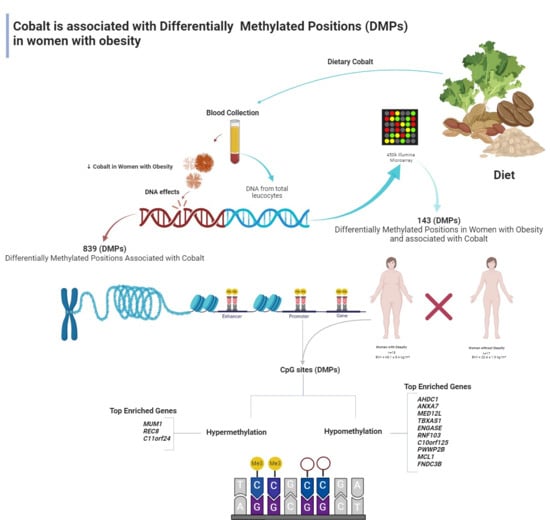
Serum Cobalt Concentration and DNA Methylation Signatures in Women with Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Population and Sampling
2.2. Ethical Aspects
2.3. Epigenetic Analysis
2.4. Bioinformatic Analysis
2.5. Cobalt Assessment
2.6. Statistical Analysis
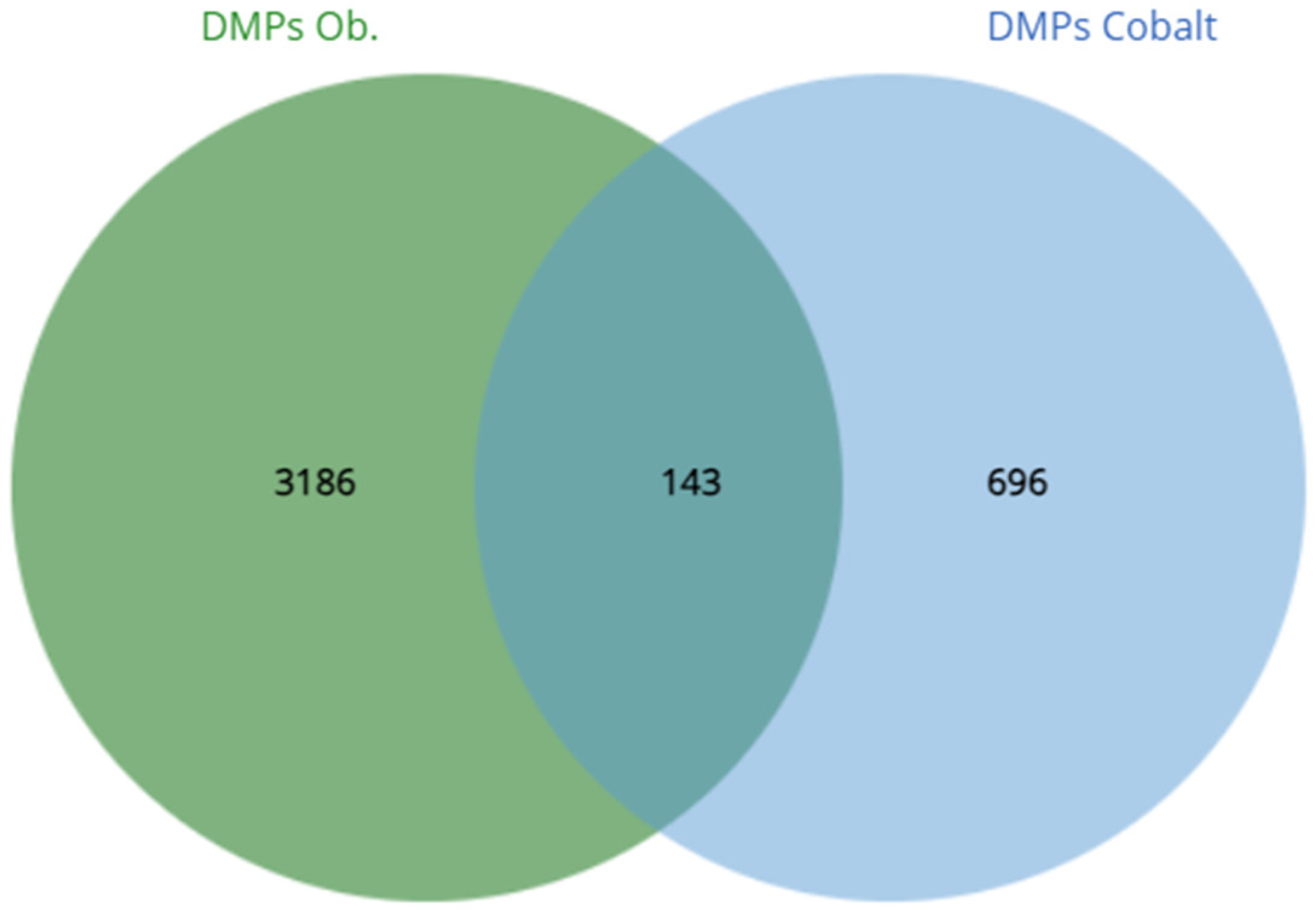
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Obesity and Overweight. [Internet]. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight#:~:text=In%202022%2C%202.5%20billion%20adults,age%20of%205%20were%20overweight (accessed on 25 March 2024).
- Obesity. [Internet]. Available online: https://www.cdc.gov/obesity/basics/index.html (accessed on 15 March 2024).
- Lin, X.; Li, H. Obesity: Epidemiology, Pathophysiology, and Therapeutics. Front. Endocrinol. 2021, 12, 706978. [Google Scholar] [CrossRef]
- Smita, R.M.; Shuvo, A.P.R.; Sabbir, R.; Jahan, R.; Simin, A.F.; Rahman, A.; Biswas, S.; Salem, L.; Sagor, M.A.T. The Role of Mineral Deficiencies in Insulin Resistance and Obesity. Curr. Diabetes Rev. 2022, 18, e171121197987. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Liu, W.; Bi, S.; Zhou, L.; Li, L. Association between minerals intake and childhood obesity: A cross-sectional study of the NHANES database in 2007–2014. PLoS ONE 2023, 18, e0295765. [Google Scholar] [CrossRef] [PubMed]
- Alloway, B.J. Sources of Heavy Metals and Metalloids in Soils. In Heavy Metals in Soils; Alloway, B., Ed.; Environmental Pollution; Springer: Dordrecht, The Netherlands, 2013; Volume 22. [Google Scholar] [CrossRef]
- Genchi, G.; Lauria, G.; Catalano, A.; Carocci, A.; Sinicropi, M.S. Prevalence of Cobalt in the Environment and Its Role in Biological Processes. Biology 2023, 12, 1335. [Google Scholar] [CrossRef] [PubMed]
- Giedyk, M.; Goliszewska, K.; Gryko, D. Vitamin B12 catalysed reactions. Chem. Soc. Rev. 2015, 44, 3391–3404. [Google Scholar] [CrossRef] [PubMed]
- Shao, W.; Liu, Q.; He, X.; Liu, H.; Gu, A.; Jiang, Z. Association between level of urinary trace heavy metals and obesity among children aged 6–19 years: NHANES 1999–2011. Environ. Sci. Pollut. Res. 2017, 24, 11573–11581. [Google Scholar] [CrossRef] [PubMed]
- Vrijheid, M.; Fossati, S.; Maitre, L.; Márquez, S.; Roumeliotaki, T.; Agier, L.; Andrusaityte, S.; Cadiou, S.; Casas, M.; de Castro, M.; et al. Early-Life Environmental Exposures and Childhood Obesity: An Exposome-Wide Approach. Environ. Health Perspect. 2020, 128, 67009. [Google Scholar] [CrossRef] [PubMed]
- Padilla, M.A.; Elobeid, M.; Ruden, D.M.; Allison, D.B. An examination of the association of selected toxic metals with total and central obesity indices: NHANES 99-02. Int. J. Environ. Res. Public Health 2010, 7, 3332–3347. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Mukherjee, B.; Park, S.K. Associations of cumulative exposure to heavy metal mixtures with obesity and its comorbidities among U.S. adults in NHANES 2003–2014. Environ. Int. 2018, 121, 683–694. [Google Scholar] [CrossRef]
- Yu, G.; Wu, L.; Su, Q.; Ji, X.; Zhou, J.; Wu, S.; Tang, Y.; Li, H. Neurotoxic effects of heavy metal pollutants in the environment: Focusing on epigenetic mechanisms. Environ. Pollut. 2024, 345, 123563. [Google Scholar] [CrossRef]
- Pidsley, R.; Zotenko, E.; Peters, T.J.; Lawrence, M.G.; Risbridger, G.P.; Molloy, P.; Van Djik, S.; Muhlhausler, B.; Stirzaker, C.; Clark, S.J. Critical evaluation of the Illumina MethylationEPIC BeadChip microarray for whole-genome DNA methylation profiling. Genome Biol. 2016, 17, 208. [Google Scholar] [CrossRef] [PubMed]
- Yuan, T.; Morris, T.J.; Webster, A.P.; Yang, Z.; Beck, S.; Feber, A.; Teschendorff, A.E. ChAMP: Updated methylation analysis pipeline for Illumina BeadChips. Bioinformatics 2017, 33, 3982–3984. [Google Scholar] [CrossRef] [PubMed]
- Noronha, N.Y.; Rodrigues, G.S.; Pinhel, M.A.S.; Cazier, J.B.; Watanabe, L.M.; Menezes, A.N.; Bueno, C.R.; Nicoletti, C.F.; de Oliveira, B.A.; Schineider, I.M.; et al. Sample Preparation to Bioinformatics Analysis of DNA Methylation: Association Strategy for Obesity and Related Trait Studies. J. Vis. Exp. 2022, 6, e62598. [Google Scholar] [CrossRef] [PubMed]
- Noronha, N.Y.; Rodrigues, G.S.; Noma, I.H.Y.; Brandão, C.F.C.; Rodrigues, K.P.; Colello, B.A.; Sae-Lee, C.; Moriguchi Watanabe, L.; Augusta de Souza Pinhel, M.; Mello Schineider, I.; et al. 14-weeks combined exercise epigenetically modulated 118 genes of menopausal women with prediabetes. Front. Endocrinol. 2022, 13, 895489. [Google Scholar] [CrossRef] [PubMed]
- Batista, B.; Rodrigues, J.; Tormen, L.; Curtius, A.; Barbosa, F., Jr. Reference concentrations for trace elements in urine for the Brazilian population based on q-ICP-MS with a simple dilute-and-shoot procedure. J. Braz. Chem. Soc. 2009, 20. [Google Scholar] [CrossRef]
- Arain, A.L.; Neitzel, R.L. A Review of Biomarkers Used for Assessing Human Exposure to Metals from E-Waste. Int. J. Environ. Res. Public Health 2019, 16, 1802. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Shen, T.; Zhong, L.; Ji, G.; Chen, B.; Liao, M.; Li, L.; Huang, H.; Li, J.; Wei, Y.; Wu, S.; et al. Associations between metal(loid) exposure with overweight and obesity and abdominal obesity in the general population: A cross-sectional study in China. Chemosphere 2024, 350, 140963. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Huang, H.; He, X.; Duan, W.; Mo, X. Sex differences in the link between blood cobalt concentrations and insulin resistance in adults without diabetes. Environ. Health Prev. Med. 2021, 26, 42. [Google Scholar] [CrossRef]
- Hu, J.; Cao, J.; Xu, Q.; Lu, M. Dose-response relationships between urinary cobalt concentrations and obesity, insulin resistance, and metabolic-related disorders in the general population. Environ. Sci. Pollut. Res. Int. 2022, 29, 29682–29688. [Google Scholar] [CrossRef]
- Do, W.L.; Nguyen, S.; Yao, J.; Guo, X.; Whitsel, E.A.; Demerath, E.; Rotter, J.I.; Rich, S.S.; Lange, L.; Ding, J.; et al. Associations between DNA methylation and BMI vary by metabolic health status: A potential link to disparate cardiovascular outcomes. Clin. Epigenetics 2021, 13, 230. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cho, S.; Lee, E.H.; Kim, H.; Lee, J.M.; So, M.H.; Ahn, J.J.; Lee, H.Y. Validation of BMI genetic risk score and DNA methylation in a Korean population. Int. J. Leg. Med. 2021, 135, 1201–1212. [Google Scholar] [CrossRef] [PubMed]
- Weyde, K.V.F.; Olsen, A.K.; Duale, N.; Kamstra, J.H.; Skogheim, T.S.; Caspersen, I.H.; Engel, S.M.; Biele, G.; Xia, Y.; Meltzer, H.M.; et al. Gestational blood levels of toxic metal and essential element mixtures and associations with global DNA methylation in pregnant women and their infants. Sci. Total Environ. 2021, 787, 147621. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Zan, G.; Feng, X.; Bao, Y.; Huang, S. The associations of multiple metals mixture with accelerated DNA methylation aging. Environ. Pollut. 2021, 269, 116230. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Shea, T.B. Folate and homocysteine metabolism in neural plasticity and neurodegenerative disorders. Trends Neurosci. 2003, 26, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Gibb, H.J.; Howe, P.; Sheffer, M. Cobalt and Inorganic Cobalt Compounds; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- Froese, D.S.; Fowler, B.; Baumgartner, M.R. Vitamin B12, folate, and the methionine remethylation cycle—Biochemistry, pathways, and regulation. J. Inherit. Metab. Dis. 2019, 42, 673–685. [Google Scholar] [CrossRef]
- Choi, M.J.; Kim, S.C.; Kim, A.N.; Kwon, H.B.; Ahn, R.S. Effect of endocrine disruptors on the oocyte maturation and ovulation in amphibians, Rana dybowskii. Integr. Biosci. 2007, 11, 1–8. [Google Scholar] [CrossRef]
- Pulido, M. Metal-induced apoptosis: Mechanisms. Mutat. Res./Fundam. Mol. Mech. Mutagen. 2003, 533, 227–241. [Google Scholar] [CrossRef]
- Kawakami, T.; Hanao, N.; Nishiyama, K.; Kadota, Y.; Inoue, M.; Sato, M.; Suzuki, S. Differential effects of cobalt and mercury on lipid metabolism in the white adipose tissue of high-fat diet-induced obesity mice. Toxicol. Appl. Pharmacol. 2012, 258, 32–42. [Google Scholar] [CrossRef]
- Tetsuka, J.; Matsukawa, T.; Yokoyama, K.; Yamasaki, S.; Ando, S.; Nishida, A.; Hiraiwa-Hasegawa, M.A.; Kasai, K. Effects of Trace Elements on Anthropometric Characteristics of Children: Cobalt and Childhood Body Mass Index. Juntendo Med. J. 2022, 68, 251–260. [Google Scholar] [CrossRef]
| Essential | Obesity (n = 16) | Normal Weight (n = 17) | Reference |
|---|---|---|---|
| Age | 38.6 ± 11.6 | 39.1 ± 13.4 | - |
| BMI | 45.1 ± 5.4 a | 22.6 ± 1.9 | Obesity: BMI ≥ 30 kg/m2 Normal weight: 18.5 > BMI < 25 kg/m2 |
| Co (μg/L) | 0.46 ± 0.08 a | 0.64 ± 0.14 | 0.30–1.20 b |
| Metal | DMPs (Effect) | GR (%) | DHS Enhancer BOTH | FDR1 | Genes (n) | Analysis | Termescription | Term ID | FDR 2 |
|---|---|---|---|---|---|---|---|---|---|
| Cobalt | 839 | 728 (86.8%) | 143 92 21 | 4.2 × 10−3 | 712 | Keywords | Methylation Chromatin regulator Transcription regulation | KW-0488 KW-0156 KW-0805 | 0.0024 0.0272 0.0392 |
| Metal | CpG ID | CHR | Gene | Feature | Δβ | p-Value | Methylation Status | p-Adjusted Value |
|---|---|---|---|---|---|---|---|---|
| Cobalt | cg16020249 | 1 | AHDC1 | TSS1500 | −0.02 | <0.001 | Hypomethylated | 0.048 |
| cg16108132 | 10 | ANXA7 | TSS1500 | −0.02 | <0.001 | Hypomethylated | 0.048 | |
| cg02951206 | 3 | MED12L | TSS1500 | −0.02 | <0.001 | Hypomethylated | 0.031 | |
| cg09658576 | 7 | TBXAS1 | TSS200 | −0.02 | <0.001 | Hypomethylated | 0.016 | |
| cg22280792 | 17 | ENGASE | TSS1500 | −0.02 | <0.001 | Hypomethylated | 0.049 | |
| cg02860394 | 2 | RNF103 | TSS200 | −0.02 | <0.001 | Hypomethylated | 0.038 | |
| cg19921261 | 10 | C10orf125 | TSS200 | −0.02 | <0.001 | Hypomethylated | 0.025 | |
| cg05010219 | 10 | PWWP2B | TSS1500 | −0.02 | <0.001 | Hypomethylated | 0.046 | |
| cg13175981 | 1 | MCL1 | TSS1500 | −0.02 | <0.001 | Hypomethylated | 0.012 | |
| cg00986800 | 3 | FNDC3B | TSS200 | −0.02 | <0.001 | Hypomethylated | 0.008 | |
| cg23057220 | 19 | MUM1 | TSS200 | 0.07 | <0.001 | Hypermethylated | 0.048 | |
| cg24127861 | 14 | REC8 | TSS1500 | 0.06 | <0.001 | Hypermethylated | 0.044 | |
| cg10477989 | 11 | C11orf24 | TSS200 | 0.05 | <0.001 | Hypermethylated | 0.012 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noronha, N.Y.; Diani, L.M.; Rodrigues, G.d.S.; Noma, I.H.Y.; Pereira, V.A.B.; Pinhel, M.A.d.S.; Watanabe, L.M.; Morais, D.A.; Barbosa, F., Jr.; Nonino, C.B. Serum Cobalt Concentration and DNA Methylation Signatures in Women with Obesity. Obesities 2024, 4, 85-92. https://doi.org/10.3390/obesities4020009
Noronha NY, Diani LM, Rodrigues GdS, Noma IHY, Pereira VAB, Pinhel MAdS, Watanabe LM, Morais DA, Barbosa F Jr., Nonino CB. Serum Cobalt Concentration and DNA Methylation Signatures in Women with Obesity. Obesities. 2024; 4(2):85-92. https://doi.org/10.3390/obesities4020009
Chicago/Turabian StyleNoronha, Natália Yumi, Luísa Maria Diani, Guilherme da Silva Rodrigues, Isabela Harumi Yonehara Noma, Vanessa Aparecida Batista Pereira, Marcela Augusta de Souza Pinhel, Lígia Moriguchi Watanabe, Déborah Araújo Morais, Fernando Barbosa, Jr., and Carla Barbosa Nonino. 2024. "Serum Cobalt Concentration and DNA Methylation Signatures in Women with Obesity" Obesities 4, no. 2: 85-92. https://doi.org/10.3390/obesities4020009
APA StyleNoronha, N. Y., Diani, L. M., Rodrigues, G. d. S., Noma, I. H. Y., Pereira, V. A. B., Pinhel, M. A. d. S., Watanabe, L. M., Morais, D. A., Barbosa, F., Jr., & Nonino, C. B. (2024). Serum Cobalt Concentration and DNA Methylation Signatures in Women with Obesity. Obesities, 4(2), 85-92. https://doi.org/10.3390/obesities4020009