Biosourced Polysaccharide-Based Superabsorbents
Abstract
:1. Introduction
2. Definition and Classification
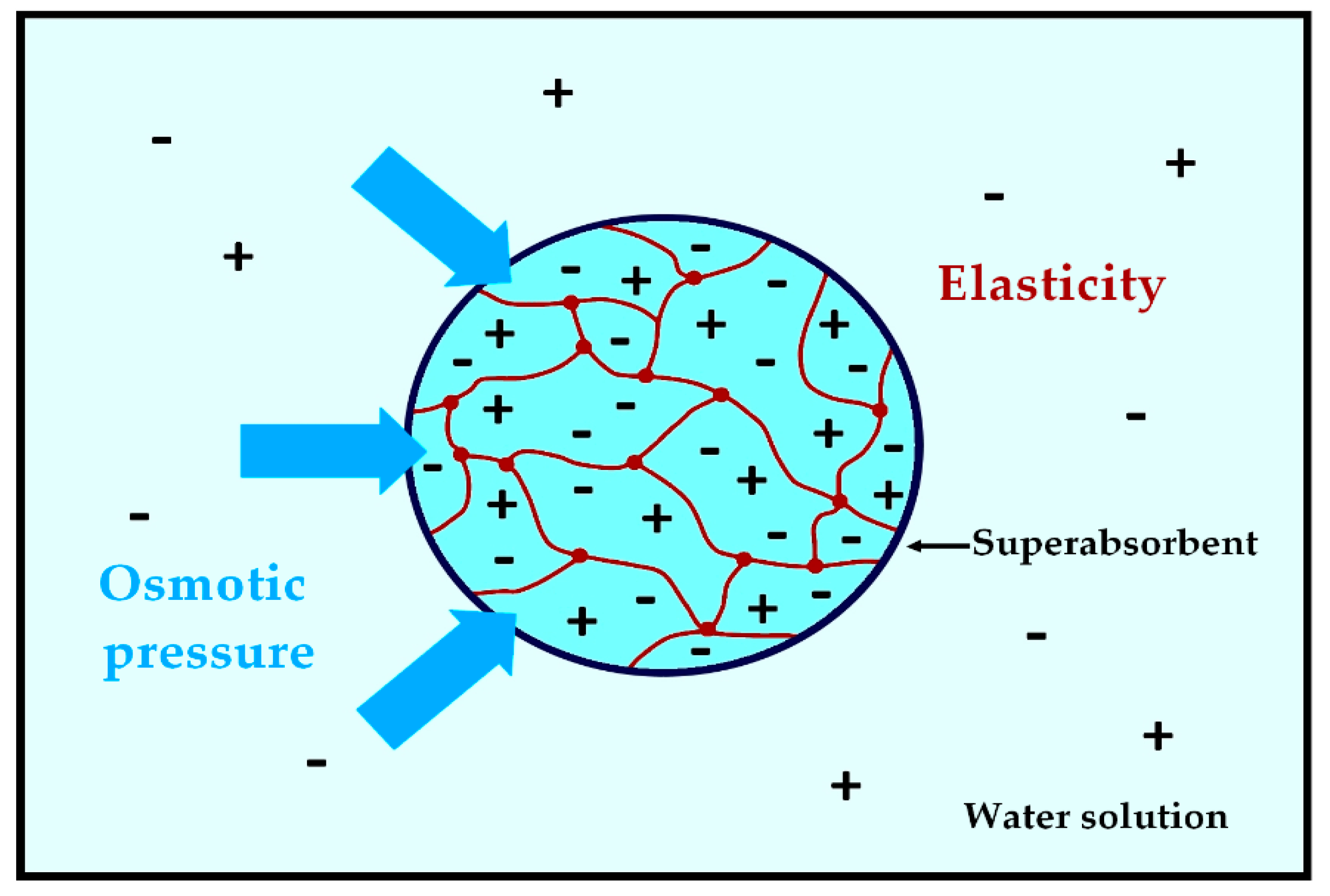
2.1. What Is a Superabsorbent and a Hydrogel?
2.2. Classification of Superabsorbents
2.2.1. Classification Based on Physical Appearance
2.2.2. Classification Based on Sources
2.2.3. Classification Based on Crosslinking
2.2.4. Classification Based on Electrical Charges
2.2.5. Classification Based on Respond to Stimuli
2.2.6. Classification According to the Polymeric Composition
2.2.7. Classification Based on Chain Configuration
2.3. Polysaccharides Used for Superabsorbents
3. Main Properties of Polysaccharide-Based Superabsorbents
3.1. Swelling and Absorption Capacities
3.2. Mechanical and Thermal Properties
3.3. Biodegradability and Biocompatibility
| Type and Name of Superabsorbent | Maximum Swelling in Distilled Water (g/g) | Maximum Swelling in NaCl Solution (g/g) | Reference |
|---|---|---|---|
| Pectin-based superabsorbent hydrogel | 484 | 74 (0.05 M NaCl) | [38] |
| Pectin-based superabsorbent hydrogel | 500 | 15 (0.9% NaCl) | [39] |
| Modified cashew-gum with acrylamide | 1500 | / | [6] |
| Vinyl-modified starch superabsorbent hydrogel | 150 | / | [6] |
| Starch-based superabsorbent polymers | ~110 (Maize) ~126 (Potato) | / | [21] |
| Vinylated Arabic gum superabsorbent hydrogel | 500 | / | [6] |
| Kappa-carrageenan with graft acrylic acid superabsorbent hydrogel | 789 | / | [40] |
| Canola protein-based with graft acrylic acid monomers superabsorbent hydrogel | 448 | ~20 (0.9 M NaCl) | [41] |
| Cellulose nanofibrils and chitosan-graft-poly(acrylic acid) superabsorbent hydrogel | 486 | / | [42] |
| Cellulose-Chitosan crosslinked superabsorbent hydrogels | 610 | 85 (0.9% NaCl) | [43] |
| Hemicellulose-based hydrogel with graft acrylic acid and acrylic amide | 1128 | 132 (0.9% NaCl) | [44] |
| Wheat straw with graft acrylic acid, acrylic amide and dimethyl diallyl ammonium superabsorbent | 133.76 | 33.83 (0.9% NaCl) | [34] |
| Ammonia ethyl chitosan with acrylic acid superabsorbent polymer | 644 | 99 (0.9% NaCl) | [35] |
| Alginate-based superabsorbent hydrogel | 450 | 60 (0.9% NaCl) | [39] |
| Acrylic-based superabsorbent hydrogel | 822 | 66 (0.9% NaCl) | [45] |
| Poly(acrylic acid-acrylamide) superabsorbent | 1019 | 49 (0.9% NaCl) | [46] |
3.4. Parameters That Affect Swelling Properties
3.4.1. Physical Stimuli
3.4.2. Chemical Stimuli
Effect of pH
Effect of Ionic Strength
Effect of Concentration of Monomer and Initiator
Effect of Neutralization
Effect of Crosslinker and Crosslinking Density
4. Preparation and Synthesis of Polysaccharide-Based Superabsorbents
4.1. Components and Material
4.2. Crosslinking Methods
4.2.1. Chemical Crosslinking
4.2.2. Physical Crosslinking
5. Techniques for Characterizing a Superabsorbent
5.1. Swelling Measurements
5.2. Fourier Transform Infrared Spectroscopy (FTIR)
5.3. Thermogravimetric Analysis (TGA)
5.4. Differential Scanning Calorimetry (DSC)
5.5. Scanning Electron Microscopy (SEM)
5.6. Absorbency Under Load (AUL)
5.7. Other Techniques
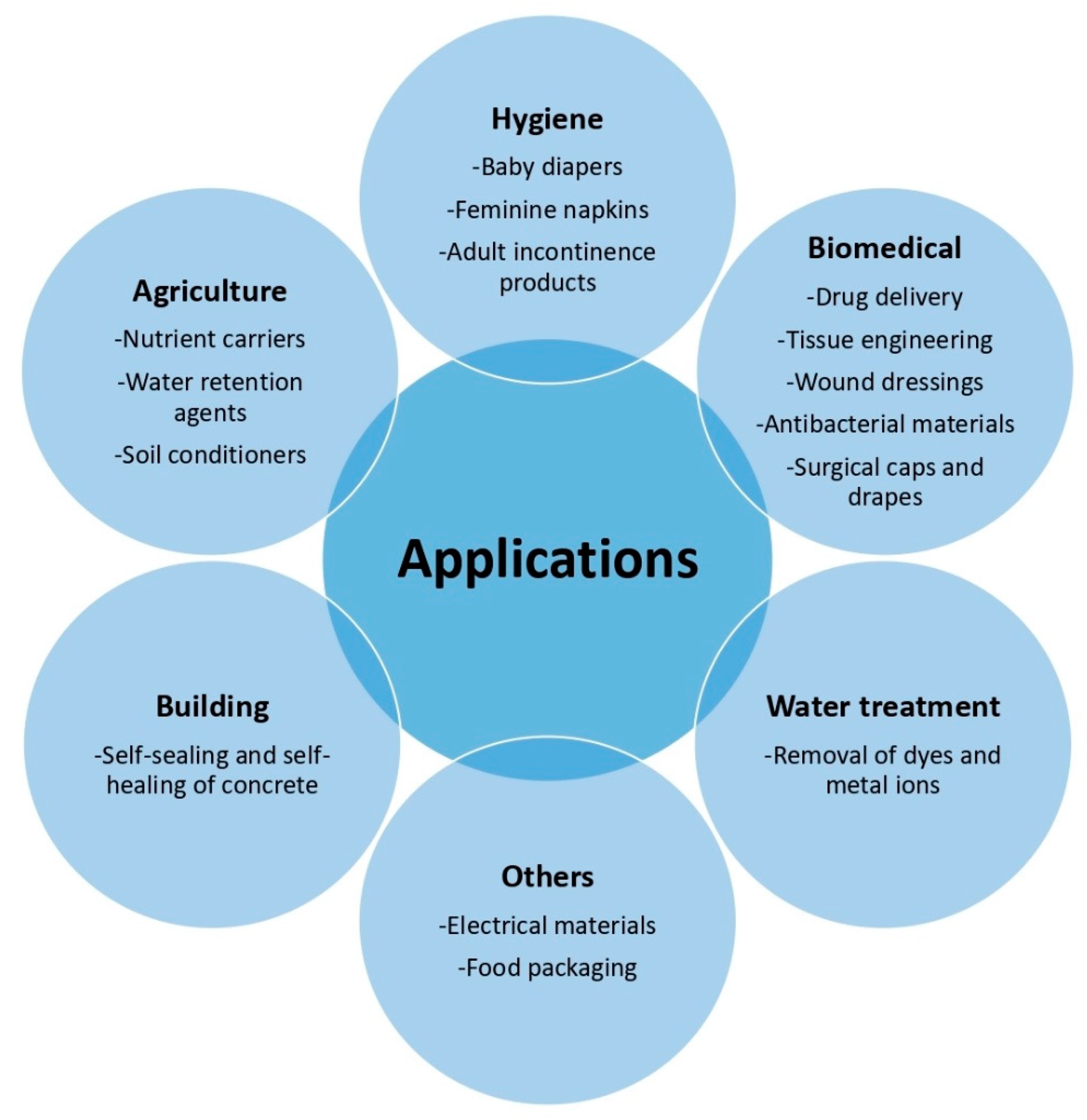
6. Applications
6.1. Hygiene Products
6.2. Biomedical Applications
6.3. Agriculture Applications
6.4. Water Treatment
6.5. Building Applications
6.6. Other Applications
7. Conclusions and Future Prospectives
Funding
Conflicts of Interest
References
- Snoeck, D. Self-Healing and Microstructure of Cementitious Materials with Microfibres and Superabsorbent Polymers; Ghent University: Ghent, Belgium, 2015. [Google Scholar]
- Wichterle, O.; Lím, D. Hydrophilic Gels for Biological Use. Nat. Cell Biol. 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Gibas, I.; Janik, H. Review: Synthetic polymer hydrogels for biomedical applications. Chem. Chem. Technol. 2010, 4, 297–304. [Google Scholar]
- Lim, F.; Sun, A.M. Microencapsulated islets as bioartificial endocrine pancreas. Science 1980, 210, 908–910. [Google Scholar] [CrossRef] [PubMed]
- Yannas, I.V.; Lee, E.; Orgill, D.P.; Skrabut, E.M.; Murphy, G.F. Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin. Proc. Natl. Acad. Sci. USA 1989, 86, 933–937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guilherme, M.R.; Aouada, F.A.; Fajardo, A.R.; Martins, A.F.; Paulino, A.T.; Davi, M.F.; Rubira, A.F.; Muniz, E.C. Superabsorbent hydrogels based on polysaccharides for application in agriculture as soil conditioner and nutrient carrier: A review. Eur. Polym. J. 2015, 72, 365–385. [Google Scholar] [CrossRef] [Green Version]
- Sinha, S. 14—Biodegradable superabsorbents: Methods of preparation and application—A review. In Fundamental Biomaterials: Polymers; Thomas, S., Balakrishnan, P., Sreekala, M.S., Eds.; Woodhead Publishing: Cambridge, UK, 2018; pp. 307–322. [Google Scholar] [CrossRef]
- Vasile, C.; Pamfil, D.; Stoleru, E.; Baican, M. New Developments in Medical Applications of Hybrid Hydrogels Containing Natural Polymers. Molecules 2020, 25, 1539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y. Hydrogels Based on Natural Polymers; Elsevier: Amsterdam, The Netherlands, 2020; p. 552. [Google Scholar] [CrossRef]
- Mehr, M.J.Z.; Omidian, H.; Doroudiani, S.; Kabiri, K. Advances in non-hygienic applications of superabsorbent hydrogel materials. J. Mater. Sci. 2010, 45, 5711–5735. [Google Scholar] [CrossRef]
- Pérez-Álvarez, L.; Ruiz-Rubio, L.; Lizundia, E.; Vilas-Vilela, J.L. Polysaccharide-Based Superabsorbents: Synthesis, Properties, and Applications. In Cellulose-Based Superabsorbent Hydrogels; Mondal, M.I.H., Ed.; Springer Nature: Cham, Switzerland, 2019; pp. 1393–1431. [Google Scholar] [CrossRef]
- Díez-Pascual, A.M. Synthesis and Applications of Biopolymer Composites. Int. J. Mol. Sci. 2019, 20, 2321. [Google Scholar] [CrossRef] [Green Version]
- Jiang, F.; Hsieh, Y.-L. Amphiphilic superabsorbent cellulose nanofibril aerogels. J. Mater. Chem. A 2014, 2, 6337–6342. [Google Scholar] [CrossRef] [Green Version]
- Jeddi, M.K.; Laitinen, O.; Liimatainen, H. Magnetic superabsorbents based on nanocellulose aerobeads for selective removal of oils and organic solvents. Mater. Des. 2019, 183, 108115. [Google Scholar] [CrossRef]
- Mignon, A.; De Belie, N.; Dubruel, P.; Van Vlierberghe, S. Superabsorbent polymers: A review on the characteristics and applications of synthetic, polysaccharide-based, semi-synthetic and ‘smart’ derivatives. Eur. Polym. J. 2019, 117, 165–178. [Google Scholar] [CrossRef]
- Capezza, A.J.; Wu, Q.; Newson, W.R.; Olsson, R.T.; Espuche, E.; Johansson, E.; Hedenqvist, M.S. Superabsorbent and Fully Biobased Protein Foams with a Natural Cross-Linker and Cellulose Nanofibers. ACS Omega 2019, 4, 18257–18267. [Google Scholar] [CrossRef] [PubMed]
- Horie, K.; Barón, M.; Fox, R.B.; He, J.; Hess, M.; Kahovec, J.; Kitayama, T.; Kubisa, P.; Maréchal, E.; Mörmann, W.; et al. Definitions of terms relating to reactions of polymers and to functional polymeric materials (IUPAC Recommendations 2003). Pure Appl. Chem. 2004, 76, 889–906. [Google Scholar] [CrossRef]
- Bhatia, J.K.; Kaith, B.S.; Kalia, S. Polysaccharide Hydrogels: Synthesis, Characterization, and Applications. In Polysaccharide Based Graft Copolymers; Kalia, S., Sabaa, M.W., Eds.; Springer: Heidelberg, Germany, 2013; pp. 271–290. [Google Scholar] [CrossRef]
- Mignon, A. Effect of pH-Responsive Superabsorbent Polymers on the Self-Sealing and Self-Healing of Cracks in Concrete; Ghent University: Ghent, Belgium, 2016. [Google Scholar]
- Yabuki, A.; Tanabe, S.; Fathona, I.W. Self-healing polymer coating with the microfibers of superabsorbent polymers provides corrosion inhibition in carbon steel. Surf. Coat. Technol. 2018, 341, 71–77. [Google Scholar] [CrossRef]
- Qiao, D.; Liu, H.; Yu, L.; Bao, X.; Simon, G.P.; Petinakis, E.; Chen, L. Preparation and characterization of slow-release fertilizer encapsulated by starch-based superabsorbent polymer. Carbohydr. Polym. 2016, 147, 146–154. [Google Scholar] [CrossRef]
- Aiouaz, N. Synthèse Et Propriétés De Superabsorbants Composites A Base De Polysaccharides; University of Science and Technology Houari Boumediene: Bab Ezzouar, Algeria, 2013. [Google Scholar]
- Boumalha, H. Elaboration De Materiaux Composites Polymeres Superabsorbants/Additifs Et Etude Leurs Performances, Pour Une Application Dans Les Produits D’hygiene; University of Science and Technology Houari Boumediene: Bab Ezzouar, Algeria, 2019. [Google Scholar]
- Pourjavadi, A.; Zeidabadi, F.; Barzegar, S. Alginate-based biodegradable superabsorbents as candidates for diclofenac sodium delivery systems. J. Appl. Polym. Sci. 2010, 118, 2015–2023. [Google Scholar] [CrossRef]
- Ahmed, E.M. Hydrogel: Preparation, characterization, and applications: A review. J. Adv. Res. 2015, 6, 105–121. [Google Scholar] [CrossRef] [Green Version]
- Olad, A.; Doustdar, F.; Gharekhani, H. Starch-based semi-IPN hydrogel nanocomposite integrated with clinoptilolite: Preparation and swelling kinetic study. Carbohydr. Polym. 2018, 200, 516–528. [Google Scholar] [CrossRef]
- Rinaudo, M. Main properties and current applications of some polysaccharides as biomaterials. Polym. Int. 2008, 57, 397–430. [Google Scholar] [CrossRef]
- Yadav, H.; Karthikeyan, C. 1—Natural polysaccharides: Structural features and properties. In Polysaccharide Carriers for Drug Delivery; Maiti, S., Jana, S., Eds.; Woodhead Publishing: Sawston, UK; Cambridge, UK, 2019; pp. 1–17. [Google Scholar] [CrossRef]
- Roberts, J.J.; Martens, P.J. 9—Engineering biosynthetic cell encapsulation systems. In Biosynthetic Polymers for Medical Applications; Poole-Warren, L., Martens, P., Green, R., Eds.; Woodhead Publishing: Cambridge, UK, 2016; pp. 205–239. [Google Scholar] [CrossRef]
- Sayah, M.Y.; Chabir, R.; Benyahia, H.; Kandri, Y.R.; Chahdi, F.O.; Touzani, H.; Errachidi, F. Yield, Esterification Degree and Molecular Weight Evaluation of Pectins Isolated from Orange and Grapefruit Peels under Different Conditions. PLoS ONE 2016, 11, e0161751. [Google Scholar] [CrossRef] [Green Version]
- Hadrich, A. Nouveaux Hydrogels A Base De Polysaccharide Obtenus Par Voie Biomimetique Ou Par Photoreticulation; University of Rouen Normandy: Rouen Normandy, France, 2019. [Google Scholar]
- Warson, H. Modern Superabsorbent Polymer Technology. Polym. Int. 2000, 49, 1548. [Google Scholar] [CrossRef]
- Chang, C.; Duan, B.; Cai, J.; Zhang, L. Superabsorbent hydrogels based on cellulose for smart swelling and controllable delivery. Eur. Polym. J. 2010, 46, 92–100. [Google Scholar] [CrossRef]
- Li, Q.; Ma, Z.; Yue, Q.; Gao, B.; Li, W.; Xu, X. Synthesis, characterization and swelling behavior of superabsorbent wheat straw graft copolymers. Bioresour. Technol. 2012, 118, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Sudarsan, S.; Franklin, D.; Sakthivel, M.; Guhanathan, S. Non toxic, antibacterial, biodegradable hydrogels with pH-stimuli sensitivity: Investigation of swelling parameters. Carbohydr. Polym. 2016, 148, 206–215. [Google Scholar] [CrossRef]
- Fang, S.; Wang, G.; Li, P.; Xing, R.; Liu, S.; Qin, Y.; Yu, H.; Chen, X.; Li, K. Synthesis of chitosan derivative graft acrylic acid superabsorbent polymers and its application as water retaining agent. Int. J. Biol. Macromol. 2018, 115, 754–761. [Google Scholar] [CrossRef]
- Das, N. Preparation methods and properties of hydrogel: A review. Int. J. Pharm. Pharm. Sci. 2013, 5, 12–117. [Google Scholar]
- Pourjavadi, A.; Barzegar, S. Synthesis and Evaluation of pH and Thermosensitive Pectin-Based Superabsorbent Hydrogel for Oral Drug Delivery Systems. Starch Stärke 2009, 61, 161–172. [Google Scholar] [CrossRef]
- Yoshimura, T.; Sengoku, K.; Fujioka, R. Pectin-based surperabsorbent hydrogels crosslinked by some chemicals: Synthesis and characterization. Polym. Bull. 2005, 55, 123–129. [Google Scholar] [CrossRef]
- Pourjavadi, A.; Harzandi, A.; Hosseinzadeh, H. Modified carrageenan 3. Synthesis of a novel polysaccharide-based superabsorbent hydrogel via graft copolymerization of acrylic acid onto kappa-carrageenan in air. Eur. Polym. J. 2004, 40, 1363–1370. [Google Scholar] [CrossRef]
- Shi, W.; Dumont, M.-J.; Ly, E.B. Synthesis and properties of canola protein-based superabsorbent hydrogels. Eur. Polym. J. 2014, 54, 172–180. [Google Scholar] [CrossRef]
- Spagnol, C.; Rodrigues, F.H.; Pereira, A.G.; Fajardo, A.R.; Rubira, A.F.; Muniz, E.C. Superabsorbent hydrogel composite made of cellulose nanofibrils and chitosan-graft-poly(acrylic acid). Carbohydr. Polym. 2012, 87, 2038–2045. [Google Scholar] [CrossRef] [Green Version]
- Alam, N.; Christopher, L.P. Natural Cellulose-Chitosan Cross-Linked Superabsorbent Hydrogels with Superior Swelling Properties. ACS Sustain. Chem. Eng. 2018, 6, 8736–8742. [Google Scholar] [CrossRef]
- Zhang, J.; Xiao, H.; Li, N.; Ping, Q.; Zhang, Y. Synthesis and characterization of super-absorbent hydrogels based on hemicellulose. J. Appl. Polym. Sci. 2015, 132, 132. [Google Scholar] [CrossRef]
- Kabiri, K.; Omidian, H.; Hashemi, S.; Zohuriaan-Mehr, M. Synthesis of fast-swelling superabsorbent hydrogels: Effect of crosslinker type and concentration on porosity and absorption rate. Eur. Polym. J. 2003, 39, 1341–1348. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, Y.; Wang, P.; Yang, Z.; Yasin, A.; Zhang, L. Preparation and Applications of Salt-Resistant Superabsorbent Poly (Acrylic Acid-Acrylamide/Fly Ash) Composite. Materials 2019, 12, 596. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, F.H.A.; Fajardo, A.R.; Pereira, A.G.B.; Ricardo, N.M.P.S.; Feitosa, J.P.A.; Muniz, E.C. Chitosan-graft-poly(acrylic acid)/rice husk ash based superabsorbent hydrogel composite: Preparation and characterization. J. Polym. Res. 2012, 19, 1–10. [Google Scholar] [CrossRef]
- Liu, T.; Wang, Y.; Li, B.; Deng, H.; Huang, Z.; Qian, L.; Wang, X. Urea free synthesis of chitin-based acrylate superabsorbent polymers under homogeneous conditions: Effects of the degree of deacetylation and the molecular weight. Carbohydr. Polym. 2017, 174, 464–473. [Google Scholar] [CrossRef]
- Aday, A.N.; Srubar, W.V. 2—Biobased polymers for mitigating early- and late-age cracking in concrete. In Bio-Based Materials and Biotechnologies for Eco-Efficient Construction; Pacheco-Torgal, F., Ivanov, V., Tsang, D.C.W., Eds.; Woodhead Publishing: Cambridge, UK, 2020; pp. 19–41. [Google Scholar] [CrossRef]
- Jenkins, A.D.; Kratochvíl, P.; Stepto, R.F.T.; Suter, U.W. Glossary of basic terms in polymer science (IUPAC Recommendations 1996). Pure Appl. Chem. 1996, 68, 2287–2311. [Google Scholar] [CrossRef]
- Akhtar, M.F.; Hanif, M.; Ranjha, N.M. Methods of synthesis of hydrogels A review. Saudi Pharm. J. 2016, 24, 554–559. [Google Scholar] [CrossRef] [Green Version]
- Jiang, F.; Hsieh, Y.-L. Super water absorbing and shape memory nanocellulose aerogels from TEMPO-oxidized cellulose nanofibrils via cyclic freezing–thawing. J. Mater. Chem. A 2014, 2, 350–359. [Google Scholar] [CrossRef] [Green Version]
- Zohuriaan, M.J.-M.; Kabiri, K. Superabsorbent Polymer Materials: A Review. Iran. Polym. J. Engl. Ed. 2008, 17, 451–447. [Google Scholar]
- Mechtcherine, V.; Snoeck, D.; Schröfl, C.; De Belie, N.; Klemm, A.J.; Ichimiya, K.; Moon, J.; Wyrzykowski, M.; Lura, P.; Toropovs, N.; et al. Testing superabsorbent polymer (SAP) sorption properties prior to implementation in concrete: Results of a RILEM Round-Robin Test. Mater. Struct. 2018, 51, 28. [Google Scholar] [CrossRef]
- Shahi, S.; Motasadizadeh, H.R.; Mehr, M.J.Z. Surface Modification of Superabsorbing Hydrogels via a Feasible Esterification Reaction: Towards Tunable Superabsorbent for Hygienic Applications. Int. J. Polym. Mater. 2017, 66, 544–557. [Google Scholar] [CrossRef]
- Sharma, P.R.; Chattopadhyay, A.; Zhan, C.; Sharma, S.K.; Geng, L.; Hsiao, B.S. Lead removal from water using carboxycellulose nanofibers prepared by nitro-oxidation method. Cellulose 2018, 25, 1961–1973. [Google Scholar] [CrossRef]
- Liu, W.; Qin, Y.; Liu, S.; Xing, R.; Yu, H.; Chen, X.; Li, K.; Li, P. C-coordinated O-carboxymethyl chitosan metal complexes: Synthesis, characterization and antifungal efficacy. Int. J. Biol. Macromol. 2018, 106, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y. 6—Superabsorbent polymers and their medical applications. In Medical Textile Materials; Qin, Y., Ed.; Woodhead Publishing: Cambridge, UK, 2016; pp. 71–88. [Google Scholar] [CrossRef]
- Kosemund, K.; Schlatter, H.; Ochsenhirt, J.L.; Krause, E.L.; Marsman, D.S.; Erasala, G.N. Safety evaluation of superabsorbent baby diapers. Regul. Toxicol. Pharmacol. 2009, 53, 81–89. [Google Scholar] [CrossRef]
- Srinivas, S.M.; Dhar, S. Advances in diaper technology. Indian J. Paediatr. Dermatol. 2016, 17, 83. [Google Scholar] [CrossRef]
- Sannino, A.; Demitri, C.; Madaghiele, M. Biodegradable Cellulose-based Hydrogels: Design and Applications. Materials 2009, 2, 353–373. [Google Scholar] [CrossRef]
- Cheng, B.; Pei, B.; Wang, Z.; Hu, Q. Advances in chitosan-based superabsorbent hydrogels. RSC Adv. 2017, 7, 42036–42046. [Google Scholar] [CrossRef] [Green Version]
- Huppe, S.; Maheux, M.-E.; Chevigny, S.; Quirion, F. Glass-Like Polysaccharide Useful as Absorbent for Liquids. WO2000CA00555, 11 May 2000. [Google Scholar]
- De France, K.J.; Hoare, T.; Cranston, E.D. Review of Hydrogels and Aerogels Containing Nanocellulose. Chem. Mater. 2017, 29, 4609–4631. [Google Scholar] [CrossRef]
- Hoggarth, A.; Bugedo, A.; Hardy, C. Wound Dressing. WO2016/174399A1, 4 August 2016. [Google Scholar]
- Hardy, G.; Hoggarth, A.; Warde, D. Wound Dressing. CA2943012A1, 24 September 2015. [Google Scholar]
- Hua, S.; Wang, A. Synthesis, characterization and swelling behaviors of sodium alginate-g-poly(acrylic acid)/sodium humate superabsorbent. Carbohydr. Polym. 2009, 75, 79–84. [Google Scholar] [CrossRef]
- Nascimento, D.M.; Nunes, Y.L.; Figueirêdo, M.C.B.; De Azeredo, H.M.C.; Aouada, F.A.; Feitosa, J.P.A.; Rosa, M.D.F.; Dufresne, A. Nanocellulose nanocomposite hydrogels: Technological and environmental issues. Green Chem. 2018, 20, 2428–2448. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Chen, G. Agricultural waste-derived superabsorbent hydrogels: Preparation, performance, and socioeconomic impacts. J. Clean. Prod. 2020, 251, 119669. [Google Scholar] [CrossRef]
- Yang, X.; Cranston, E.D. Chemically Cross-Linked Cellulose Nanocrystal Aerogels with Shape Recovery and Superabsorbent Properties. Chem. Mater. 2014, 26, 6016–6025. [Google Scholar] [CrossRef]
- Sharma, P.R.; Sharma, S.K.; Borges, W.; Chen, H.; Hsiao, B.S. Remediation of UO2 2+ from Water by Nitro-Oxidized Carboxycellulose Nanofibers: Performance and Mechanism. In Contaminants in Our Water: Identification and Remediation Methods; Ahuja, S., Loganathan, B.G., Eds.; American Chemical Society: Washington, DC, USA, 2020; Volume 1352, pp. 269–283. [Google Scholar] [CrossRef]
- Bortolin, A.; Serafim, A.R.; Aouada, F.A.; Mattoso, L.H.C.; Ribeiro, C. Macro- and Micronutrient Simultaneous Slow Release from Highly Swellable Nanocomposite Hydrogels. J. Agric. Food Chem. 2016, 64, 3133–3140. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Wu, Q.; Lei, T.; Negulescu, I.I. Adsorption kinetic and equilibrium studies for methylene blue dye by partially hydrolyzed polyacrylamide/cellulose nanocrystal nanocomposite hydrogels. Chem. Eng. J. 2014, 251, 17–24. [Google Scholar] [CrossRef]
- Keller, H.; Wissemeier, A.; Weigelt, W.; Sanz-Gomez, J.; Yamamoto, M. Polysaccharide Hydrogels. WO2015097033, 2 July 2015. [Google Scholar]
- Savich, M.H.; Olson, G.S.; Clark, E.W. Superabsorbent Polymer Suspension for Use in Agriculture. CA2693796A1, 5 January 2016. [Google Scholar]
- Borriello, A.; Baldino, L.; Cardea, S.; Reverchon, E.; Nicolais, L. Process for Preparing A Superabsorbent Aerogel. WO2019167013, 6 September 2019. [Google Scholar]
- Sharma, P.R.; Sharma, S.K.; Lindström, T.; Hsiao, B.S. Nanocellulose-Enabled Membranes for Water Purification: Perspectives. Adv. Sustain. Syst. 2020, 4, 1900114. [Google Scholar] [CrossRef]
- Peng, N.; Hu, D.; Zeng, J.; Li, Y.; Liang, L.; Chang, C. Superabsorbent Cellulose–Clay Nanocomposite Hydrogels for Highly Efficient Removal of Dye in Water. ACS Sustain. Chem. Eng. 2016, 4, 7217–7224. [Google Scholar] [CrossRef]
- Chen, H.; Sharma, S.K.; Sharma, P.R.; Yeh, H.; Johnson, K.; Hsiao, B.S. Arsenic (III) Removal by Nanostructured Dialdehyde Cellulose–Cysteine Microscale and Nanoscale Fibers. ACS Omega 2019, 4, 22008–22020. [Google Scholar] [CrossRef] [Green Version]
- Sharma, P.R.; Sharma, S.K.; Antoine, R.; Hsiao, B.S. Efficient Removal of Arsenic Using Zinc Oxide Nanocrystal-Decorated Regenerated Microfibrillated Cellulose Scaffolds. ACS Sustain. Chem. Eng. 2019, 7, 6140–6151. [Google Scholar] [CrossRef]
- Ge, H.; Hua, T. Synthesis and characterization of poly (maleic acid)-grafted crosslinked chitosan nanomaterial with high uptake and selectivity for Hg (II) sorption. Carbohydr. Polym. 2016, 153, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, R.; Ray, S.K. Micro- and nano-sized bentonite filled composite superabsorbents of chitosan and acrylic copolymer for removal of synthetic dyes from water. Appl. Clay Sci. 2014, 101, 510–520. [Google Scholar] [CrossRef]



| Polysaccharides | Compositions | Glycosidic Linkages | Origins | Molecular Weights | Properties and Characteristics | Applications | References |
|---|---|---|---|---|---|---|---|
| Alginate | l-guluronic acid and d-mannuronic acid. | α-(1,4) and β-(1,4) | Brown algae | 50–106 kg/mol | Biocompatible, immunogenic and gel-forming property. | In drug release, tissue engineering and cells encapsulation), food industry (stabilizers and emulsifiers). | [27] [28] |
| Agar | Agarose and agaropectin with mainly d- and l-galactopyranose. | α-(1,3) and β-(1,4) | Red algae | / | Gelling properties. | In tissue engineering, to encapsulate cells and as growth medium. | [15] [27] |
| Agarose | d-galactopyranose and 3,6-anhydro-l-galactopyranose. | α-(1,3) and β-(1,4) | Red algae | / | Agarose is biocompatible and has high water absorption capacity. | For gel electrophoresis, immobilization of enzymes, cells encapsulation and as growth medium. | [15] [27] [29] |
| Pectin | d-galacturonic acid with rhamnogalacturonan regions. | α-(1,4) | Cell walls of terrestrial plants and from citrus fruits | Around 102 kg/mol | / | In food industry as colloidal stabilizer and gelling agent. | [11] [27] [30] |
| Starch | Amylose and branched amylopectin with d-glucopyranose. | α-(1,4) and α-(1,6). | Green plants | Amylopectin: 107–109 and amylose: 105–106 g/mol | Abundant availability in nature, biocompatible, good mechanical resistance and plasticity. | In products for drug delivery and release. Also used as food thickeners, cosmetic creams and in adhesive and paper industries. | [9] [28] |
| Hyaluronic acid | Disaccharide with d-glucuronic acid and N-acetyl-d-glucosamine. | β-(1,4) and β-(1,3) | Vertebrates tissues or produced by bacteria | 50–2.106 kg/mol | Biocompatible and mucoadhesivity. | In tissue engineering, drug delivery and wound healing. | [9] [28] |
| Carrageenan | d-galactose and d-anhydrogalactose with ester sulfate groups. | α-(1,4) and β-(1,3) | Red algae | Above 100 kg/mol | Biocompatible, water-retention and gel-forming properties. | In drug delivery, tissue engineering and wound healing. In food industry, as emulsifier, thickener and stabilizer. | [15] [28] |
| Dextran | d-glucopyranose. | α-(1,6) and α-(1,3) | Produced by lactic acid bacteria | 9.103–5.105 kg/mol | High cost and nonavailability. | In biomedical for protein and drug delivery, tissue engineering and as bioadhesive. | [9] [28] |
| Chitin | N-acetylglucosamine. | β-(1,4) | Exoskeleton of animals and cell walls of fungi | / | Biocompatibility, proteins affinity, antibacterial and gel-forming properties. | In tissue engineering, drug delivery and wound healing. | [27] [28] |
| Chitosan | d-glucosamine and N-acetyl-d-glucosamine. | β-(1,4) | Exoskeleton of animals and cell walls of fungi | / | Biocompatible, immunostimulating and mucoadhesive properties. | In drug delivery, wound dressing, tissue engineering and cell encapsulation. In water treatment as absorbent. | [28] |
| Cellulose | d-glucose. | β-(1,4) | Cell wall of terrestrial plants, algae and bacteria | / | Biocompatibility, thermal and chemical stability and high hydrophilicity. | In drug delivery, tissue engineering and wound dressing. And in paper and textile industries. | [9] [28] |
| Guar gum | d-mannopyranose and d-galactopyranose. | β-(1,4) and α-(1,6) | Seeds of terrestrial plants | / | Thickens spontaneously. | In the oil industry as thickener, in food as food supplement and in drug delivery. And as binder, emulsifier and stabilizer. | [9] [28] |
| Xanthan gum | d-glucose with d-mannose and d-glucuronic acid. | α-(1,3), β-(1,4) and β-(1,2) | Produced by bacterial fermentation | Around 103 kg/mol | Good stability in aqueous solution, suspending and thickening properties. | In food industry as stabilizer and suspending agent but also in biomedical for drug release. | [9] [27] [28] |
| Arabic gum | d-galactopyranose with l-arabinofuranose, l-rhamnopyranose and d-glucopyranose. | (1,3) and (1,6) | Stems and branches of terrestrial plants | / | Emulsifying, film-forming, stabilizing and thickening properties. | In industries as fiber and food additive as well as biomaterial for drug delivery and tissue engineering. | [28] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Llanes, L.; Dubessay, P.; Pierre, G.; Delattre, C.; Michaud, P. Biosourced Polysaccharide-Based Superabsorbents. Polysaccharides 2020, 1, 51-79. https://doi.org/10.3390/polysaccharides1010005
Llanes L, Dubessay P, Pierre G, Delattre C, Michaud P. Biosourced Polysaccharide-Based Superabsorbents. Polysaccharides. 2020; 1(1):51-79. https://doi.org/10.3390/polysaccharides1010005
Chicago/Turabian StyleLlanes, Ludovic, Pascal Dubessay, Guillaume Pierre, Cédric Delattre, and Philippe Michaud. 2020. "Biosourced Polysaccharide-Based Superabsorbents" Polysaccharides 1, no. 1: 51-79. https://doi.org/10.3390/polysaccharides1010005
APA StyleLlanes, L., Dubessay, P., Pierre, G., Delattre, C., & Michaud, P. (2020). Biosourced Polysaccharide-Based Superabsorbents. Polysaccharides, 1(1), 51-79. https://doi.org/10.3390/polysaccharides1010005








