Assessing the Effect of Mineralogy and Reaction Pathways on Geological Hydrogen (H2) Generation in Ultramafic and Mafic (Basaltic) Rocks
Abstract
1. Introduction
2. Methodology
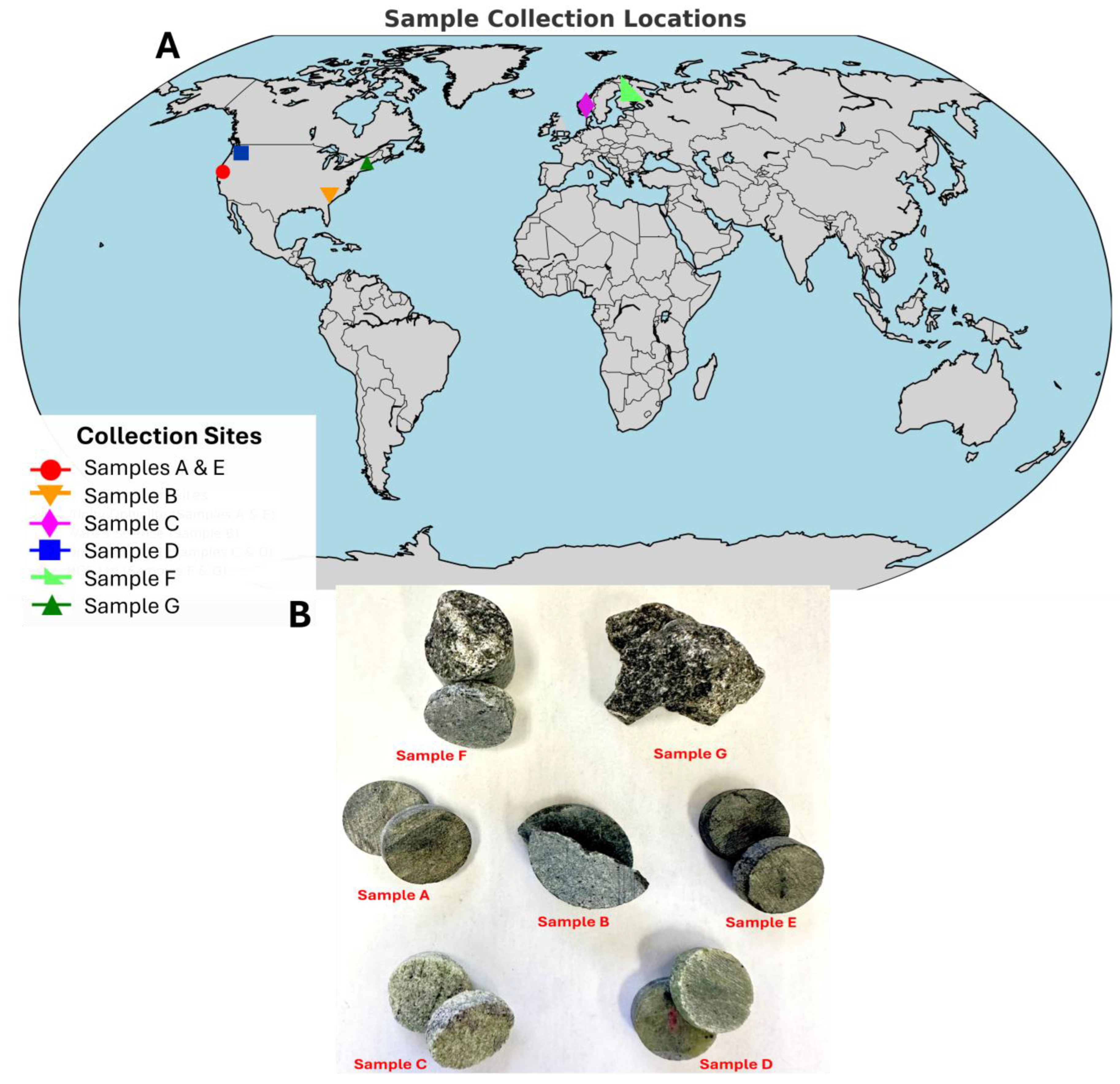
2.1. Rock Samples
2.2. Experimental Description
2.2.1. Fluids Analysis
2.2.2. Mineral Characterization Using X-Ray Diffraction (XRD)
2.2.3. X-Ray Photoelectron Spectroscopy (XPS)
3. Results
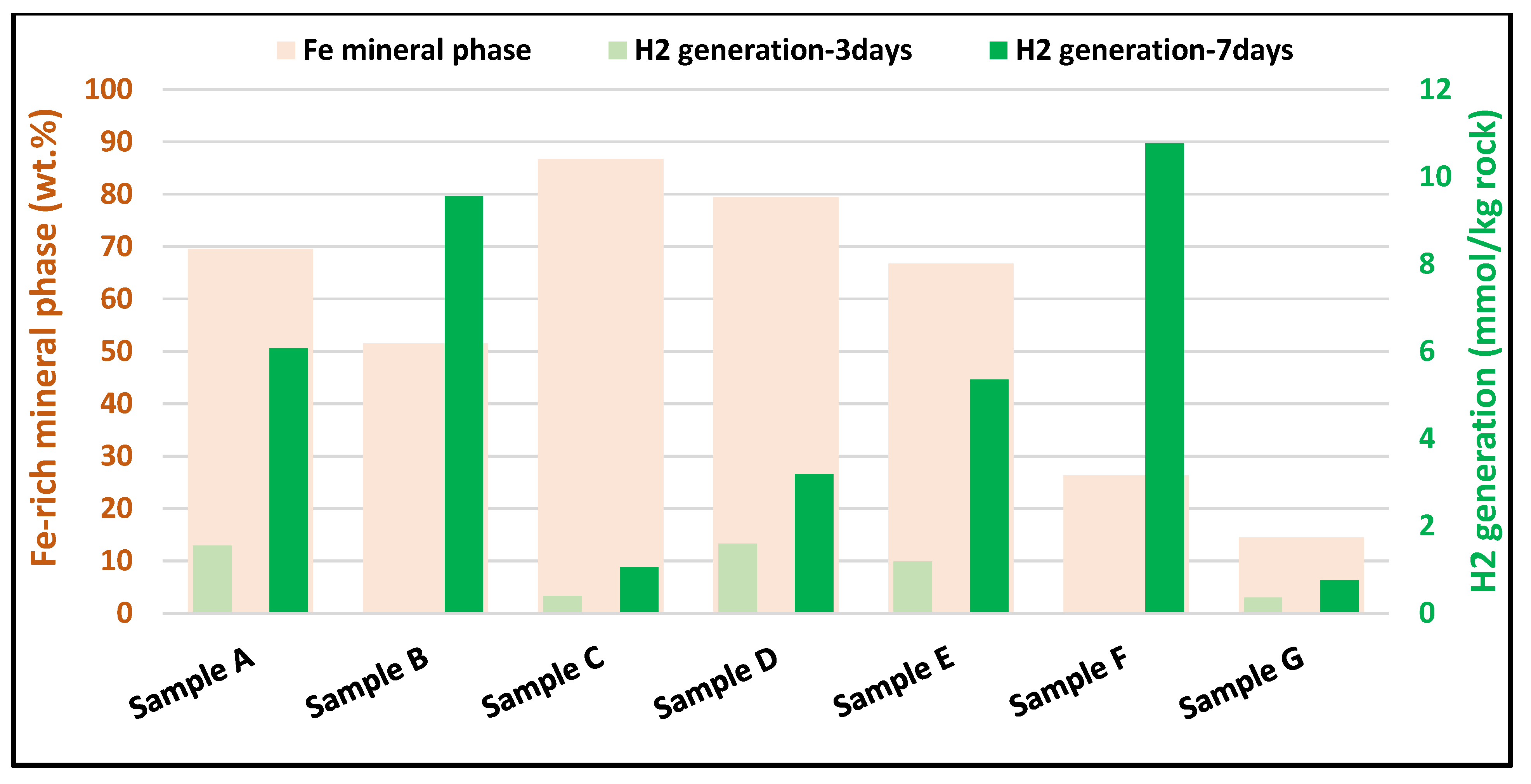
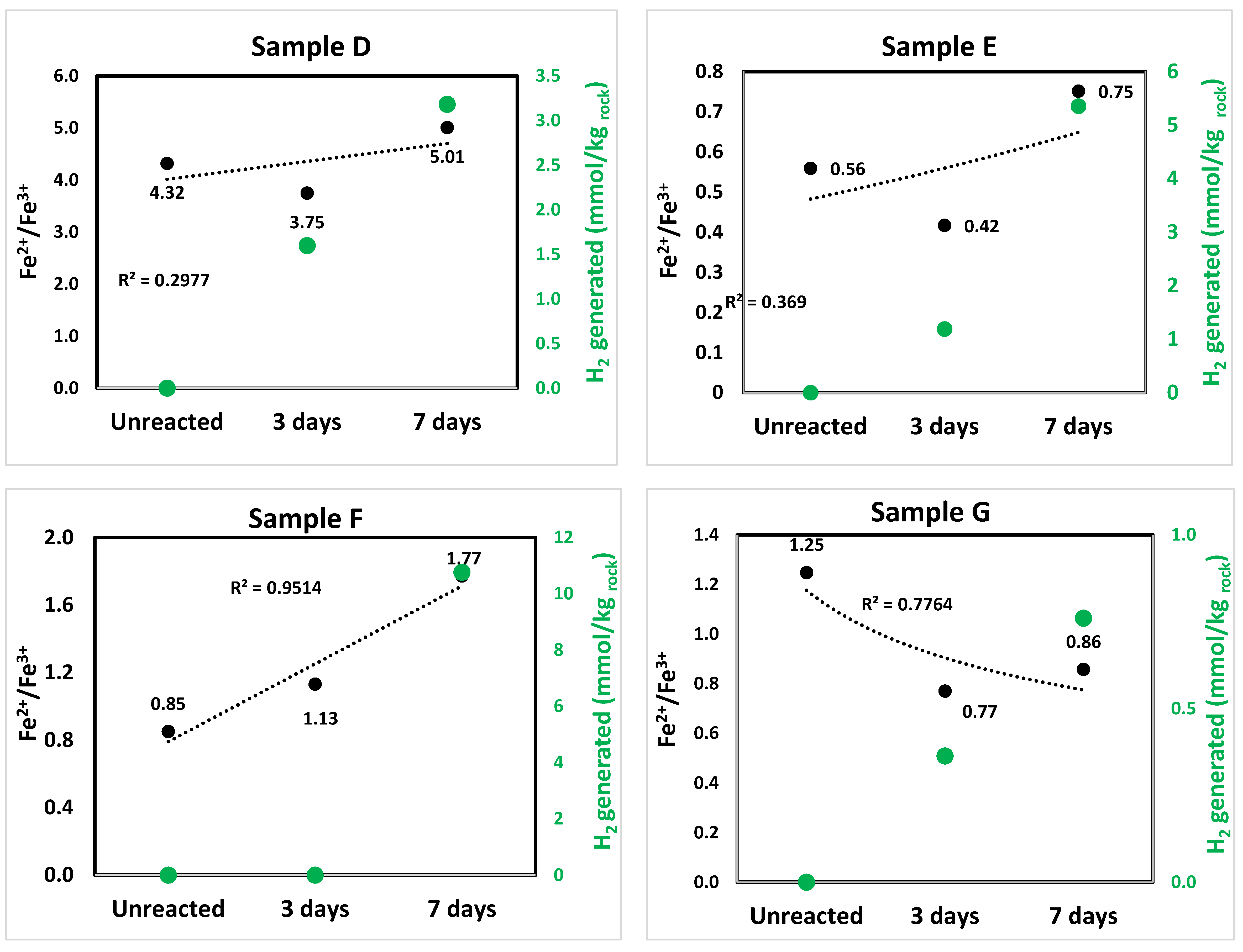
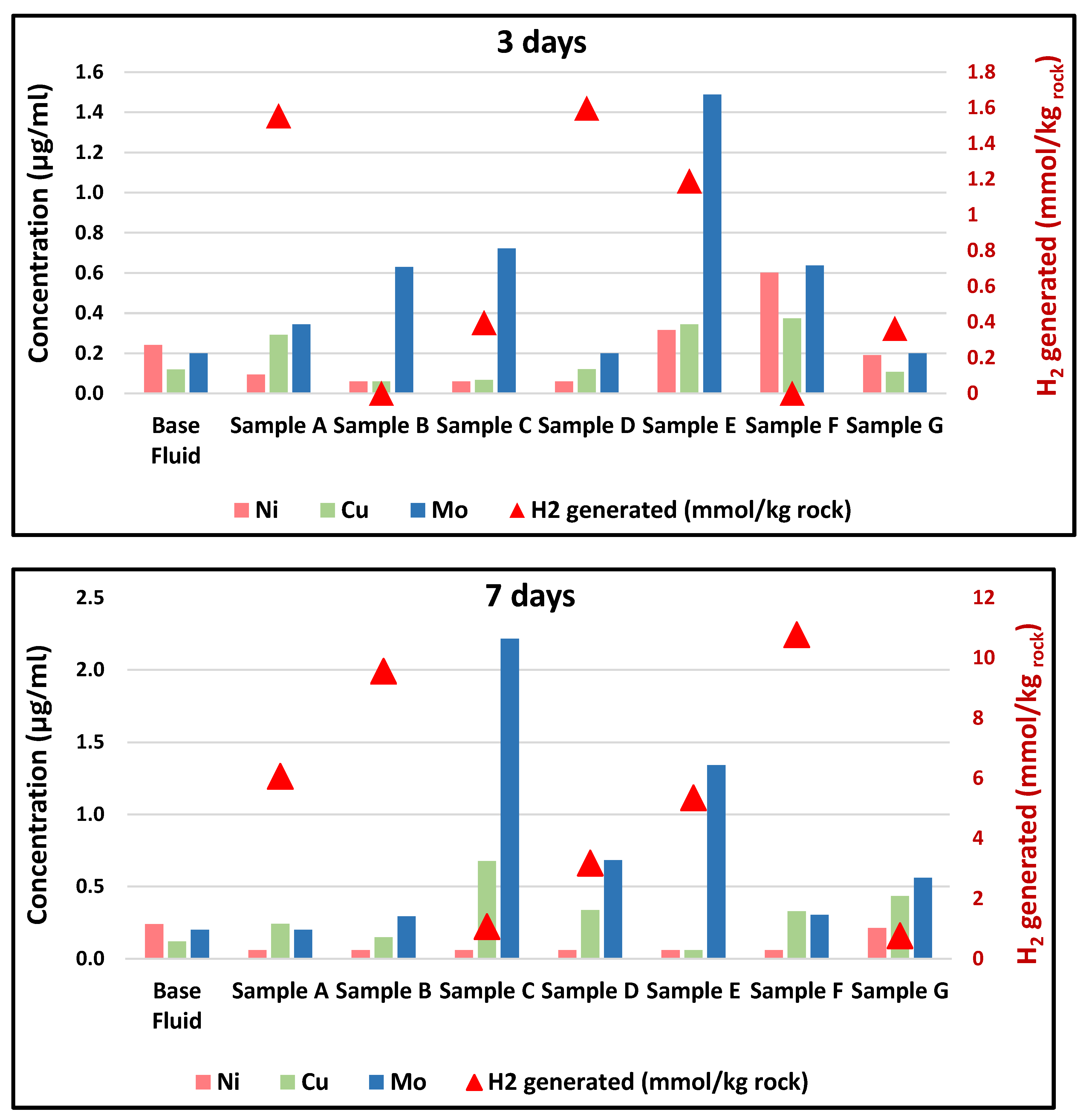
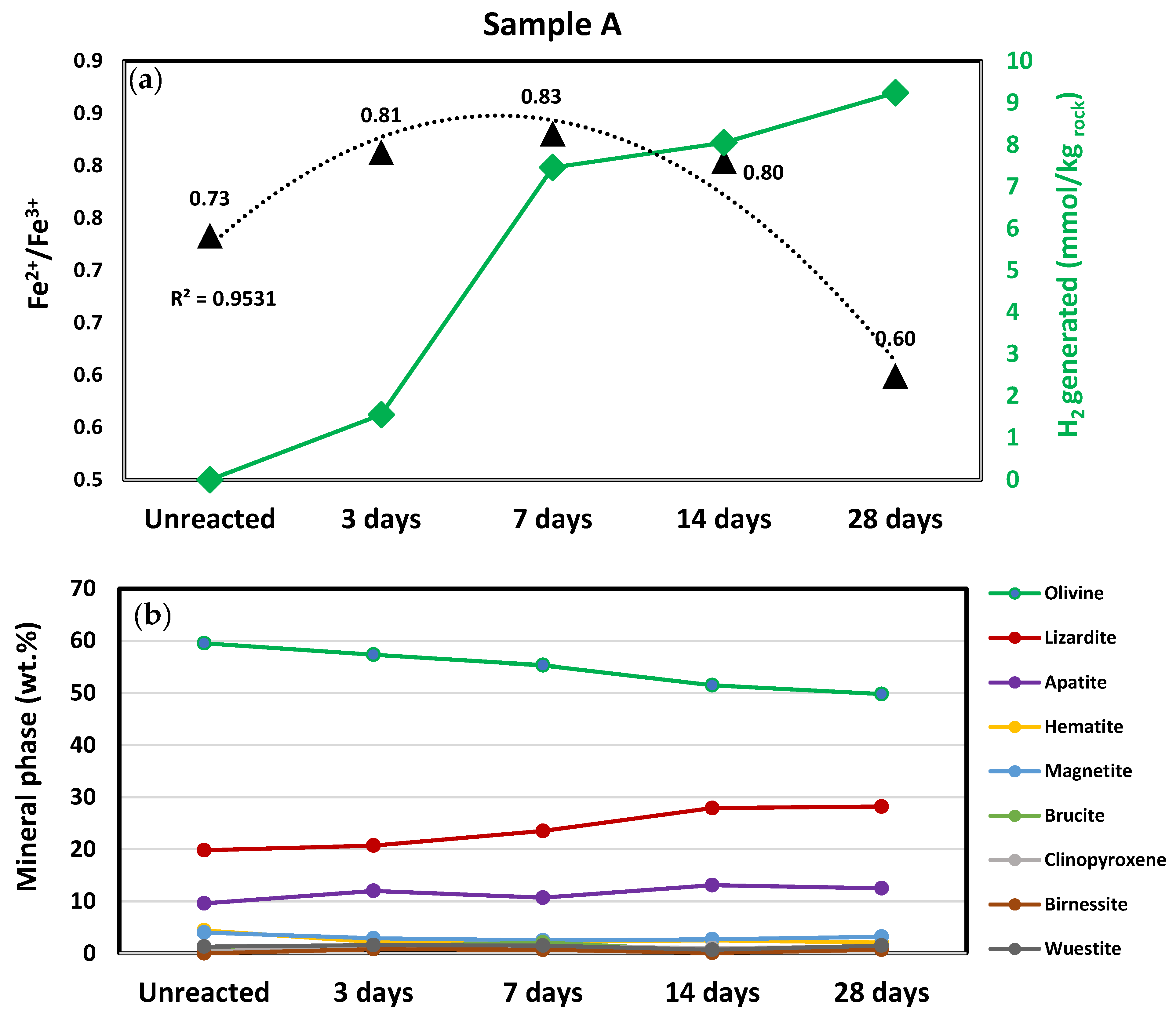
3.1. Hydrogen Generation
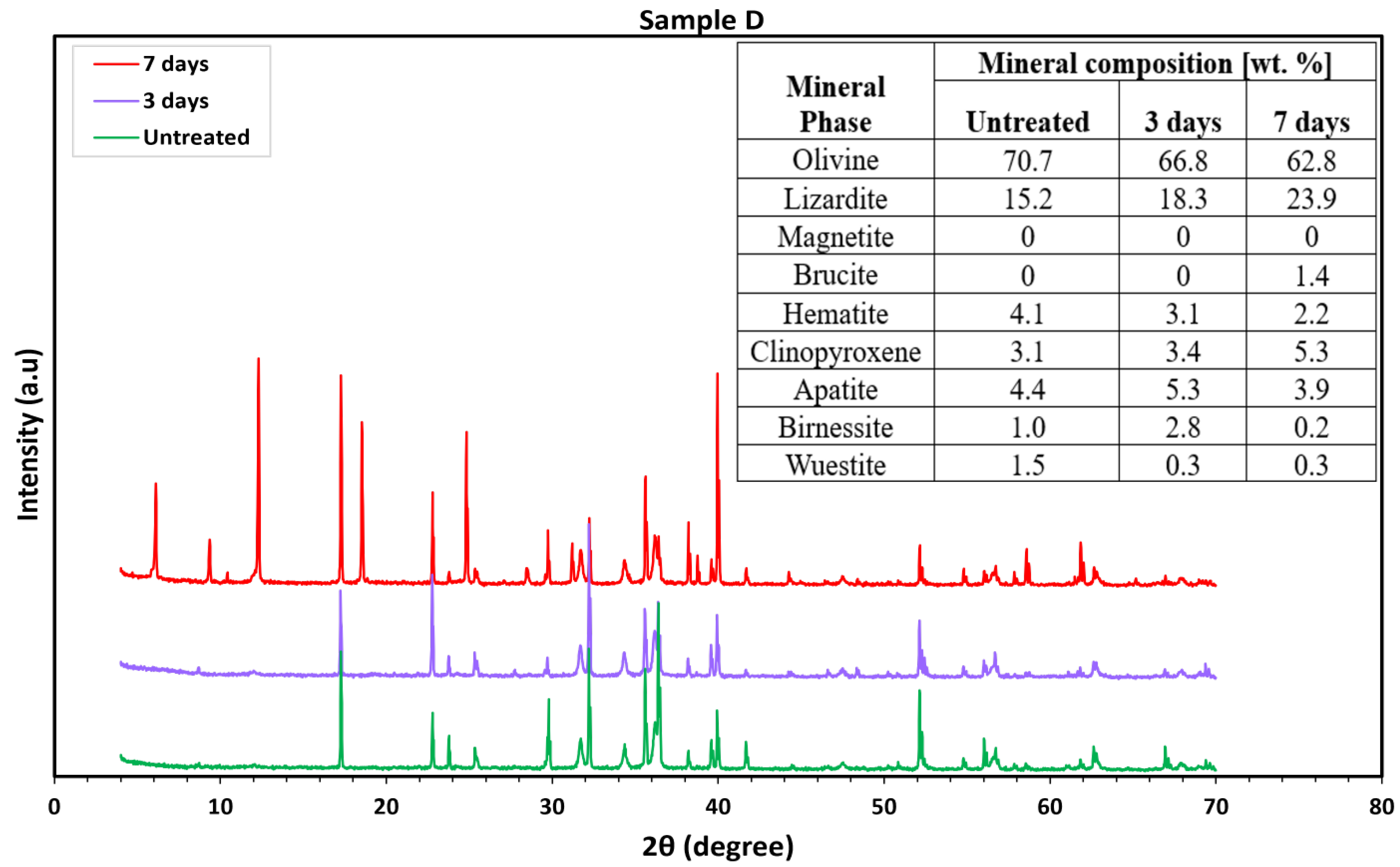
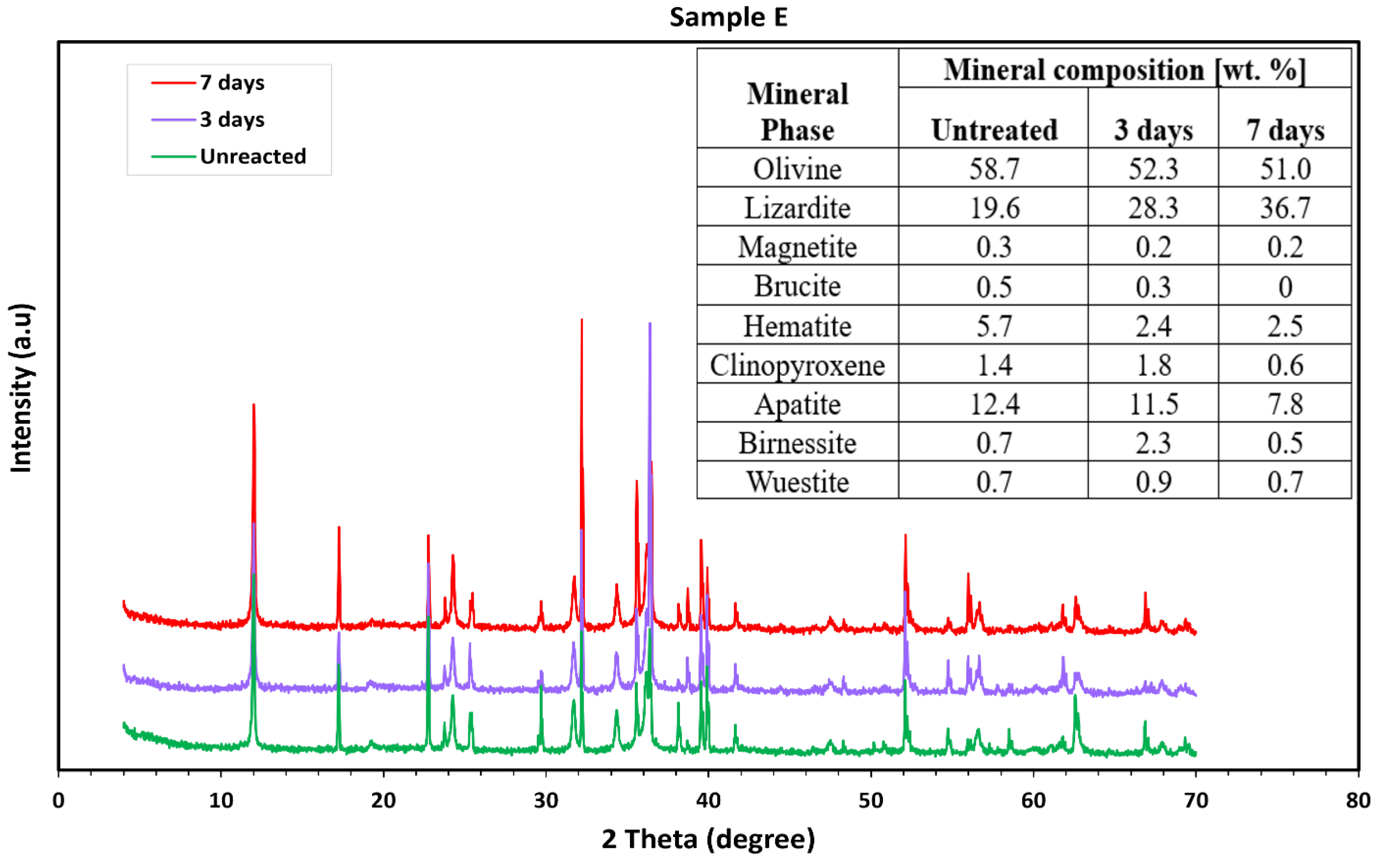
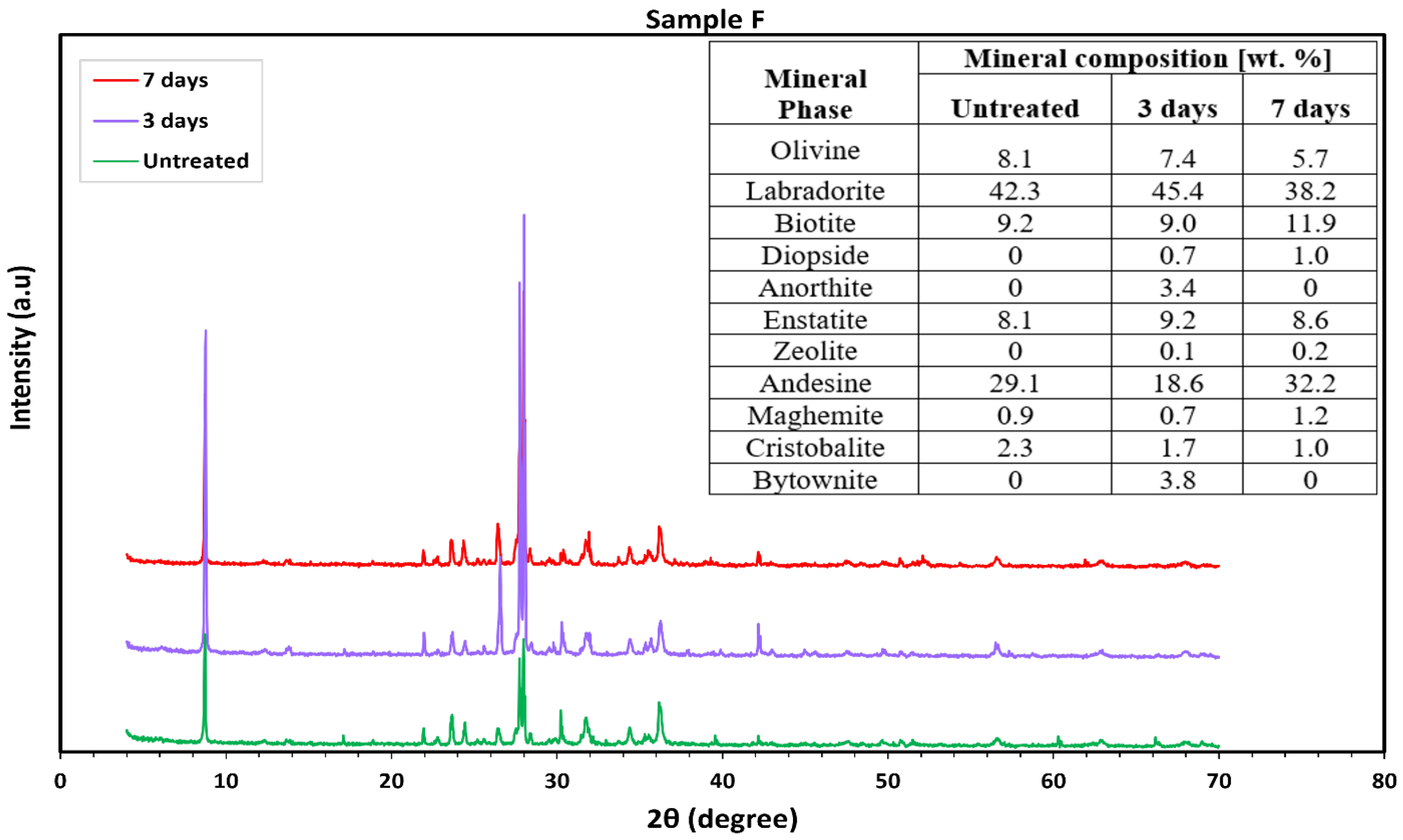
3.2. Mineralogical Characterization
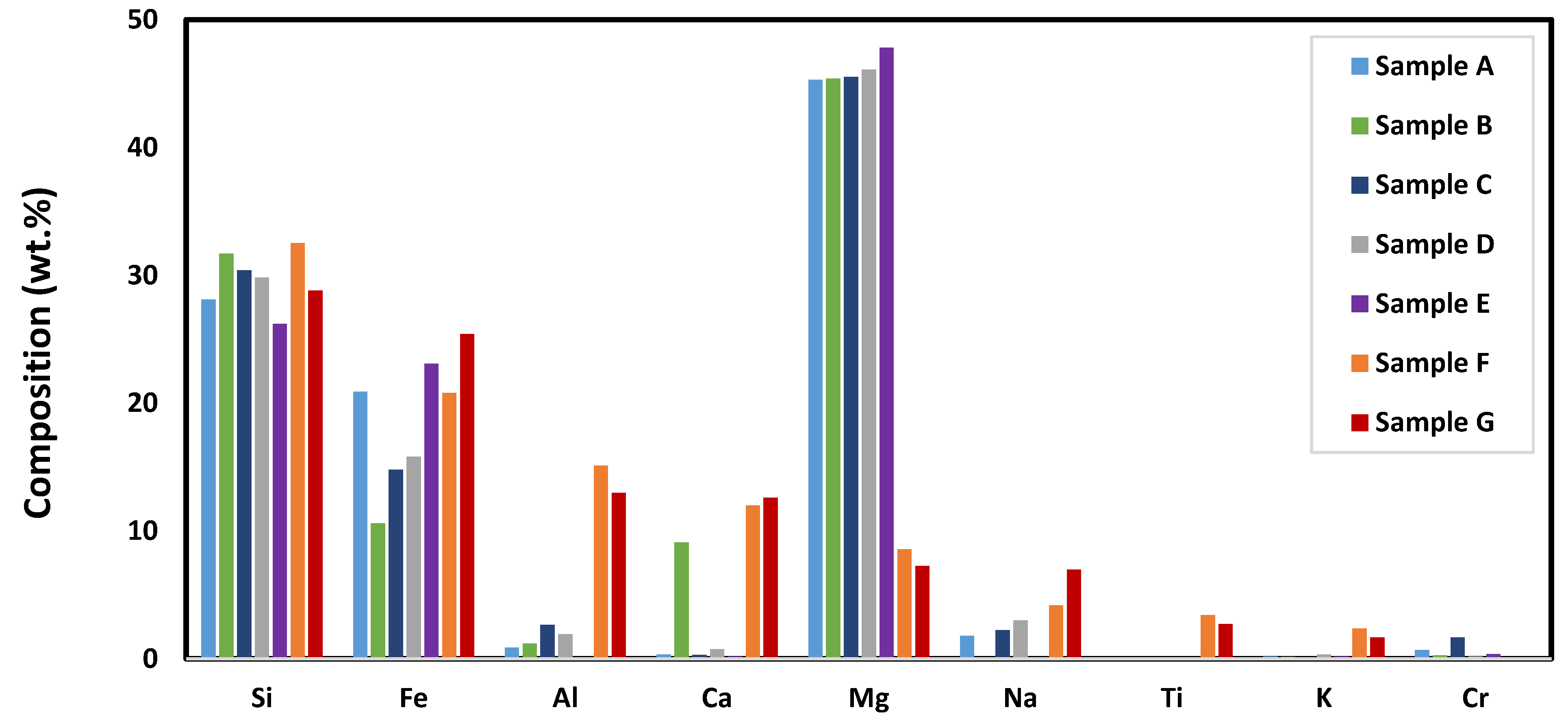
3.2.1. Elemental Composition
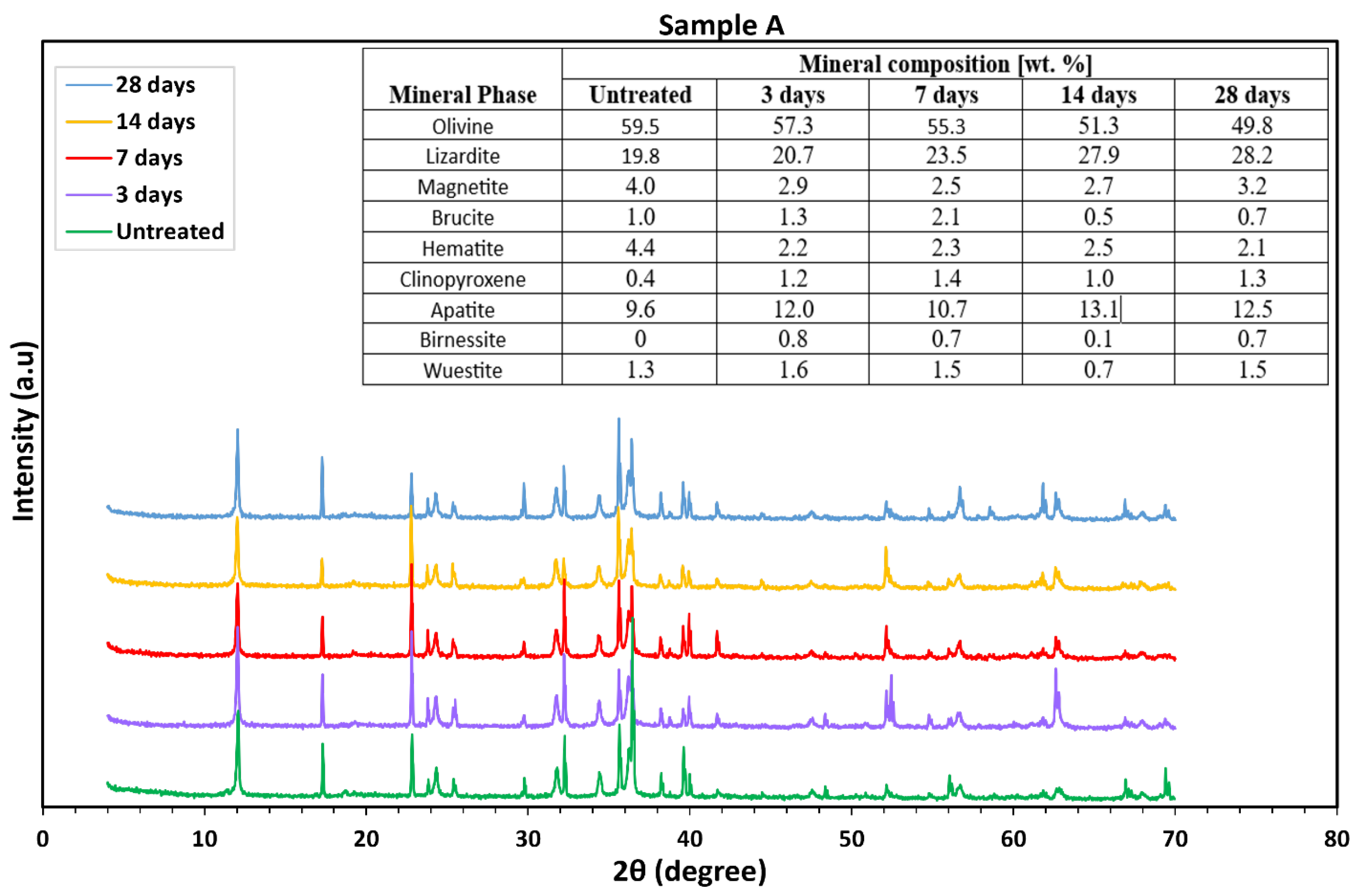
3.2.2. Ultramafic Rocks
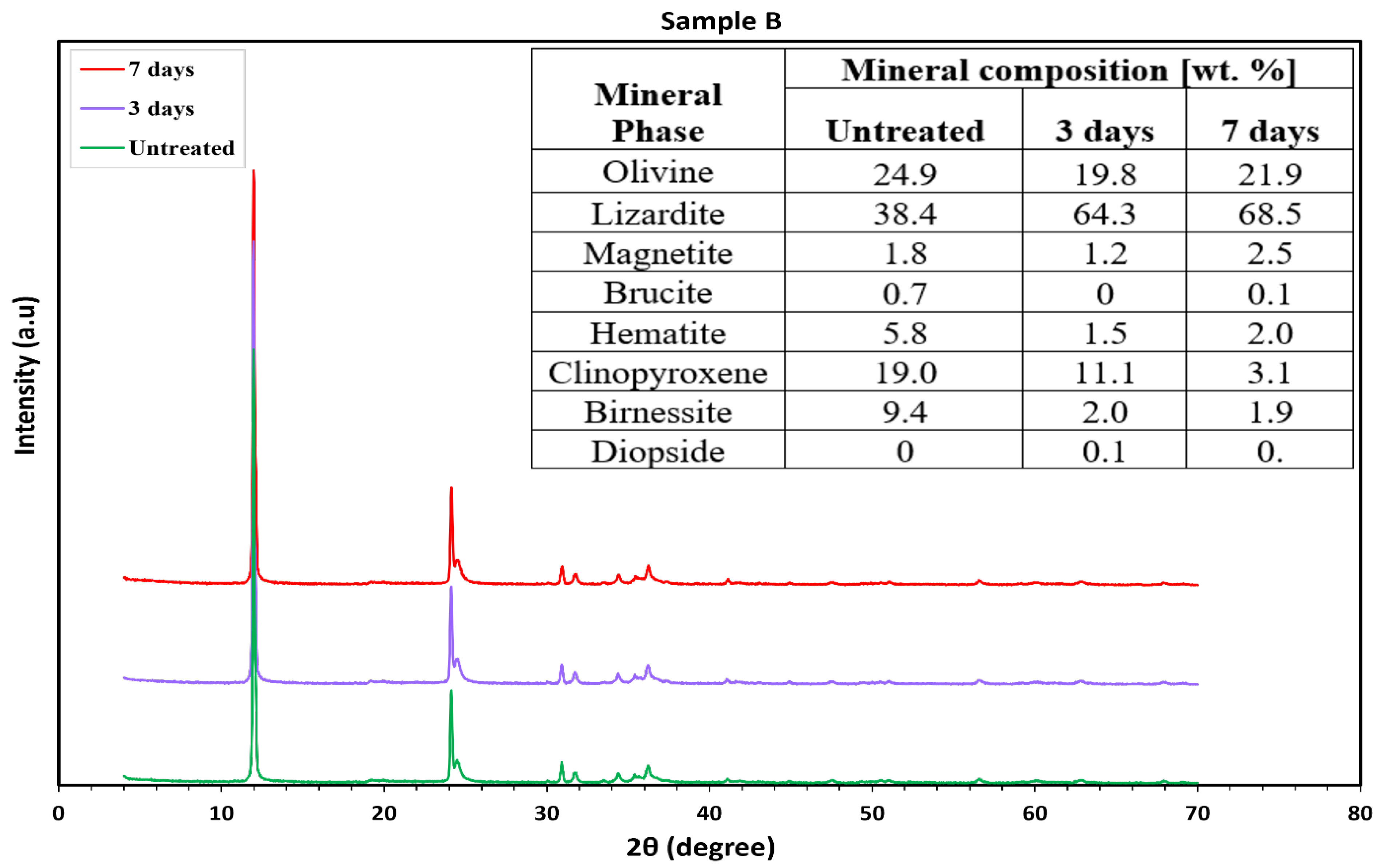
3.2.3. Mafic (Basaltic) Rocks
3.3. Elemental Analysis and Iron (Fe) Oxidation State
3.3.1. XPS Elemental Analysis
3.3.2. Iron (Fe) Oxidation States and H2 Generation
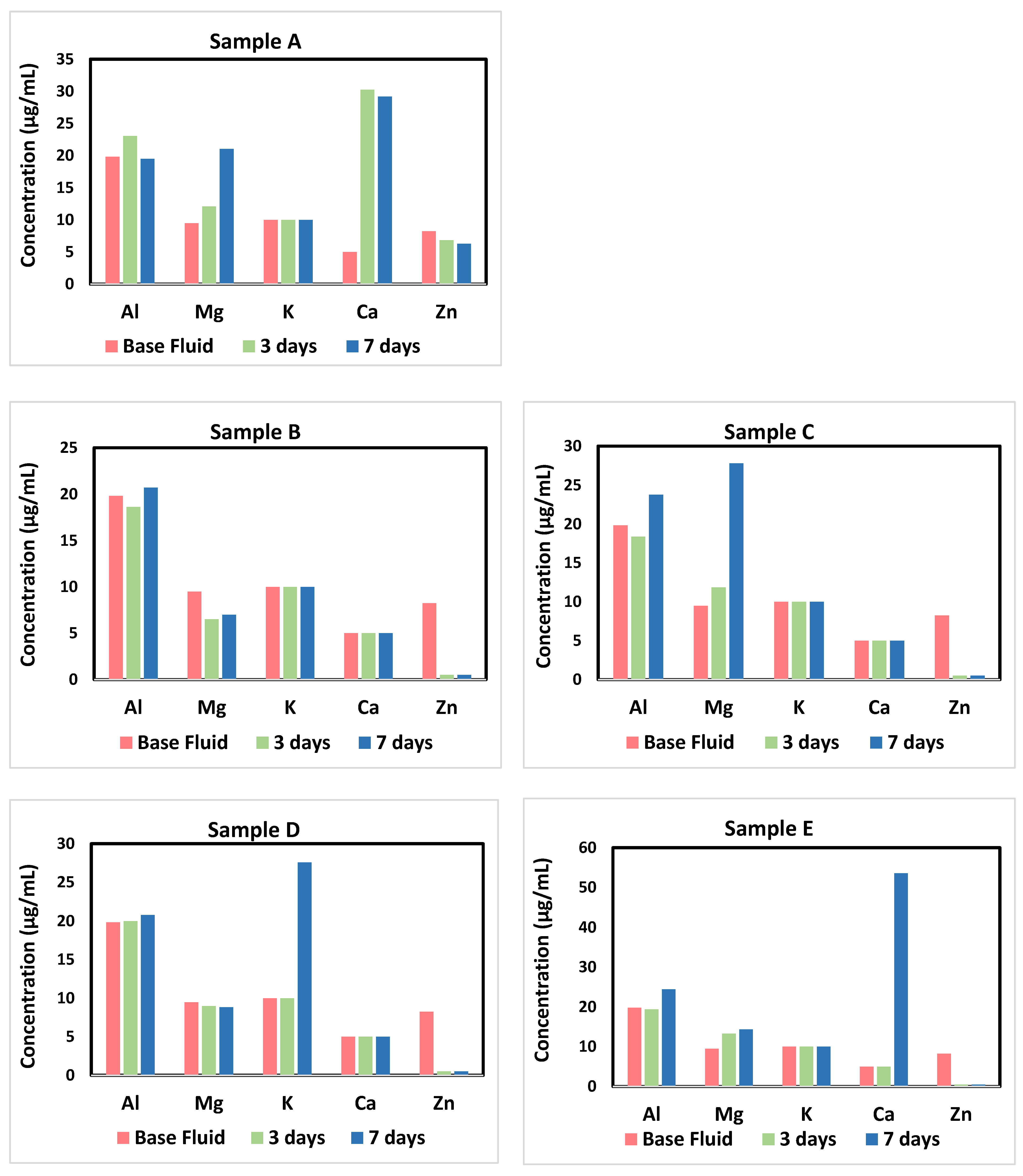
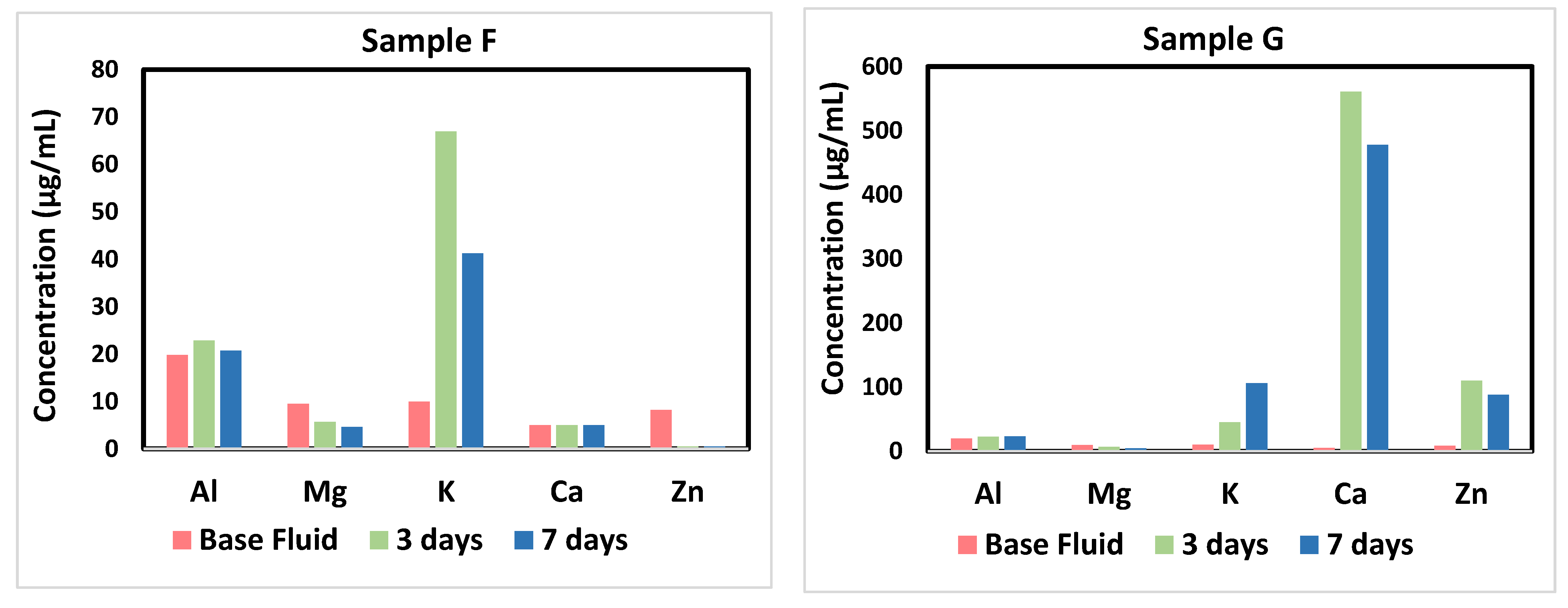
3.4. Aqueous Fluids Analysis
3.4.1. Dissolution/Precipitation and Components Partitioning
3.4.2. Other Trace Elements
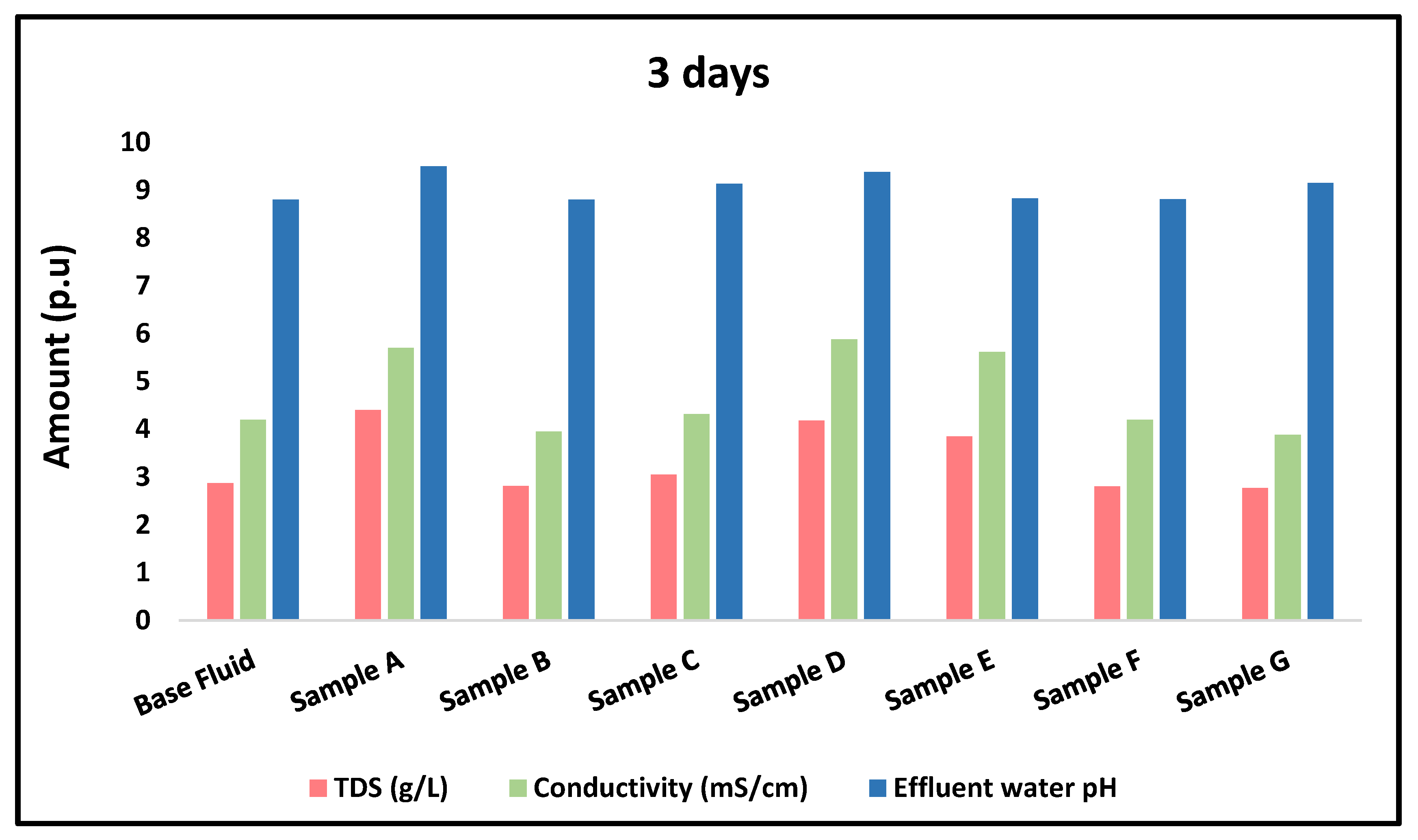
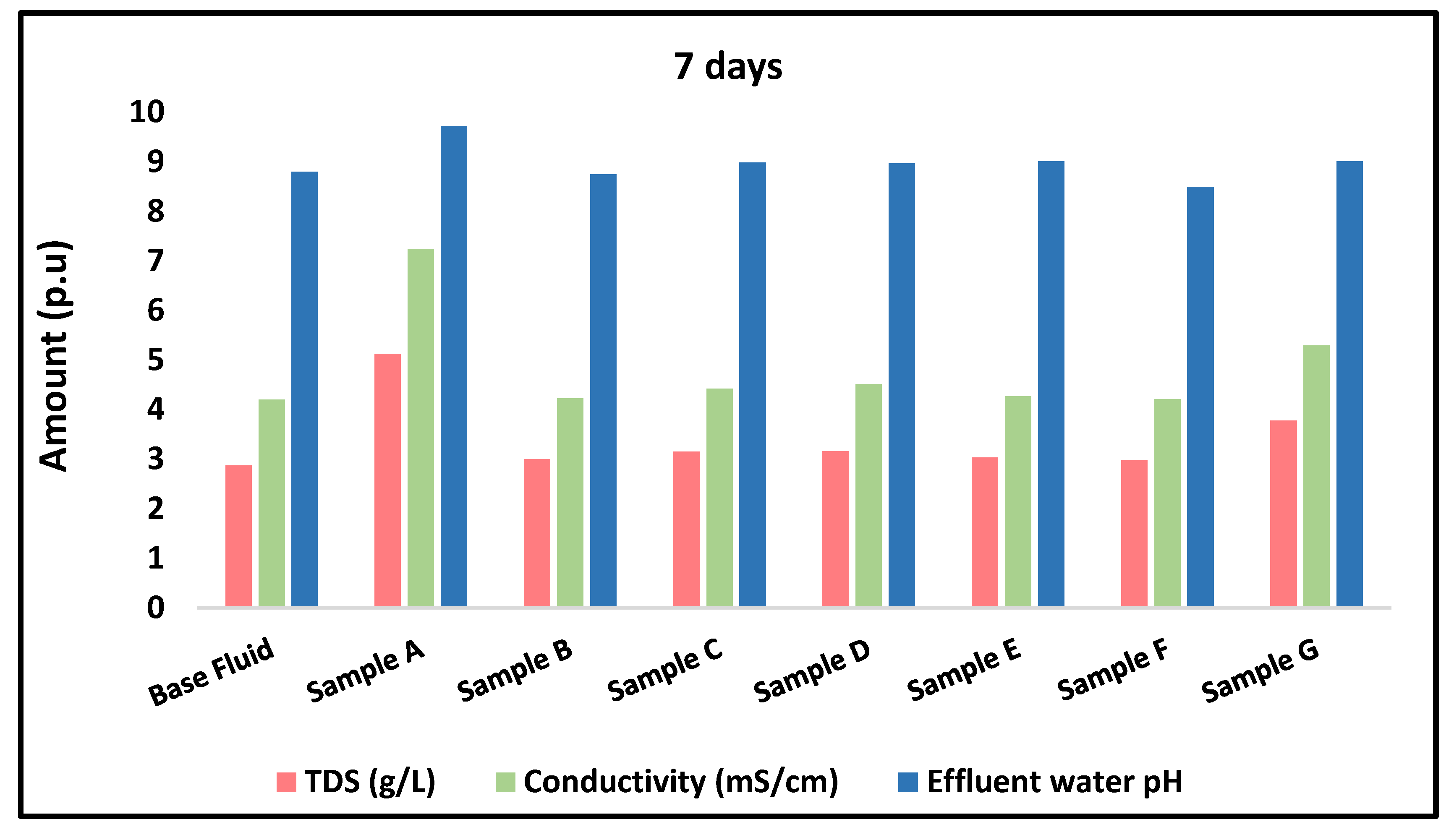
3.4.3. Physicochemical Properties
4. Discussion
4.1. H2 Generation in Ultramafic and Mafic (Basaltic) Rocks
4.1.1. Reaction Pathways in Ultramafic Rocks
4.1.2. Reaction Pathways in Mafic (Basalts) Rocks
4.2. Extended Reactions of Sample A and Its Impact on H2 Generation
4.2.1. Early-Stage H2 Generation (0–7 Days)
4.2.2. Intermediate-Stage H2 Generation (7–28 Days)
4.3. Potential Geocatalysis During H2 Generation
5. Conclusions
6. Future Works
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Keir, R.S. A note on the fluxes of abiogenic methane and hydrogen from mid-ocean ridges. Geophys. Res. Lett. 2010, 37, 24609. [Google Scholar] [CrossRef]
- Maiga, O.; Deville, E.; Laval, J.; Prinzhofer, A.; Diallo, A.B. Characterization of the spontaneously recharging natural hydrogen reservoirs of Bourakebougou in Mali. Sci. Rep. 2023, 13, 11876. [Google Scholar] [CrossRef]
- Wetzel, L.R.; Shock, E.L. Distinguishing ultramafic-from basalt-hosted submarine hydrothermal systems by comparing calculated vent fluid compositions. J. Geophys. Res. Solid Earth 2000, 105, 8319–8340. [Google Scholar] [CrossRef]
- Zhang, M.; Perkovich, N.; Li, Y.; Weihermann, J.; Crummett, R.N. A geophysical investigation of the fairy circles in Nebraska for geologic hydrogen exploration. Sci. Rep. 2025, 15, 22344. [Google Scholar] [CrossRef]
- Truche, L.; Bourdelle, F.; Salvi, S.; Lefeuvre, N.; Zug, A.; Lloret, E. Hydrogen generation during hydrothermal alteration of peralkaline granite. Geochim. Cosmochim. Acta 2021, 308, 42–59. [Google Scholar] [CrossRef]
- Martin, B.; Fyfe, W.S. Some experimental and theoretical observations on the kinetics of hydration reactions with particular reference to serpentinization. Chem. Geol. 1970, 6, 185–202. [Google Scholar] [CrossRef]
- Kita, I.; Marsuo, S.; Wakita, H. H2 generation by reaction between H2O and crushed rock: An experimental study on H2 degassing from the active fault zone. J. Geophys. Res. Solid Earth 1982, 87, 10789–10795. [Google Scholar] [CrossRef]
- Liu, Q.; Wei, Y.; Li, P.; Huang, X.; Meng, Q.; Wu, X.; Zhu, D.; Xu, H.; Fu, Y.; Zhu, D.; et al. Natural hydrogen in the volcanic-bearing sedimentary basin: Origin, conversion, and production rates. Sci. Adv. 2025, 11, eadr6771. [Google Scholar] [CrossRef]
- Wei, Y.; Liu, Q.; Zhu, D.; Meng, Q.; Xu, H.; Zhang, W.; Wu, X.; Li, P.; Huang, X.; Mou, Y.; et al. Helium and natural hydrogen in the Bohai Bay Basin, China: Occurrence, resources, and exploration prospects. Appl. Energy 2025, 383, 125398. [Google Scholar] [CrossRef]
- Vacquand, C.; Deville, E.; Beaumont, V.; Guyot, F.; Sissmann, O.; Pillot, D.; Arcilla, C.; Prinzhofer, A. Reduced gas seepages in ophiolitic complexes: Evidences for multiple origins of the H2-CH4-N2 gas mixtures. Geochim. Cosmochim. Acta 2018, 223, 437–461. [Google Scholar] [CrossRef]
- Marcaillou, C.; Muñoz, M.; Vidal, O.; Parra, T.; Harfouche, M. Mineralogical evidence for H2 degassing during serpentinization at 300 °C/300 bar. Earth Planet. Sci. Lett. 2011, 303, 281–290. [Google Scholar] [CrossRef]
- Mayhew, L.E.; Ellison, E.T.; McCollom, T.M.; Trainor, T.P.; Templeton, A.S. Hydrogen generation from low-temperature water–rock reactions. Nat. Geosci. 2013, 6, 478–484. [Google Scholar] [CrossRef]
- Klein, F.; Bach, W.; McCollom, T.M. Compositional controls on hydrogen generation during serpentinization of ultramafic rocks. Lithos 2013, 178, 55–69. [Google Scholar] [CrossRef]
- McCollom, T.M.; Klein, F.; Moskowitz, B.; Berquó, T.S.; Bach, W.; Templeton, A.S. Hydrogen generation and iron partitioning during experimental serpentinization of an olivine–pyroxene mixture. Geochim. Cosmochim. Acta 2020, 282, 55–75. [Google Scholar] [CrossRef]
- McCollom, T.M.; Klein, F.; Moskowitz, B.; Solheid, P. Experimental serpentinization of iron-rich olivine (hortonolite): Implications for hydrogen generation and secondary mineralization on Mars and icy moons. Geochim. Cosmochim. Acta 2022, 335, 98–110. [Google Scholar] [CrossRef]
- Greber, N.D.; Dauphas, N.; Bekker, A.; Ptáček, M.P.; Bindeman, I.N.; Hofmann, A. Titanium isotopic evidence for felsic crust and plate tectonics 3.5 billion years ago. Science 2017, 357, 1271–1274. [Google Scholar] [CrossRef]
- Tang, M.; Chen, K.; Rudnick, R.L. Archean upper crust transition from mafic to felsic marks the onset of plate tectonics. Science 2016, 351, 372–375. [Google Scholar] [CrossRef]
- Leong, J.A.M.; Ely, T.; Shock, E.L. Decreasing extents of Archean serpentinization contributed to the rise of an oxidized atmosphere. Nat. Commun. 2021, 12, 7341. [Google Scholar] [CrossRef]
- Leong, J.A.; Nielsen, M.; McQueen, N.; Karolytė, R.; Hillegonds, D.J.; Ballentine, C.; Darrah, T.; McGillis, W.; Kelemen, P. H2 and CH4 outgassing rates in the Samail ophiolite, Oman: Implications for low-temperature, continental serpentinization rates. Geochim. Cosmochim. Acta 2023, 347, 1–15. [Google Scholar] [CrossRef]
- Rezaee, R. Assessment of natural hydrogen systems in Western Australia. Int. J. Hydrogen Energy 2021, 46, 33068–33077. [Google Scholar] [CrossRef]
- Saidy, K.; Fawad, M.; Whattam, S.A.; Al-Shuhail, A.A.; Al-Shuhail, A.A.; Campos, M.; Sulistyohariyanto, F.A. Unlocking the H2 potential in Saudi Arabia: Exploring serpentinites as a source of H2 production. Int. J. Hydrogen Energy 2024, 89, 1482–1491. [Google Scholar] [CrossRef]
- Templeton, A.S.; Ellison, E.T.; Kelemen, P.B.; Leong, J.; Boyd, E.S.; Colman, D.R.; Matter, J.M. Low-temperature hydrogen production and consumption in partially-hydrated peridotites in Oman: Implications for stimulated geological hydrogen production. Front. Geochem. 2024, 2, 1366268. [Google Scholar] [CrossRef]
- McCollom, T.M.; Bach, W. Thermodynamic constraints on hydrogen generation during serpentinization of ultramafic rocks. Geochim. Cosmochim. Acta 2009, 73, 856–875. [Google Scholar] [CrossRef]
- Klein, F.; Bach, W.; Jöns, N.; McCollom, T.; Moskowitz, B.; Berquó, T. Iron partitioning and hydrogen generation during serpentinization of abyssal peridotites from 15°N on the Mid-Atlantic Ridge. Geochim. Cosmochim. Acta 2009, 73, 6868–6893. [Google Scholar] [CrossRef]
- Tutolo, B.M.; Seyfried, W.E.; Tosca, N.J. A seawater throttle on H2 production in Precambrian serpentinizing systems. Proc. Natl. Acad. Sci. USA 2020, 117, 14756–14763. [Google Scholar] [CrossRef] [PubMed]
- Foustoukos, D.I.; Seyfried, W.E. Hydrocarbons in Hydrothermal Vent Fluids: The Role of Chromium-Bearing Catalysts. Science 2004, 304, 1002–1005. [Google Scholar] [CrossRef]
- Geymond, U.; Briolet, T.; Combaudon, V.; Sissmann, O.; Martinez, I.; Duttine, M.; Moretti, I. Reassessing the role of magnetite during natural hydrogen generation. Front. Earth Sci. 2023, 11, 1169356. [Google Scholar] [CrossRef]
- Goldstein, T.P. Geocatalytic Reactions in Formation and Maturation of Petroleum. AAPG Bull. 1983, 67, 152–159. [Google Scholar] [CrossRef]
- Liu, J.; Yang, T.; Peng, Q.; Yang, Y.; Li, Y.W.; Wen, X.D. Theoretical exploration of the interaction between hydrogen and pyrite-type FeS2 surfaces. Appl. Surf. Sci. 2021, 537, 147900. [Google Scholar] [CrossRef]
- Neubeck, A.; Duc, N.T.; Bastviken, D.; Crill, P.; Holm, N.G. Formation of H2 and CH4 by weathering of olivine at temperatures between 30 and 70 °C. Geochem. Trans. 2011, 12, 6. [Google Scholar] [CrossRef]
- Schoonen, M.A.A.; Xu, Y.; Strongin, D.R. An introduction to geocatalysis. J. Geochem. Explor. 1998, 62, 201–215. [Google Scholar] [CrossRef]
- Truche, L.; Berger, G.; Albrecht, A.; Domergue, L. Engineered materials as potential geocatalysts in deep geological nuclear waste repositories: A case study of the stainless steel catalytic effect on nitrate reduction by hydrogen. Appl. Geochem. 2013, 35, 279–288. [Google Scholar] [CrossRef]
- Corre, M.; Brunet, F.; Schwartz, S.; Gautheron, C.; Agranier, A.; Lesimple, S. Quaternary low-temperature serpentinization and carbonation in the New Caledonia ophiolite. Sci. Rep. 2023, 13, 19413. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Sun, W.; Ding, X.; Zhao, Y.; Song, M. Effect of pressure on the kinetics of peridotite serpentinization. Phys. Chem. Miner. 2020, 47, 33. [Google Scholar] [CrossRef]
- Pens, M.; Andreani, M.; Daniel, I.; Perrillat, J.P.; Cardon, H. Contrasted effect of aluminum on the serpentinization rate of olivine and orthopyroxene under hydrothermal conditions. Chem. Geol. 2016, 441, 256–264. [Google Scholar] [CrossRef]
- Malvoisin, B.; Brunet, F.; Carlut, J.; Rouméjon, S.; Cannat, M. Serpentinization of oceanic peridotites: 2. Kinetics and processes of San Carlos olivine hydrothermal alteration. J. Geophys. Res. 2012, 117, 1–13. [Google Scholar] [CrossRef]
- Biesinger, M.C.; Payne, B.P.; Grosvenor, A.P.; Lau, L.W.M.; Gerson, A.R.; Smart, R.S.C. Resolving surface chemical states in XPS analysis of first row transition metals, oxides and hydroxides: Cr, Mn, Fe, Co and Ni. Appl. Surf. Sci. 2011, 257, 2717–2730. [Google Scholar] [CrossRef]
- Grosvenor, A.P.; Kobe, B.A.; Biesinger, M.C.; McIntyre, N.S. Investigation of multiplet splitting of Fe 2p XPS spectra and bonding in iron compounds. Surf. Interface Anal. 2004, 36, 1564–1574. [Google Scholar] [CrossRef]
- Molchan, I.S.; Thompson, G.E.; Skeldon, P.; Lindsay, R.; Walton, J.; Kouvelos, E.; Romanos, G.E.; Falaras, P.; Kontos, A.G.; Arfanis, M.; et al. Microscopic study of the corrosion behaviour of mild steel in ionic liquids for CO2 capture applications. RSC Adv. 2015, 5, 35181–35194. [Google Scholar] [CrossRef]
- Ali, M.; Isah, A.; Yekeen, N.; Hassanpouryouzband, A.; Sarmadivaleh, M.; Okoroafor, R.; Al Kobaisi, M.; Mahmoud, M.; Vahrenkamp, V.; Hoteit, H. Recent Progress in Underground Hydrogen Storage. Energy Environ. Sci. 2025, 18, 5740–5810. [Google Scholar] [CrossRef]
- Mohammed, I.; Isah, A.; Bello, A.; Mahmoud, M.; Al Shehri, D.; Raza, A.; Alafnan, S. Iron-Rich Rocks for Hydrogen Geo-Storage: Induced Storage Capacity by Active Sorption. Energy Fuels 2024, 38, 16795–16808. [Google Scholar] [CrossRef]
- Herzberg, C.; Asimow, P.D.; Arndt, N.; Niu, Y.; Lesher, C.M.; Fitton, J.G.; Cheadle, M.J.; Saunders, A.D. Temperatures in ambient mantle and plumes: Constraints from basalts, picrites, and komatiites. Geochem. Geophys. Geosyst. 2007, 8, Q02006. [Google Scholar] [CrossRef]
- Stevens, T.O.; McKinley, J.P. Abiotic controls on H2 production from Basalt—Water reactions and implications for aquifer biogeochemistry. Environ. Sci. Technol. 2000, 34, 826–831. [Google Scholar] [CrossRef]
- Carlin, W.; Malvoisin, B.; Brunet, F.; Lanson, B.; Findling, N.; Lanson, M.; Fargetton, T.; Jeannin, L.; Lhote, O. Kinetics of low-temperature H2 production in ultramafic rocks by ferroan brucite oxidation. Geochem. Perspect. Lett. 2024, 29, 27–32. [Google Scholar] [CrossRef]
- Templeton, A.S.; Ellison, E.T. Formation and loss of metastable brucite: Does Fe(II)-bearing brucite support microbial activity in serpentinizing ecosystems? Philos. Trans. R. Soc. A 2020, 378, 20180423. [Google Scholar] [CrossRef]
- Bach, W.; Paulick, H.; Garrido, C.J.; Ildefonse, B.; Meurer, W.P.; Humphris, S.E. Unraveling the sequence of serpentinization reactions: Petrography, mineral chemistry, and petrophysics of serpentinites from MAR 15°N (ODP Leg 209, Site 1274). Geophys. Res. Lett. 2006, 33, 13306. [Google Scholar] [CrossRef]
- Jöns, N.; Kahl, W.A.; Bach, W. Reaction-induced porosity and onset of low-temperature carbonation in abyssal peridotites: Insights from 3D high-resolution microtomography. Lithos 2017, 268–271, 274–284. [Google Scholar] [CrossRef]
- Deer, W.A.; Howie, R.A.; Zussman, J. Ortho- and Ring Silicates; Longmans: London, UK, 1962. [Google Scholar]
- Al-Yaseri, A.; Desouky, M.; Aljawad, M.S.; Hassanpouryouzband, A. Evidence of hydrogen release during CO2 sequestration in basalt. Int. J. Hydrogen Energy 2025, 101, 1183–1190. [Google Scholar] [CrossRef]
- Otake, T.; Wesolowski, D.J.; Anovitz, L.M.; Allard, L.F.; Ohmoto, H. Experimental evidence for non-redox transformations between magnetite and hematite under H2-rich hydrothermal conditions. Earth Planet. Sci. Lett. 2007, 257, 60–70. [Google Scholar] [CrossRef]
- Preiner, M.; Xavier, J.C.; Sousa, F.L.; Zimorski, V.; Neubeck, A.; Lang, S.Q.; Greenwell, H.C.; Kleinermanns, K.; Tüysüz, H.; McCollom, T.M.; et al. Serpentinization: Connecting geochemistry, ancient metabolism and industrial hydrogenation. Life 2018, 8, 41. [Google Scholar] [CrossRef]
- Schwander, L.; Brabender, M.; Mrnjavac, N.; Wimmer, J.L.; Preiner, M.; Martin, W.F. Serpentinization as the source of energy, electrons, organics, catalysts, nutrients and pH gradients for the origin of LUCA and life. Front. Microbiol. 2023, 14, 1257597. [Google Scholar] [CrossRef]
- Song, H.; Ou, X.; Han, B.; Deng, H.; Zhang, W.; Tian, C.; Chai, C.; Lu, A.; Lin, Z.; Chai, L. An overlooked natural hydrogen evolution pathway: Ni2+ boosting H2O reduction by Fe(OH)2 oxidation during low-temperature serpentinization. Angew. Chem. Int. Ed. 2021, 60, 24054–24058. [Google Scholar] [CrossRef]
- Beyazay, T.; Ochoa-Hernández, C.; Song, Y.; Belthle, K.S.; Martin, W.F.; Tüysüz, H. Influence of composition of nickel-iron nanoparticles for abiotic CO2 conversion to early prebiotic organics. Angew. Chem. 2023, 135, e202218189. [Google Scholar] [CrossRef]
- Andreani, M.; Daniel, I.; Pollet-Villard, M. Aluminum speeds up the hydrothermal alteration of olivine. Am. Mineral. 2013, 98, 1738–1744. [Google Scholar] [CrossRef]
- Zähr, J.; Oswald, S.; Türpe, M.; Ullrich, H.J.; Füssel, U. Characterisation of oxide and hydroxide layers on technical aluminum materials using XPS. Vacuum 2012, 86, 1216–1219. [Google Scholar] [CrossRef]




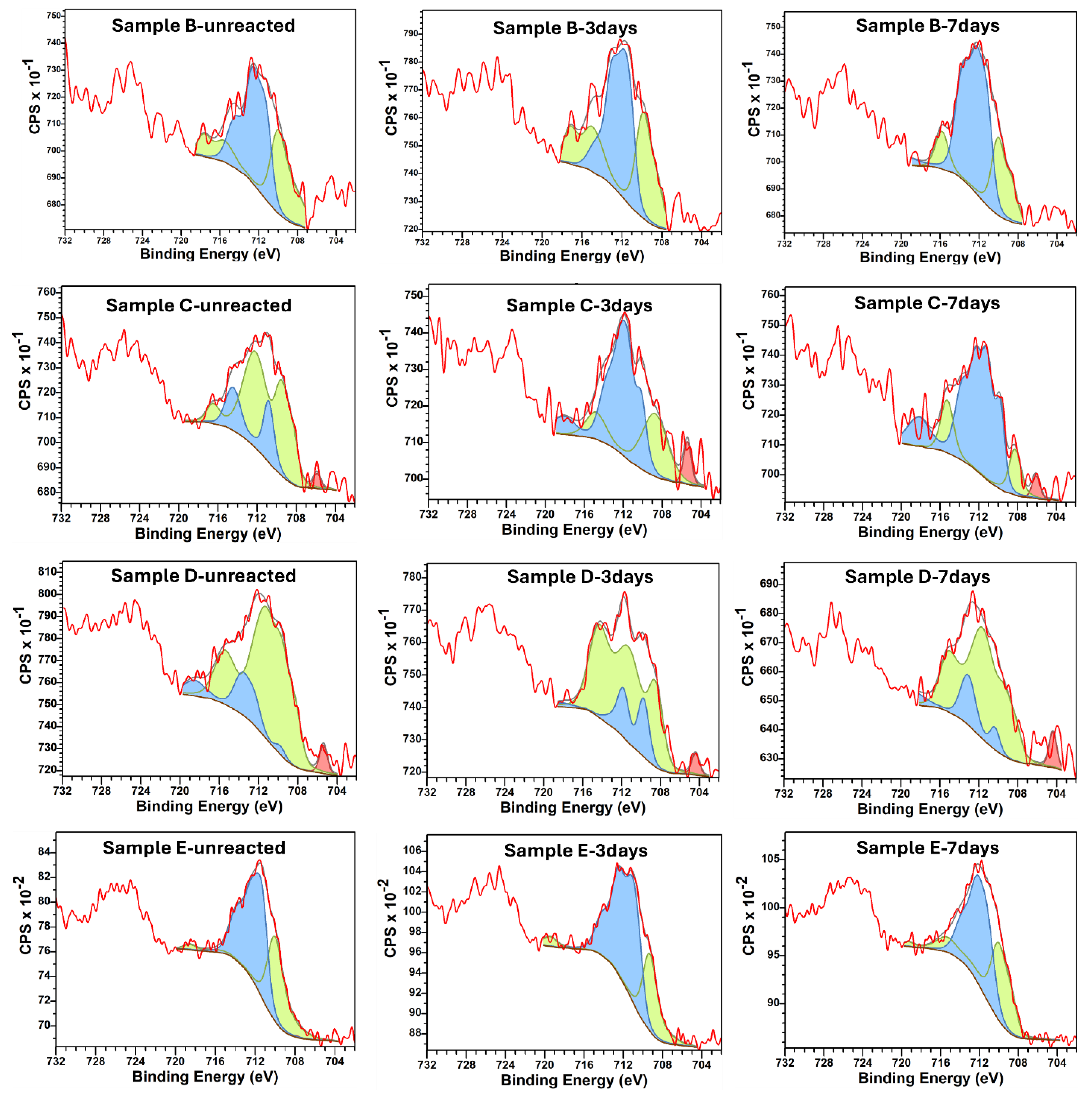
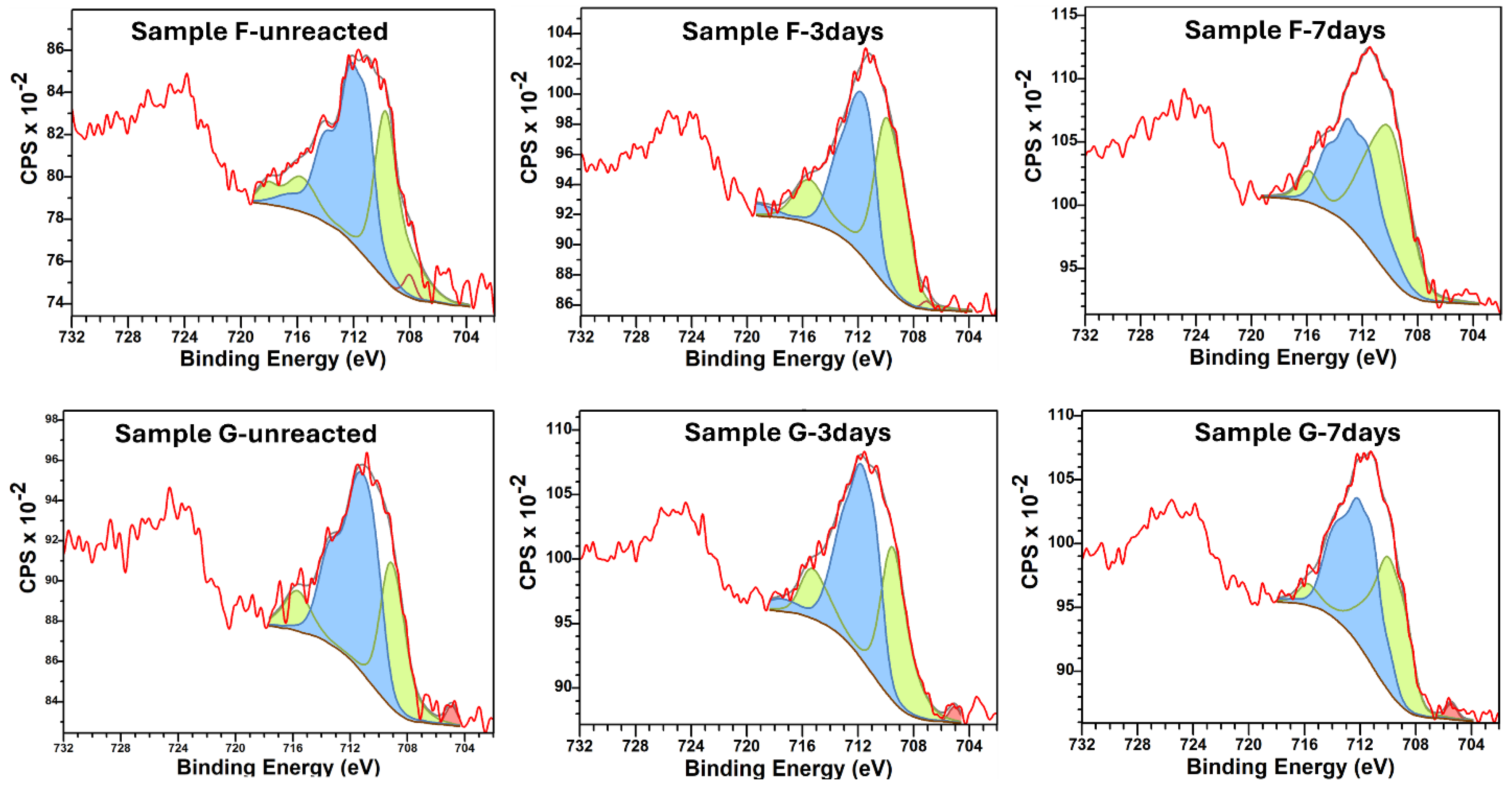

| S/# | Name | Pos. Constraint (eV) | FWHM Constraint (eV) | Area Constraint | Position (eV) | FWHM (eV) |
|---|---|---|---|---|---|---|
| A | Fe | 729.108, 704.398 | 0.9, 0.95 | 0.0, 10,000,000.0 | 706.70 | 0.95 |
| B | Fe2+ 1 | 708.75, 708.25 | 1.35, 1.45 | 0.0, 10,000,000.0 | 708.48 | 1.45 |
| C | Fe2+ 2 | 710.05, 708.258 | 1.55, 1.75 | 0.0, 12,400,000.0 | 710.05 | 1.75 |
| D | Fe2+ 3 | 711.25, 708.25 | 1.55, 1.75 | 0.0, 5,990,000.0 | 708.25 | 1.75 |
| E | Fe2+ 4 | 712.45, 708.25 | 2.85, 3.05 | 0.0, 10,580,000.0 | 709.42 | 3.05 |
| F | Fe2+ 5 | 715.75, 708.25 | 2.45, 2.55 | 0.0, 2,310,000.0 | 714.36 | 2.5 |
| G | Fe3+ 1 | 710.35, 709.75 | 1.15, 1.35 | 0.0, 10,000,000.0 | 710.35 | 1.15 |
| H | Fe3+ 2 | 711.35, 709.75 | 1.25, 1.35 | 0.0, 9,520,000.0 | 711.13 | 1.25 |
| I | Fe3+ 3 | 712.25, 709.75 | 1.35, 1.55 | 0.0, 7,320,000.0 | 712.25 | 1.55 |
| J | Fe3+ 4 | 713.35, 709.75 | 1.35, 1.55 | 0.0, 3,980,000.0 | 709.75 | 1.55 |
| K | Fe3+ 5 | 714.45, 709.75 | 1.85, 1.95 | 0.0, 3,010,000.0 | 713.65 | 1.95 |
| L | Fe3+ 6 | 719.85, 709.75 | 2.65, 2.75 | 0.0, 3,270,000.0 | 719.85 | 2.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isah, A.; Samouei, H.; Okoroafor, E.R. Assessing the Effect of Mineralogy and Reaction Pathways on Geological Hydrogen (H2) Generation in Ultramafic and Mafic (Basaltic) Rocks. Hydrogen 2025, 6, 76. https://doi.org/10.3390/hydrogen6040076
Isah A, Samouei H, Okoroafor ER. Assessing the Effect of Mineralogy and Reaction Pathways on Geological Hydrogen (H2) Generation in Ultramafic and Mafic (Basaltic) Rocks. Hydrogen. 2025; 6(4):76. https://doi.org/10.3390/hydrogen6040076
Chicago/Turabian StyleIsah, Abubakar, Hamidreza Samouei, and Esuru Rita Okoroafor. 2025. "Assessing the Effect of Mineralogy and Reaction Pathways on Geological Hydrogen (H2) Generation in Ultramafic and Mafic (Basaltic) Rocks" Hydrogen 6, no. 4: 76. https://doi.org/10.3390/hydrogen6040076
APA StyleIsah, A., Samouei, H., & Okoroafor, E. R. (2025). Assessing the Effect of Mineralogy and Reaction Pathways on Geological Hydrogen (H2) Generation in Ultramafic and Mafic (Basaltic) Rocks. Hydrogen, 6(4), 76. https://doi.org/10.3390/hydrogen6040076







