-Bastardo, N.; Schlögl, R.; Ruland, H. Methane Pyrolysis for Zero-Emission Hydrogen Production: A Potential Bridge Technology from Fossil Fuels to a Renewable and Sustainable Hydrogen Economy. Ind. Eng. Chem. Res. 2021, 60, 11855–11881. [Google Scholar] [CrossRef]
- Yin, Y.; Wang, J.; Li, L. An Assessment Methodology for International Hydrogen Competitiveness: Seven Case Studies Compared. Sustainability 2024, 16, 4981. [Google Scholar] [CrossRef]
- Arcos, J.M.M.; Santos, D.M.F. The Hydrogen Color Spectrum: Techno-Economic Analysis of the Available Technologies for Hydrogen Production. Gases 2023, 3, 25–46. [Google Scholar] [CrossRef]
- Luboń, K.; Tarkowski, R.; Uliasz-Misiak, B. Impact of Depth on Underground Hydrogen Storage Operations in Deep Aquifers. Energies 2024, 17, 1268. [Google Scholar] [CrossRef]
- Noussan, M.; Raimondi, P.P.; Scita, R.; Hafner, M. The Role of Green and Blue Hydrogen in the Energy Transition—A Technological and Geopolitical Perspective. Sustainability 2020, 13, 298. [Google Scholar] [CrossRef]
- Zhao, C.; Kobayashi, L.Z.; Alquaity, A.B.S.; Monfort, J.-C.; Cenker, E.; Miralles, N.; Sarathy, S.M. Solutions for decarbonising urban bus transport: A life cycle case study in Saudi Arabia. Commun. Eng. 2024, 3, 95. [Google Scholar] [CrossRef]
- Zayat, B.; Mitra, D.; Irshad, A.; Rajan, A.S.; Narayanan, S.R. Inexpensive and robust iron-based electrode substrates for water electrolysis and energy storage. Curr. Opin. Electrochem. 2021, 25, 100628. [Google Scholar] [CrossRef]
- Brauns, J.; Turek, T. Model-Based Analysis and Optimization of Pressurized Alkaline Water Electrolysis Powered by Renewable Energy. J. Electrochem. Soc. 2023, 170, 064510. [Google Scholar] [CrossRef]
- Jin, L.; Nakashima, R.N.; Comodi, G.; Frandsen, H.L. Alkaline electrolysis for green hydrogen production: Techno-economic analysis of temperature influence and control. In Proceedings of the 36th International Conference on Efficiency, Cost, Optimization, Simulation and Environmental Impact of Energy Systems, ECOS 2023, Las Palmas de Gran Canaria, Spain, 25–30 June 2023; pp. 908–919. [Google Scholar] [CrossRef]
- Ozcan, H.; El-Emam, R.S.; Celik, S.; Amini Horri, B. Recent advances, challenges, and prospects of electrochemical water-splitting technologies for net-zero transition. Clean. Chem. Eng. 2023, 8, 100115. [Google Scholar] [CrossRef]
- Scheepers, F.; Stähler, M.; Stähler, A.; Rauls, E.; Müller, M.; Carmo, M.; Lehnert, W. Improving the efficiency of PEM electrolyzers through membrane-specific pressure optimization. Energies 2020, 13, 612. [Google Scholar] [CrossRef]
- Yan, T.; Chen, S.; Sun, W.; Liu, Y.; Pan, L.; Shi, C.; Zhang, X.; Huang, Z.-F.; Zou, J.-J. IrO2 Nanoparticle-Decorated Ir-Doped W18O49 Nanowires with High Mass Specific OER Activity for Proton Exchange Membrane Electrolysis. ACS Appl. Mater. Interfaces 2023, 15, 6912–6922. [Google Scholar] [CrossRef]
- Zarkadoulas, A.; Field, M.J.; Papatriantafyllopoulou, C.; Fize, J.; Artero, V.; Mitsopoulou, C.A. Experimental and Theoretical Insight into Electrocatalytic Hydrogen Evolution with Nickel Bis(aryldithiolene) Complexes as Catalysts. Inorg. Chem. 2016, 55, 432–444. [Google Scholar] [CrossRef]
- Choi, B.B.; Jo, J.H.; Lee, T.; Jeon, S.Y.; Kim, J.; Yoo, Y.S. Operational characteristics of high-performance kw class alkaline electrolyzer stack for green hydrogen production. J. Electrochem. Sci. Technol. 2021, 12, 302–307. [Google Scholar] [CrossRef]
- Heenan, T.M.M.; Robinson, J.B.; Lu, X.; Bailey, J.J.; Brett, D.J.L.; Shearing, P.R. Analyzing the Mechanical Performance of Solid Oxide Fuel Cells at Interfacial Anode/Electrolyte Regions Using Sub-Micron Resolution 3D X-Ray Computed Tomography. ECS Trans. 2017, 78, 2317–2321. [Google Scholar] [CrossRef]
- Sebbahi, S.; Assila, A.; Belghiti, A.A.; Laasri, S.; Kaya, S.; Hlil, E.K.; Rachidi, S.; Hajjaji, A. A comprehensive review of recent advances in alkaline water electrolysis for hydrogen production. Int. J. Hydrogen Energy 2024, 82, 583–599. [Google Scholar] [CrossRef]
- Abdin, Z.; Zafaranloo, A.; Rafiee, A.; Mérida, W.; Lipiński, W.; Khalilpour, K.R. Hydrogen as an energy vector. Renew. Sustain. Energy Rev. 2020, 120, 109620. [Google Scholar] [CrossRef]
- Taipabu, M.I.; Viswanathan, K.; Wu, W.; Hattu, N.; Atabani, A.E. A critical review of the hydrogen production from biomass-based feedstocks: Challenge, solution, and future prospect. Process Saf. Environ. Prot. 2022, 164, 384–407. [Google Scholar] [CrossRef]
- IEA. The Future of Hydrogen; IEA: Paris, France, 2019; Available online: https://www.iea.org/reports/the-future-of-hydrogen (accessed on 13 November 2024).
- Dickel, R. Blue Hydrogen as an Enabler of Green Hydrogen: The Case of Germany; Oxford Institute for Energy Studies: Oxford, UK, 2020. [Google Scholar]
- Tabat, M.E.; Omoarukhe, F.O.; Güleç, F.; Adeniyi, D.E.; Mukherjee, A.; Okoye, P.U.; Ogbaga, C.C.; Epelle, E.I.; Akande, O.; Okolie, J.A. Process design, exergy, and economic assessment of a conceptual mobile autothermal methane pyrolysis unit for onsite hydrogen production. Energy Convers. Manag. 2023, 278, 116707. [Google Scholar] [CrossRef]
- Gutiérrez Ortiz, F.J. Techno-economic assessment of supercritical processes for biofuel production. J. Supercrit. Fluids 2020, 160, 104788. [Google Scholar] [CrossRef]
- Colli, A.N.; Girault, H.H.; Battistel, A. Non-Precious Electrodes for Practical Alkaline Water Electrolysis. Materials 2019, 12, 1336. [Google Scholar] [CrossRef]
- Squadrito, G.; Nicita, A.; Maggio, G. A size-dependent financial evaluation of green hydrogen-oxygen co-production. Renew. Energy 2021, 163, 2165–2177. [Google Scholar] [CrossRef]
- Yusof, N.K.; Abas, P.E.; Mahlia, T.M.I.; Hannan, M.A. Techno-Economic Analysis and Environmental Impact of Electric Buses. World Electr. Veh. J. 2021, 12, 31. [Google Scholar] [CrossRef]
- Danial, M.; Azis, F.A.; Abas, P.E. Techno-economic analysis and feasibility studies of electric vehicle charging station. World Electr. Veh. J. 2021, 12, 264. [Google Scholar] [CrossRef]
- Dincer, I.; AlZahrani, A.A. 4.25 Electrolyzers. Compr. Energy Syst. 2018, 4, 985–1025. [Google Scholar] [CrossRef]
- Sánchez, M.; Amores, E.; Abad, D.; Rodríguez, L.; Clemente-Jul, C. Aspen Plus model of an alkaline electrolysis system for hydrogen production. Int. J. Hydrogen Energy 2020, 45, 3916–3929. [Google Scholar] [CrossRef]
- Jeddizahed, J.; Webley, P.A.; Hughes, T.J. Integrating alkaline electrolysis with oxyfuel combustion for hydrogen and electricity production. Appl. Energy 2024, 361, 122856. [Google Scholar] [CrossRef]
- Zeng, X.; Zheng, C.; Li, A.; Fan, G.; Xiao, Y.; Yan, C. Numerical study on a new separator for downhole gas-liquid separation. Chem. Eng. Res. Des. 2025, 214, 317–326. [Google Scholar] [CrossRef]
- Zeng, X.; Fan, G.; Xu, J.; Liu, A.; Xu, Y.; Yan, C. Experimental study on a new gas–liquid separator for a wide range of gas volume fraction. Chem. Eng. Res. Des. 2020, 160, 561–570. [Google Scholar] [CrossRef]
- Trap Efficiency of Reservoirs: Concept, Review and Application. Available online: https://www.researchgate.net/publication/366042934_Trap_Efficiency_of_Reservoirs_Concept_Review_and_Application (accessed on 9 March 2025).
- Arfan, M.; Eriksson, O.; Wang, Z.; Soam, S. Life cycle assessment and life cycle costing of hydrogen production from biowaste and biomass in Sweden. Energy Convers. Manag. 2023, 291, 117262. [Google Scholar] [CrossRef]
- Udemu, C.; Font-Palma, C. Potential cost savings of large-scale blue hydrogen production via sorption-enhanced steam reforming process. Energy Convers. Manag. 2024, 302, 118132. [Google Scholar] [CrossRef]
- Di Nardo, A.; Portarapillo, M.; Russo, D.; Di Benedetto, A. Hydrogen production via steam reforming of different fuels: Thermodynamic comparison. Int. J. Hydrogen Energy 2024, 55, 1143–1160. [Google Scholar] [CrossRef]
- IEA. Putting CO2 to Use Creating Value from Emissions; IEA: Paris, France, 2019. [Google Scholar]
- Wan, L.; Pang, M.; Le, J.; Xu, Z.; Zhou, H.; Xu, Q.; Wang, B. Oriented intergrowth of the catalyst layer in membrane electrode assembly for alkaline water electrolysis. Nat. Commun. 2022, 13, 7956. [Google Scholar] [CrossRef]
- Zainul, R.; Qayoom, A.; Ahmad, M.S.; Selvaraj, J.; Rahim, N.A. Investigative study of cost-effective ZrO 2 nanoparticles/acrylic based diaphragm for alkaline electrolyzers. IOP Conf. Ser. Earth Environ. Sci. 2023, 1281, 012046. [Google Scholar] [CrossRef]
- Pg Haji Omar Ali, D.N.H.A.; Suhaimi, H.; Abas, P.E. Membrane-Based Hydrogen Production: A Techno-Economic Evaluation of Cost and Feasibility. Hydrogen 2025, 6, 9. [Google Scholar] [CrossRef]
- Dou, W.W.; Ji, Y.; Wu, W. Competition, profitability, and discount rates. J. Financ. Econ. 2021, 140, 582–620. [Google Scholar] [CrossRef]
- Kumar, R. Perspectives on value and valuation. Valuation 2016, 3–46. [Google Scholar] [CrossRef]
- Navarro, I.; Yepes, V.; Martí, J. Life Cycle Cost Assessment of Preventive Strategies Applied to Prestressed Concrete Bridges Exposed to Chlorides. Sustainability 2018, 10, 845. [Google Scholar] [CrossRef]
- Shirasaki, Y.; Tsuneki, T.; Ota, Y.; Yasuda, I.; Tachibana, S.; Nakajima, H.; Kobayashi, K. Development of membrane reformer system for highly efficient hydrogen production from natural gas. Int. J. Hydrogen Energy 2009, 34, 4482–4487. [Google Scholar] [CrossRef]
- Wang, M.; Deng, C.; Chen, H.; Wang, X.; Liu, B.; Sun, C.; Chen, G.; El-Halwagi, M.M. An analytical investigation on the energy efficiency of integration of natural gas hydrate exploitation with H2 production (by in situ CH4 reforming) and CO2 sequestration. Energy Convers. Manag. 2020, 216, 112959. [Google Scholar] [CrossRef]
- El-Shafie, M. Hydrogen production by water electrolysis technologies: A review. Results Eng. 2023, 20, 101426. [Google Scholar] [CrossRef]
- de Groot, M.T.; Kraakman, J.; Garcia Barros, R.L. Optimal operating parameters for advanced alkaline water electrolysis. Int. J. Hydrogen Energy 2022, 47, 34773–34783. [Google Scholar] [CrossRef]
- Todoroki, N.; Wadayama, T. Heterolayered Ni–Fe Hydroxide/Oxide Nanostructures Generated on a Stainless-Steel Substrate for Efficient Alkaline Water Splitting. ACS Appl. Mater. Interfaces 2019, 11, 44161–44169. [Google Scholar] [CrossRef]
- Mandal, B.; Sirkar, A.; De, P.; Baran Kuila, S. Studies on the Effect of Electrolyte Concentration on Alkaline Electrolysis and Ion Exchange Membrane Water Splitting for Production of Hydrogen. Available online: http://www.esatjournals.org (accessed on 9 March 2025).
- Kumar, S.S.; Himabindu, V. Hydrogen production by PEM water electrolysis—A review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar] [CrossRef]
- Sakr, M.; Mahrous, A.-F.M.; Balabel, A.; Ibrahim, K. Experimental Investigation into Hydrogen Production Through Alkaline Water Electrolysis. ERJ. Eng. Res. J. 2011, 34, 37–41. [Google Scholar] [CrossRef]
- Kim, D.; Oh, L.S.; Park, J.H.; Kim, H.J.; Lee, S.; Lim, E. Perovskite-based electrocatalysts for oxygen evolution reaction in alkaline media: A mini review. Front. Chem. 2022, 10, 1024865. [Google Scholar] [CrossRef] [PubMed]
- Haug, P.; Koj, M.; Turek, T. Influence of process conditions on gas purity in alkaline water electrolysis. Int. J. Hydrogen Energy 2017, 42, 9406–9418. [Google Scholar] [CrossRef]
- Katebah, M.; Al-Rawashdeh, M.; Linke, P. Analysis of hydrogen production costs in Steam-Methane Reforming considering integration with electrolysis and CO2 capture. Clean. Eng. Technol. 2022, 10, 100552. [Google Scholar] [CrossRef]
- Zakariazadeh, A.; Ahshan, R.; Al Abri, R.; Al-Abri, M. Renewable energy integration in sustainable water systems: A review. Clean. Eng. Technol. 2024, 18, 100722. [Google Scholar] [CrossRef]
- Lee, B.; Choe, C.; Kim, H.; Kim, A.; Shin, Y.-U.; Haider, J.; Lim, H. Economic Parity Analysis of Green Methanol Synthesis Using Water Electrolysis Based on Renewable Energy. ACS Sustain. Chem. Eng. 2021, 9, 15807–15818. [Google Scholar] [CrossRef]
- Grafton, R.Q.; Manero, A.; Chu, L.; Wyrwoll, P. The Price and Value of Water: An Economic Review. Camb. Prism. Water 2023, 1, e3. [Google Scholar] [CrossRef]
- Ali, S.M.; Alkhatib, I.I.I.; AlHajaj, A.; Vega, L.F. How sustainable and profitable are large-scale hydrogen production plants from CH4 and H2S? J. Clean. Prod. 2023, 428, 139475. [Google Scholar] [CrossRef]
- ECONOMICS (BOUNDLESS) Boundless (Now LumenLearning) Boundless. Available online: https://LibreTexts.org (accessed on 13 November 2024).
- Pei, X.; Tian, H.; Roberts, W.L. Swirling Flame Combustion of Heavy Fuel Oil Blended with Diesel: Effect of Asphaltene Concentration. Energies 2022, 15, 6156. [Google Scholar] [CrossRef]
- Sofiev, M.; Winebrake, J.J.; Johansson, L.; Carr, E.W.; Prank, M.; Soares, J.; Vira, J.; Kouznetsov, R.; Jalkanen, J.-P.; Corbett, J.J. Cleaner fuels for ships provide public health benefits with climate tradeoffs. Nat. Commun. 2018, 9, 406. [Google Scholar] [CrossRef]
- Brauns, J.; Turek, T. Alkaline Water Electrolysis Powered by Renewable Energy: A Review. Processes 2020, 8, 248. [Google Scholar] [CrossRef]
- Chi, J.; Yu, H.; Qin, B.; Fu, L.; Jia, J.; Yi, B.; Shao, Z. Vertically Aligned FeOOH/NiFe Layered Double Hydroxides Electrode for Highly Efficient Oxygen Evolution Reaction. ACS Appl. Mater. Interfaces 2017, 9, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Lokesh, S.; Srivastava, R. CuAl layered double hydroxide as a highly efficient electrocatalyst for the electrolysis of water into hydrogen and oxygen fuels. Int. J. Hydrogen Energy 2023, 48, 35–50. [Google Scholar] [CrossRef]
- Lim, T.; Kim, S.K. Non-precious hydrogen evolution reaction catalysts: Stepping forward to practical polymer electrolyte membrane-based zero-gap water electrolyzers. Chem. Eng. J. 2022, 433, 133681. [Google Scholar] [CrossRef]
- Shi, W.; Zhu, J.; Gong, L.; Feng, D.; Ma, Q.; Yu, J.; Tang, H.; Zhao, Y.; Mu, S. Fe-Incorporated Ni/MoO2 Hollow Heterostructure Nanorod Arrays for High-Efficiency Overall Water Splitting in Alkaline and Seawater Media. Small 2022, 18, 2205683. [Google Scholar] [CrossRef]
- Pagani, G.; Hajimolana, Y.; Acar, C. Green hydrogen for ammonia production—A case for the Netherlands. Int. J. Hydrogen Energy 2024, 52, 418–432. [Google Scholar] [CrossRef]
- Wang, Y.; Williamson, N.; Dawson, R.; Bimbo, N. Electrodeposition of nickel–iron on stainless steel as an efficient electrocatalyst coating for the oxygen evolution reaction in alkaline conditions. J. Appl. Electrochem. 2023, 53, 877–892. [Google Scholar] [CrossRef]
- Hnát, J.; Plevova, M.; Tufa, R.A.; Zitka, J.; Paidar, M.; Bouzek, K. Development and testing of a novel catalyst-coated membrane with platinum-free catalysts for alkaline water electrolysis. Int. J. Hydrogen Energy 2019, 44, 17493–17504. [Google Scholar] [CrossRef]
- Shahidi, S.M.M.; Shayan, M.E.; Najafi, G.; Mazlan, M. Exergy and energy analysis of Organic Rankine Cycle integration in the carbon black industry using pinch technology. Therm. Sci. Eng. Progress. 2023, 46, 102160. [Google Scholar] [CrossRef]
- Arunachalam, M.; Han, D.S. Efficient solar-powered PEM electrolysis for sustainable hydrogen production: An integrated approach. Emergent Mater. 2024, 7, 1401–1415. [Google Scholar] [CrossRef]
- Hnát, J.; Paidar, M.; Schauer, J.; Žitka, J.; Bouzek, K. Polymer anion selective membranes for electrolytic splitting of water. Part I: Stability of ion-exchange groups and impact of the polymer binder. J. Appl. Electrochem. 2011, 41, 1043–1052. [Google Scholar] [CrossRef]
- Li, Y.; Wei, B.; Yu, Z.; Bondarchuk, O.; Araujo, A.; Amorim, I.; Zhang, N.; Xu, J.; Neves, I.C.; Liu, L. Bifunctional Porous Cobalt Phosphide Foam for High-Current-Density Alkaline Water Electrolysis with 4000-h Long Stability. ACS Sustain. Chem. Eng. 2020, 8, 10193–10200. [Google Scholar] [CrossRef]
- Wu, R.; Hu, Z.; Zhang, H.; Wang, J.; Qin, C.; Zhou, Y. Bubbles in Porous Electrodes for Alkaline Water Electrolysis. Langmuir 2024, 40, 721–733. [Google Scholar] [CrossRef] [PubMed]
- Rehberger, H.; Rezaei, M.; Aljabour, A. Challenges and Opportunities of Choosing a Membrane for Electrochemical CO2 Reduction. Membranes 2025, 15, 55. [Google Scholar] [CrossRef]
- Kodir, A.; Yim, S.D.; Lee, H.; Shin, D.; Bae, B. Comprehensive study of hydrogen-crossover reducing agents for polymer electrolyte membranes. J. Membr. Sci. 2024, 707, 122989. [Google Scholar] [CrossRef]
- Brunei Darussalam—Countries & Regions—IEA. Available online: https://www.iea.org/countries/brunei-darussalam (accessed on 9 March 2025).





| Parameter | SMR | Reference | AWE | Reference |
|---|---|---|---|---|
| Feed (kg/h) | 384.11 | Calculated | - | - |
| H2O Feed (kg/h) | 834.98 | Calculated | 2233.68 | Calculated |
| H2 Produced (kg/h) | 143.80 | Calculated | 143.70 | Calculated |
| Condensate (kg/h) | 273.82 | Calculated | - | - |
| CO2 Purge Gas (kg/h) | 905.72 | Calculated | - | - |
| Electrolyte Value (%) | - | - | 35 | [44,45,46] |
| Stack Efficiency (%) | - | - | 57.9 | [45] |
| H2 Separation Efficiency (%) | - | - | 90 | [47,48] |
| H2 Trap Efficiency (%) | - | - | 99 | [49] |
| O2 Separation Efficiency (%) | - | - | 95 | [47,48] |
| O2 Trap Efficiency (%) | - | - | 99 | [49] |
| KOH Feed (kg/h) | - | - | 1202.75 | Calculated |
| O2 Produced (kg/h) | - | - | 1149.6 | Calculated |
| Parameter | Value | Unit | Reference |
|---|---|---|---|
| Plant Lifetime | 20 | year | [50] |
| Hydrogen Production Rate | 1,259,758.1 | kg/year | Calculated |
| Oxygen Production Rate | 10,078,064.8 | kg/year | Calculated |
| Discount Rate | 8 | % | [51] |
| Hydrogen Selling Price | 5.23 | USD/kg | [41] |
| Oxygen Selling Price | 3.14 | USD/kg | [41] |
| Steam Methane Reforming (SMR) | Alkaline Water Electrolysis (AWE) | |
| Preliminary LCC (USD) | 67,707,977.96 | 408,719,639.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, C.C.; Suhainin, M.D.; Pg Haji Omar Ali, D.N.H.A.; Lim, J.Y.; Swee, P.S.; Raymundo, J.Y.; Tan, R.X.H.; Yap, M.K.; Khoo, H.F.; Suhaimi, H.; et al. A Techno-Economic Assessment of Steam Methane Reforming and Alkaline Water Electrolysis for Hydrogen Production. Hydrogen 2025, 6, 23. https://doi.org/10.3390/hydrogen6020023
Chu CC, Suhainin MD, Pg Haji Omar Ali DNHA, Lim JY, Swee PS, Raymundo JY, Tan RXH, Yap MK, Khoo HF, Suhaimi H, et al. A Techno-Economic Assessment of Steam Methane Reforming and Alkaline Water Electrolysis for Hydrogen Production. Hydrogen. 2025; 6(2):23. https://doi.org/10.3390/hydrogen6020023
Chicago/Turabian StyleChu, Ching Cheng, Muhammad Danial Suhainin, Dk Nur Hayati Amali Pg Haji Omar Ali, Jia Yuan Lim, Poh Serng Swee, Jerick Yap Raymundo, Ryan Xin Han Tan, Mei Kei Yap, Hsin Fei Khoo, Hazwani Suhaimi, and et al. 2025. "A Techno-Economic Assessment of Steam Methane Reforming and Alkaline Water Electrolysis for Hydrogen Production" Hydrogen 6, no. 2: 23. https://doi.org/10.3390/hydrogen6020023
APA StyleChu, C. C., Suhainin, M. D., Pg Haji Omar Ali, D. N. H. A., Lim, J. Y., Swee, P. S., Raymundo, J. Y., Tan, R. X. H., Yap, M. K., Khoo, H. F., Suhaimi, H., & Abas, P. E. (2025). A Techno-Economic Assessment of Steam Methane Reforming and Alkaline Water Electrolysis for Hydrogen Production. Hydrogen, 6(2), 23. https://doi.org/10.3390/hydrogen6020023








