Comparative Evaluation of Various ABO3 Perovskites (A = La, Ca, Sr; B = Mn, Fe) as Oxygen Carrier Materials in Chemical Looping Hydrogen Production
Abstract
1. Introduction
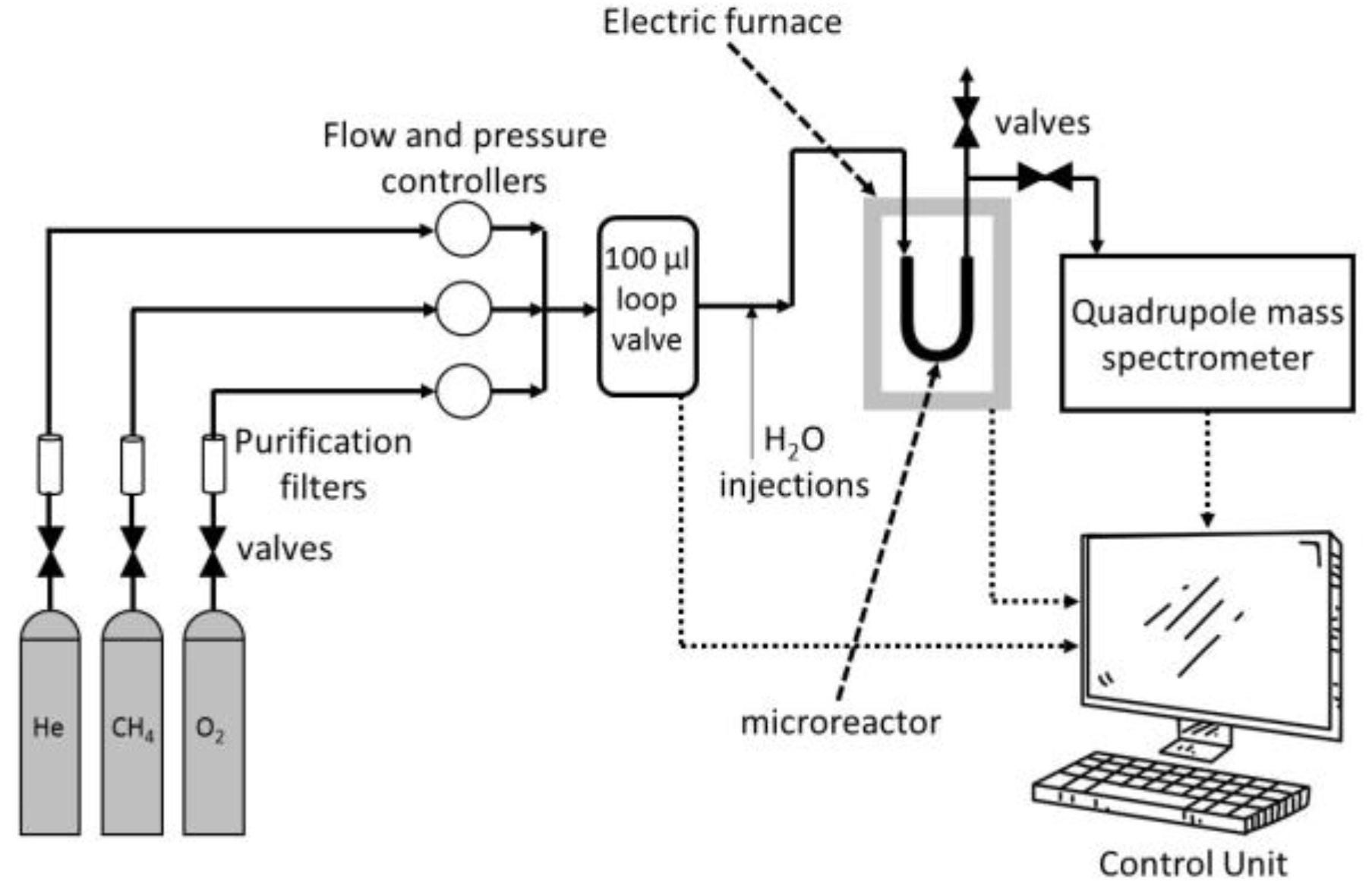
2. Materials and Methods
3. Results
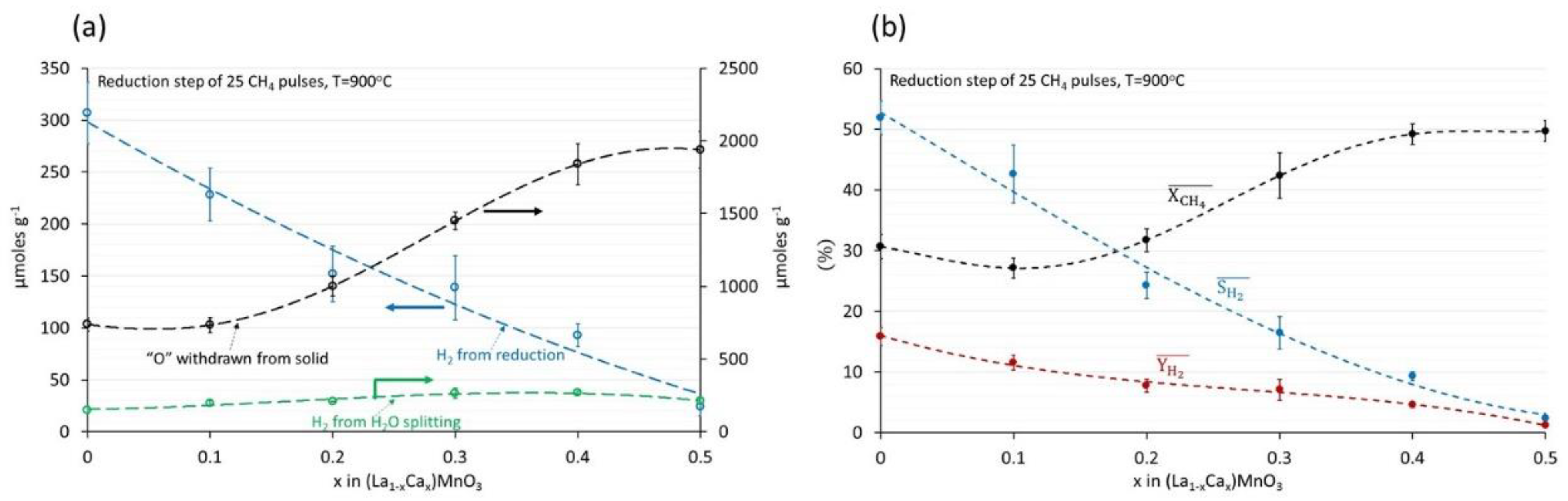
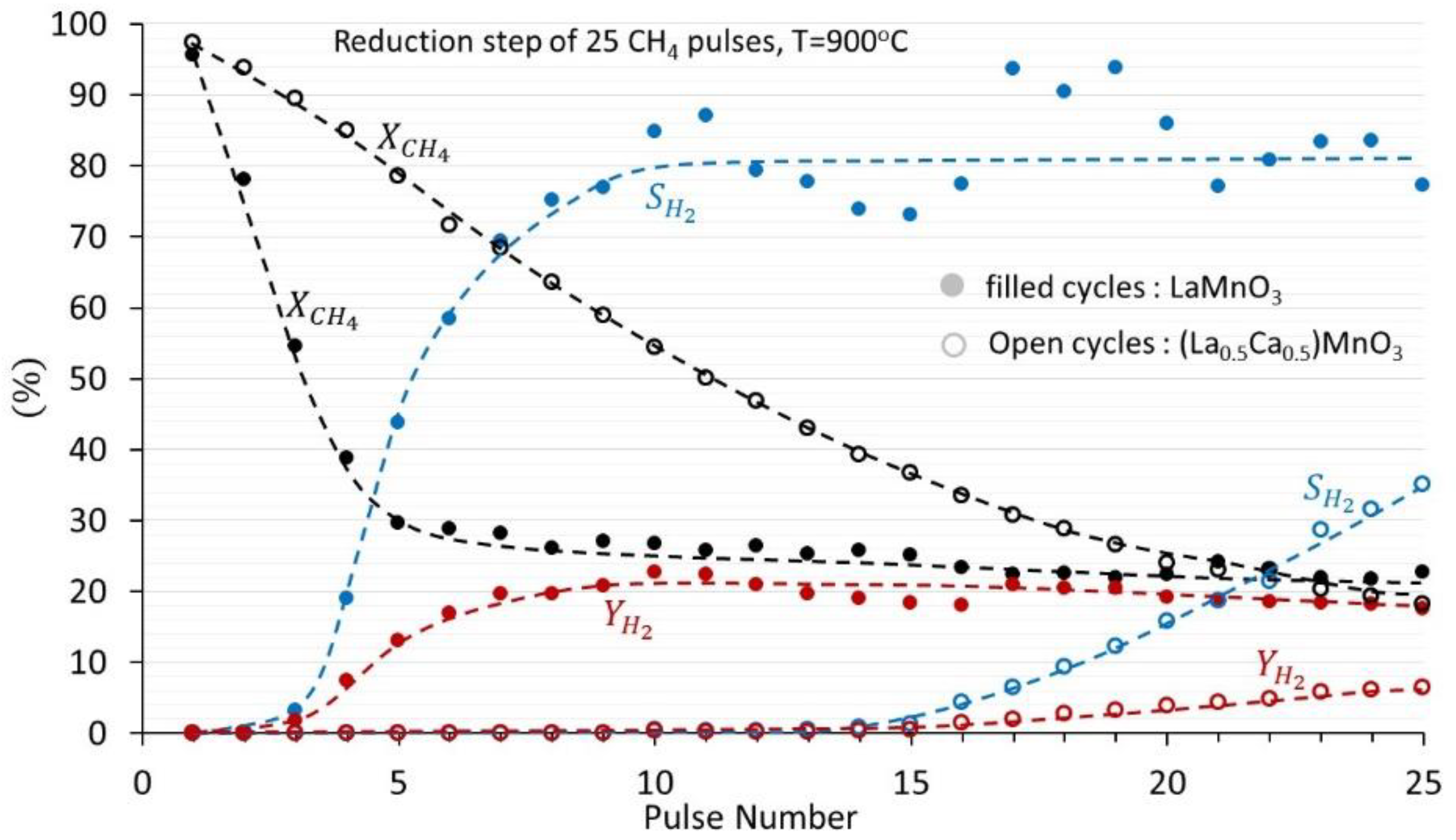
3.1. (La1−xCax)MnO3, x = 0, 0.1, 0.2, 0.3, 0.4, 0.5 Perovskites
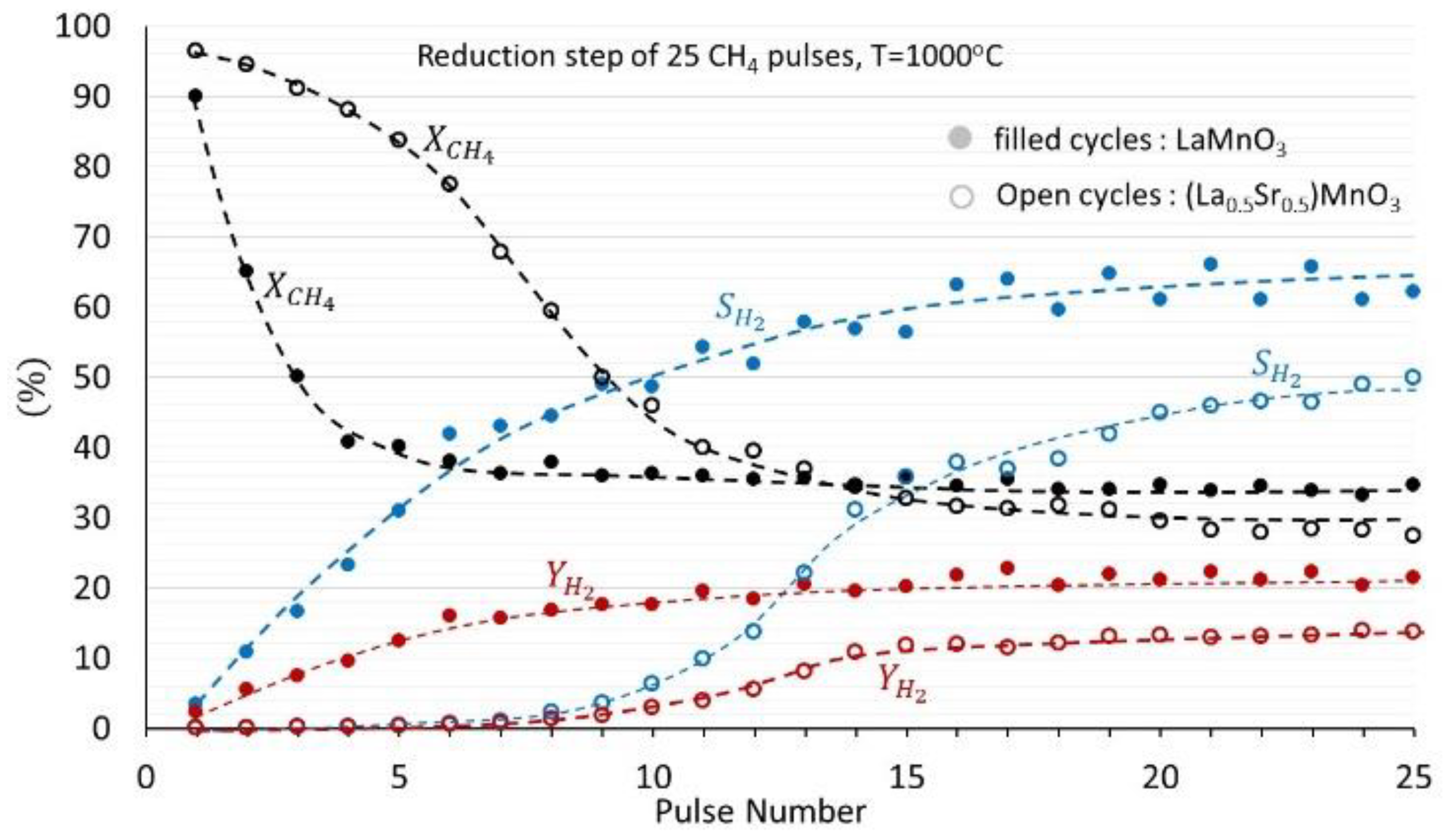
3.2. (La1−xSrx)MnO3, x = 0, 0.3, 0.5 Perovskites
3.3. (La0.6Ca0.4)(Mn1−xFex)O3, x = 0, 0.3, 0.5/LaFeO3 Perovskites
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kim, Y.; Kim, H.S.; Kim, H.; Ha, J.; Kang, D.; Lee, J.W. Recent Strategies of Oxygen Carrier Design in Chemical Looping Processes for Inherent CO2 Capture and Utilization. Korean J. Chem. Eng. 2024, 1–20. [Google Scholar] [CrossRef]
- Zheng, H.; Jiang, X.; Gao, Y.; Tong, A.; Zeng, L. Chemical looping reforming: Process fundamentals and oxygen carriers. Discov. Chem. Eng. 2022, 2, 5. [Google Scholar] [CrossRef]
- Zhu, X.; Imtiaz, Q.; Donat, F.; Müller, C.R.; Li, F. Chemical looping beyond combustion-a perspective. Energy Environ. Sci. 2020, 13, 772–804. [Google Scholar] [CrossRef]
- Das, S.; Biswas, A.; Tiwary, C.S.; Paliwal, M. Hydrogen production using chemical looping technology: A review with emphasis on H2 yield of various oxygen carriers. Int. J. Hydrogen Energy 2022, 47, 28322–28352. [Google Scholar] [CrossRef]
- Li, R.; Zeng, J.; Wei, Y.; Chen, Z. Integration of supervised machine learning for predictive evaluation of chemical looping hydrogen production and storage system. Sustain. Energy Fuels 2025, 9, 640–650. [Google Scholar] [CrossRef]
- Voitic, G.; Hacker, V. Recent advancements in chemical looping water splitting for the production of hydrogen. RSC Adv. 2016, 6, 98267. [Google Scholar] [CrossRef]
- Cho, W.C.; Lee, D.Y.; Seo, M.W.; Kim, S.D.; Kang, K.; Bae, K.K.; Kim, C.H.; Jeong, S.; Park, C.S. Continuous operation characteristics of chemical looping hydrogen production system. Appl. Energy 2014, 113, 1667–1674. [Google Scholar] [CrossRef]
- Li, M.; Qiu, Y.; Ma, L.; Cui, D.; Zhang, S.; Zeng, D.; Xiao, R. Chemical looping hydrogen storage and production: Use of binary ferrite-spinel as oxygen carrier materials. Sustain. Energy Fuels 2020, 4, 1665–1673. [Google Scholar] [CrossRef]
- Yüzbasi, N.S.; Armutlulu, A.; Huthwelker, T.; Abdala, P.M.; Müller, R.C. Na-β-Al2O3 stabilized Fe2O3 oxygen carriers for chemical looping water splitting: Correlating structure with redox stability. J. Mater. Chem. A 2022, 10, 10692–10700. [Google Scholar] [CrossRef]
- Dueso, C.; Ortiz, M.; Abad, A.; García-Labiano, F.; de Diego, L.F.; Gayán, P.; Adánez, J. Reduction and oxidation kinetics of nickel-based oxygen-carriers for chemical-looping combustion and chemical-looping reforming. Chem. Eng. J. 2012, 188, 142–154. [Google Scholar] [CrossRef]
- Yuan, H.; Huang, Z.; He, F.; Zhang, S. Thermodynamic and experimental investigation on chemical looping hydrogen of alkali lignin pyrolysis gas with NiFe2O4 oxygen carrier. Fuel 2025, 386, 134199. [Google Scholar] [CrossRef]
- Imtiaz, Q.; Armutlulu, A.; Donat, F.; Müller, C. CuO-based materials for thermochemical redox cycles: The influence of the formation of a CuO percolation network on oxygen release and oxidation kinetics. Disc. Chem. Eng. 2022, 2, 6. [Google Scholar] [CrossRef] [PubMed]
- Costa, T.; Gayán, P.; Abad, A.; García-Labiano, F.; de Diego, L.; Melo, D.; Adánez, J. Mn-based oxygen carriers prepared by impregnation for Chemical Looping Combustion with diverse fuels. Fuel Process. Technol. 2018, 178, 236–250. [Google Scholar] [CrossRef]
- Zornoza, B.; Mendiara, T.; Abad, A. Evaluation of oxygen carriers based on manganese-iron mixed oxides prepared from natural ores or industrial waste products for chemical looping processes. Fuel Process. Technol. 2022, 234, 107313. [Google Scholar] [CrossRef]
- Liu, C.; Park, J.; De Santiago, H.A.; Xu, B.; Li, W.; Zhang, D.; Zhou, L.; Qi, Y.; Luo, J.; Liu, X. Perovskite Oxide Materials for Solar Thermochemical Hydrogen Production from Water Splitting through Chemical Looping. ACS Catal. 2024, 14, 14974–15013. [Google Scholar] [CrossRef]
- De Vos, Y.; Jabobs, M.; Van Der Voort, P.; Van Driessche, I.; Snijkers, F.; Verberckmoes, A. Development of stable oxygen carrier materials for chemical looping processes-A Review. Catalysts 2020, 10, 926. [Google Scholar] [CrossRef]
- Mattison, T. Materials for Chemical-Looping with Oxygen Uncoupling. ISRN Chem. Eng. 2013, 2013, 526375. [Google Scholar] [CrossRef]
- Rydén, M.; Leion, H.; Mattison, T.; Lyngfelt, A. Combined oxides as oxygen-carrrier material for chemical-looping with oxygen uncoupling. Appl. Energy 2014, 113, 1924–1932. [Google Scholar] [CrossRef]
- Arjmand, M.; Kooiman, R.F.; Rydén, M.; Leion, H.; Mattisson, T.; Lyngfelt, A. Sulfur Tolerance of CaxMn1−yMyO3−δ (Μ = Mg, Ti) Perovskite-Type Oxygen Carriers in Chemical-Looping with Oxygen Uncoupling (CLOU). Energy Fuels 2014, 28, 1312–1324. [Google Scholar] [CrossRef]
- Evdou, A.; Zaspalis, V.; Nalbandian, L. La1−xSrxMnO3−δ perovskites as redox materials for the production of high purity hydrogen. Int. J. Hydrogen Energy 2008, 33, 5554–5562. [Google Scholar] [CrossRef]
- Evdou, A.; Georgitsis, T.; Matsuka, C.; Pacahtouridou, E.; Iliopoulou, E.; Zaspalis, V. Defect Chemistry and Chemical Looping Performance of La1−xMxMnO3 (M = Sr, Ca, (x = 0–0.5)) Perovskites. Nanomaterials 2022, 12, 3461. [Google Scholar] [CrossRef] [PubMed]
- Iliopoulou, E.F.; Matsouka, C.; Pachatouridou, E.; Papadopoulou, F.; Psarras, A.; Evdou, A.; Zaspalis, V.; Nalbandian, L. Novel La1−xCa xMnO3 perovskite materials for chemical looping combustion applications. Int. J. Energy Res. 2022, 46, 20386–20400. [Google Scholar] [CrossRef]
- Yin, X.; Wang, S.; Wang, B.; Shen, L. Chemical looping steam methane reforming using Al doped LaMnO3+δ perovskites as oxygen carriers. Int. J. Hydrogen Energy 2021, 46, 33375–33387. [Google Scholar] [CrossRef]
- Yang, Z.; Zheng, K.L.; Wang, Y.; Wang, H.; Wang, L.; Jiang, X.; Zhu, X.; Wei, Y. Chemical-looping reforming of methane over La-Mn-Fe-O oxygen carriers: Effect of calcination temperature. Chem. Eng. Sci. 2021, 229, 116085. [Google Scholar] [CrossRef]
- Yu, Z.; Yang, Y.; Yang, S.; Zhang, Q.; Zhao, J.; Fang, Y.; Hao, X.; Guan, G. Iron-based oxygen carriers in chemical looping conversions: A review. Carbon Resour. Convers. 2019, 2, 23–34. [Google Scholar] [CrossRef]
- Dawa, T.; Sajjadi, B. Exploring the potential of perovskite structures for chemical looping technology: A state-of-the-art review. Fuel Process. Technol. 2024, 253, 108022. [Google Scholar] [CrossRef]
- Yang, J.; Bjørgum, E.; Chang, H.; Zhu, K.-K.; Sui, Z.-J.; Zhou, X.-G.; Holmen, A.; Zhu, Y.-A.; Chen, D. On the ensemble requirement of fully selective chemical looping methane partial oxidation over La-Fe-based perovskites. Appl. Catal. B Environ. 2022, 301, 120788. [Google Scholar] [CrossRef]
- Kumar, S.; Cheng, Z.; Gun, S.; Qin, L.; Colijn, H.; Mohammad, Z.; Fan, L.-S. Nanoscale structural transformations in LaFeO3 oxygen carriers for enhanced reactivity in chemical looping combustion. Powder Technol. 2024, 444, 1199888. [Google Scholar] [CrossRef]
- Zhao, K.; He, F.; Huang, Z.; Zheng, A.; Li, H.; Zhao, Z. Three-dimensionally ordered macroporous LaFeO3 perovskites for chemical-looping steam reforming of methane. Int. J. Hydrogen Energy 2014, 39, 3243–3252. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Lu, C.; Li, D.; Li, Z.; Gao, J.; Wei, J.; Li, K. Enhanced performance of LaFeO3 oxygen carriers by NiO for chemical looping partial oxidation of methane. Fuel Process. Technol. 2022, 236, 107396. [Google Scholar] [CrossRef]
- Sastre, D.; Serrano, D.P.; Pizzaro, P.; Coronado, J.M. Chemical insights on the activity of La1−xSrxFeO3 perovskites for chemical looping reforming of methane coupled with CO2-splitting. J. CO2 Util. 2019, 31, 16–26. [Google Scholar] [CrossRef]
- Zhao, K.; He, F.; Huang, Z.; Wei, A.; Zheng, H.; Li, Z.; Zhao, Z. Perovskite-type oxides LaFe1−xCoxO3 for chemical looping steam methanne reforming to syngas and hydrogen co-production. Appl. Energy 2016, 168, 192–203. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, H.S.; Kang, D.; Kim, M.; Lee, J.W. Enhanced redox performance of LaFeO3 perovskite through in-situ exsolution of iridium nanoparticles for chemical looping steam methane reforming. Chem. Eng. J. 2023, 468, 143662. [Google Scholar] [CrossRef]
- Nalbandian, L.; Evdou, A.; Zaspalis, V. La1−xSrxMyFe1−yO3−δ perovskites as oxygen-carrier materials for chemical looping reforming. Int. J. Hydrogen Energy 2011, 36, 6657–6670. [Google Scholar] [CrossRef]
- Heuer, S.A.; Schierholz, R.; Alekseev, E.V.; Peters, L.; Mueller, D.N.; Duchoň, T.; Vibhu, V.; Tempel, H.; de Haart, L.G.J.; Kungl, H.; et al. Oxygen Nonstoichiometry and Valence State of Manganese in La1−xCaxMnO3+δ. ACS Omega 2021, 6, 9638–9652. [Google Scholar] [CrossRef]
- Goldyreva, E.I.; Leonidov, I.A.; Patrakeev, M.V.; Kozhevnikov, V.L. Oxygen non-stoichiometry and defect equilibria in CaMnO3−δ. J. Sol. St. Electrochem. 2012, 16, 1187–1191. [Google Scholar] [CrossRef]
- Kuo, J.H.; Anderson, H.U. Oxidation-reduction behavior of undoped and Sr-doped LaMnO3: Defect structure, electrical conductivity and thermoelectric power. J. Sol. St. Chem. 1990, 87, 55–63. [Google Scholar] [CrossRef]
- Nowotny, J.; Rekas, M. Defect chemistry of (La, Sr)MnO3. J. Am. Ceram. Soc. 1998, 81, 67–80. [Google Scholar] [CrossRef]
- Kröger, F.A. The Chemistry of Imperfect Crystals; North-Holland Publishing Company: Amsterdam, The Netherlands, 1964. [Google Scholar] [CrossRef]
- Zhang, X.; Li, H.; Li, Y.; Shen, W. Structural properties and catalytic activity of Sr-substituted LaFeO3 perovskite. Chin. J. Catal. 2012, 33, 1019–1114. [Google Scholar] [CrossRef]
- Wærnhus, I.; Grande, T.; Wiik, K. Electronic properties of polycrystalline LaFeO3. Part II: Defect modelling including Schottky defects. Sol. St. Ion. 2005, 176, 2609–2616. [Google Scholar] [CrossRef]
- Reijnen, P.J.L. Sintering behavior and microstructures of aluminates and ferrites with spinel structure with regard to deviation from stoichiometry. Sci. Ceram. 1968, 4, 169–188. [Google Scholar]
- Lee, M.; Lim, H.S.; Kim, Y.; Lee, J.W. Enhancement of highly-concentrated hydrogen productivity in chemical looping steam methane reforming using Fe-substituted LaCoO3. Energy Convers. Manag. 2020, 207, 112507. [Google Scholar] [CrossRef]
- Pecchi, G.; Reyes, P.; Zamora, R.; Campos, C.; Cadus, L.E.; Barbero, B. Effect of the preparation method on the catalytic activity of La1−xCaxFeO3 perovskite-type oxides. Catal. Today 2008, 133–135, 420–427. [Google Scholar] [CrossRef]


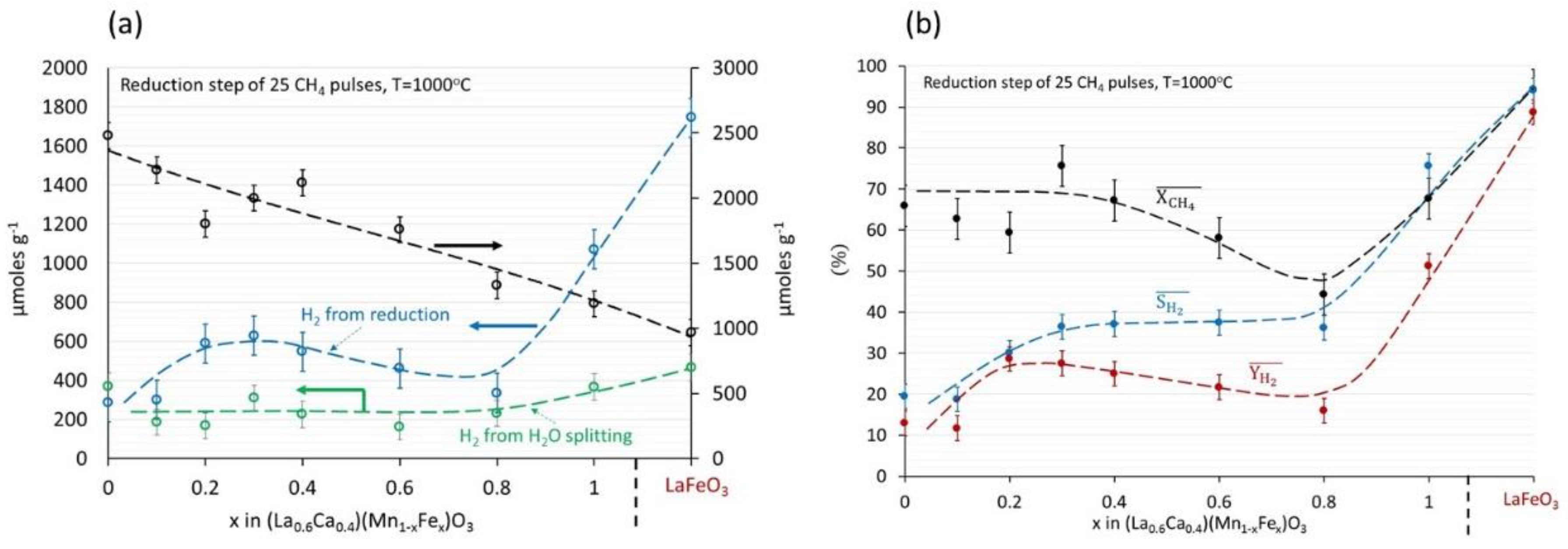

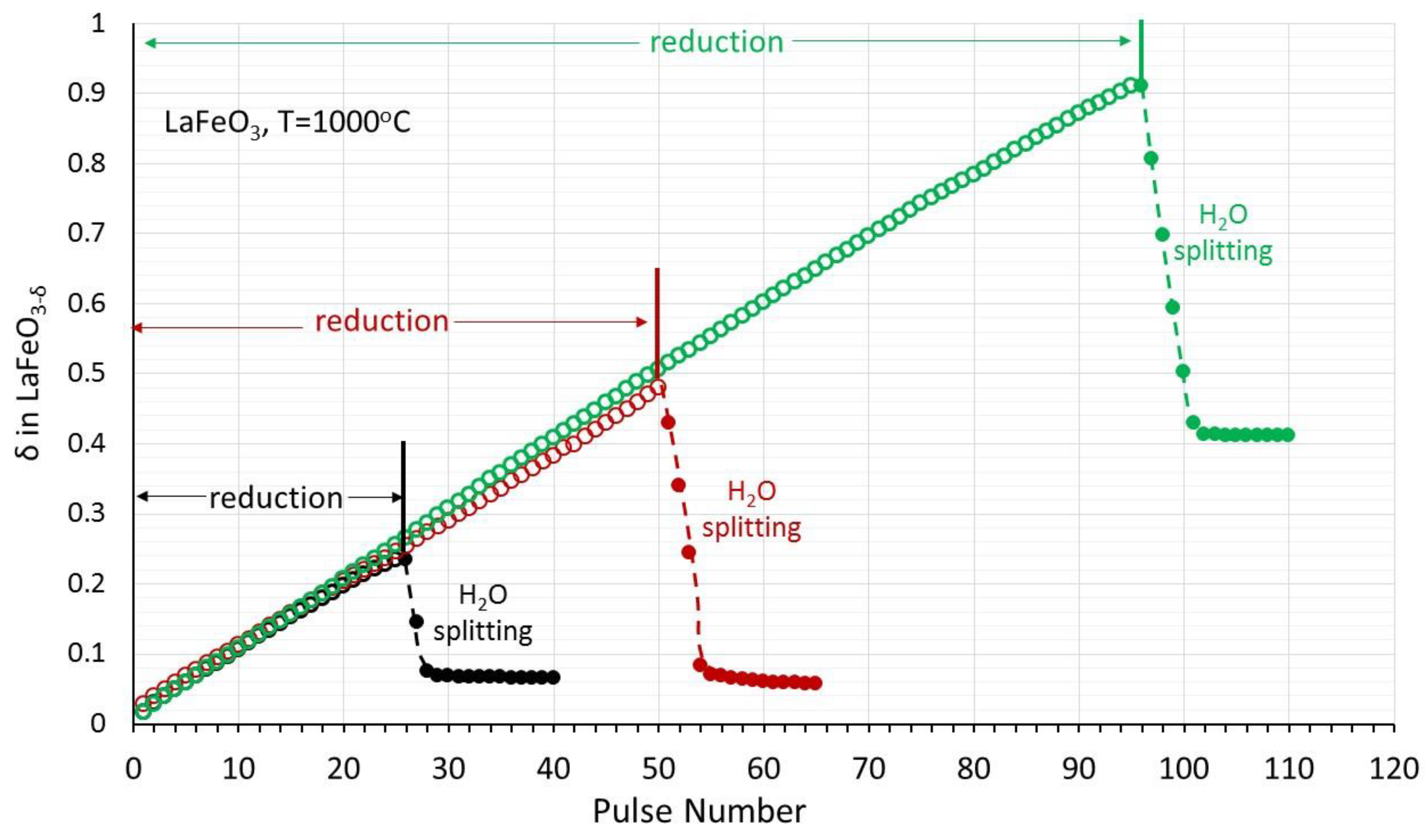
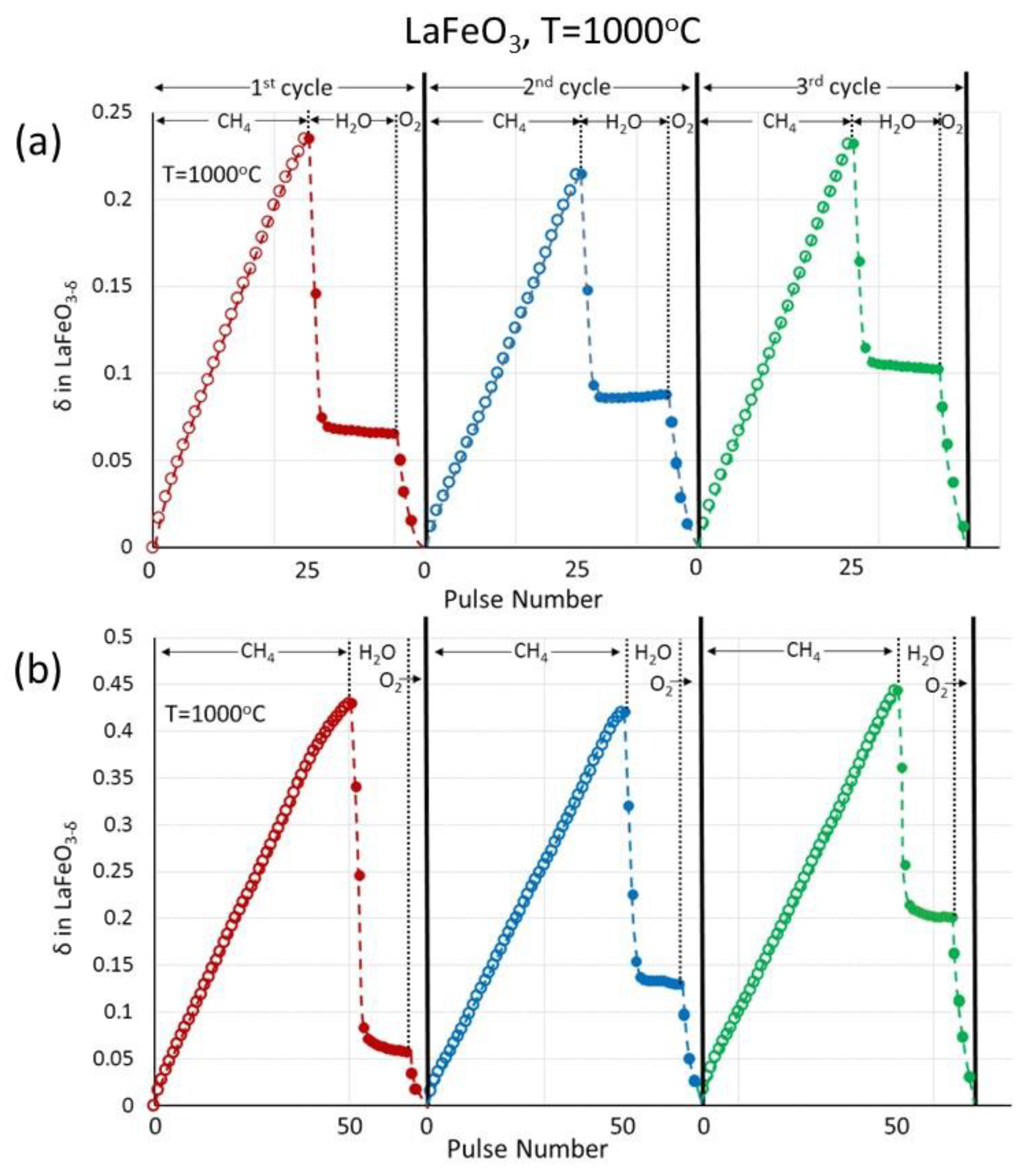
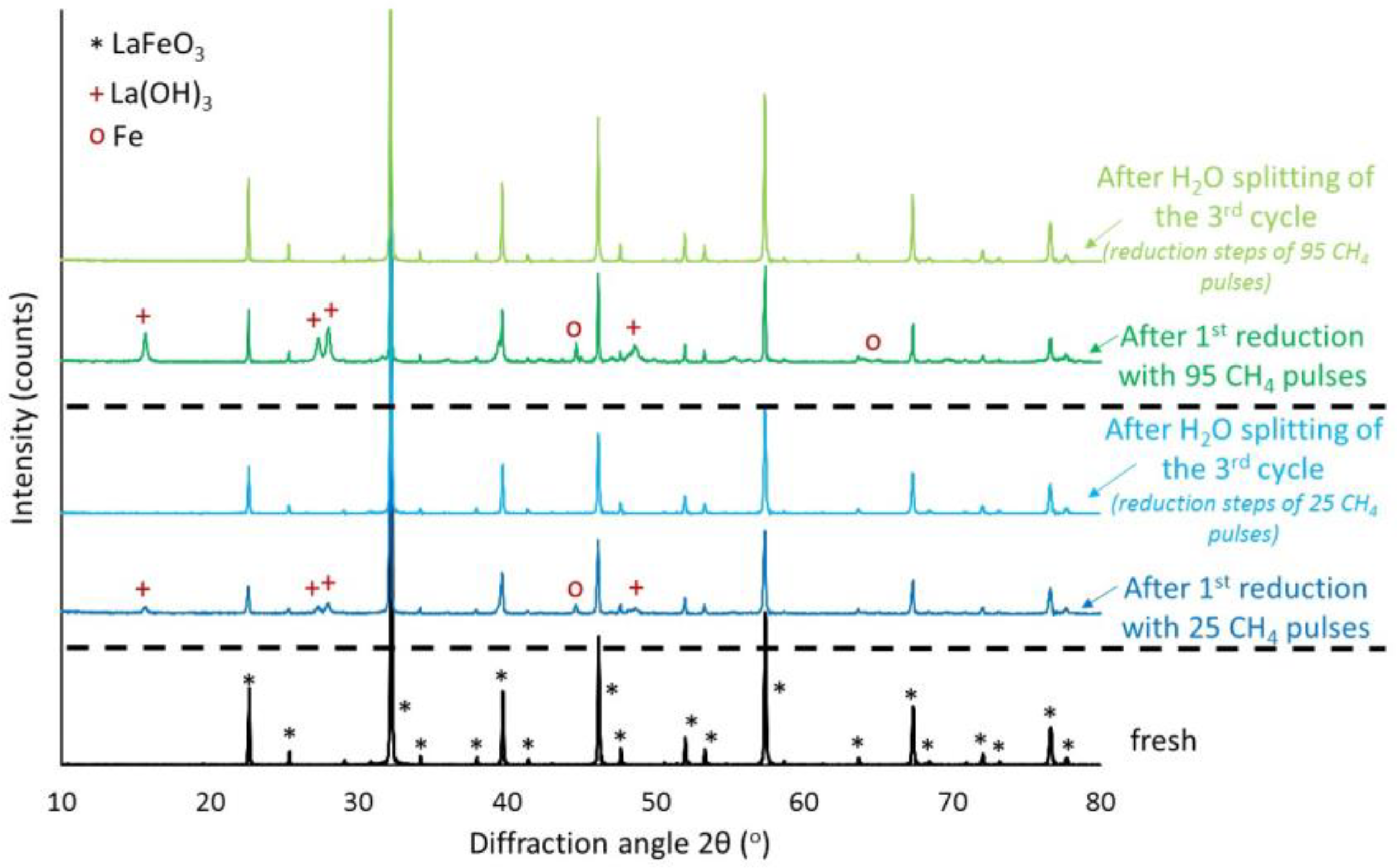

| Pulse Nr. | 25 | 50 | 95 |
|---|---|---|---|
| Reduction Step | |||
| H2 produced (μmole g−1) | 1745 | 3739 | 7748 |
| (%) | 94 | 93.5 | 90.5 |
| (%) | 98 | 99.9% | 99.9% |
| (%) | 92.5 | 95 | 92 |
| Water Splitting Step | |||
| H2 produced (μmole g−1) | 700 | 1538 | 2059 |
| (%) | 100% | 100% | 100% |
| Total H2 produced per cycle (μmole g−1) | |||
| 2445 | 5277 | 9807 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evdou, A.; Zaspalis, V. Comparative Evaluation of Various ABO3 Perovskites (A = La, Ca, Sr; B = Mn, Fe) as Oxygen Carrier Materials in Chemical Looping Hydrogen Production. Hydrogen 2025, 6, 27. https://doi.org/10.3390/hydrogen6020027
Evdou A, Zaspalis V. Comparative Evaluation of Various ABO3 Perovskites (A = La, Ca, Sr; B = Mn, Fe) as Oxygen Carrier Materials in Chemical Looping Hydrogen Production. Hydrogen. 2025; 6(2):27. https://doi.org/10.3390/hydrogen6020027
Chicago/Turabian StyleEvdou, Antigoni, and Vassilis Zaspalis. 2025. "Comparative Evaluation of Various ABO3 Perovskites (A = La, Ca, Sr; B = Mn, Fe) as Oxygen Carrier Materials in Chemical Looping Hydrogen Production" Hydrogen 6, no. 2: 27. https://doi.org/10.3390/hydrogen6020027
APA StyleEvdou, A., & Zaspalis, V. (2025). Comparative Evaluation of Various ABO3 Perovskites (A = La, Ca, Sr; B = Mn, Fe) as Oxygen Carrier Materials in Chemical Looping Hydrogen Production. Hydrogen, 6(2), 27. https://doi.org/10.3390/hydrogen6020027






