Rheological Characterization of the Influence of Pomegranate Peel Extract Addition and Concentration in Chitosan and Gelatin Coatings
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Methods
2.2.1. Chitosan Obtention and Characterization
2.2.2. Pomegranate Peel Extraction and Characterization
2.2.3. Coating Preparation
2.2.4. Rheological Measurements
2.2.5. Strain Sweep Measurements
2.2.6. Frequency Sweep Measurements
2.2.7. Temperature Sweep Measurements
2.2.8. Steady Shear Measurements
3. Statistical Analysis
4. Results and Discussion
4.1. Pomegranate Peel Extraction and Characterization
4.2. Rheological Measurements
Strain Sweep Measurements
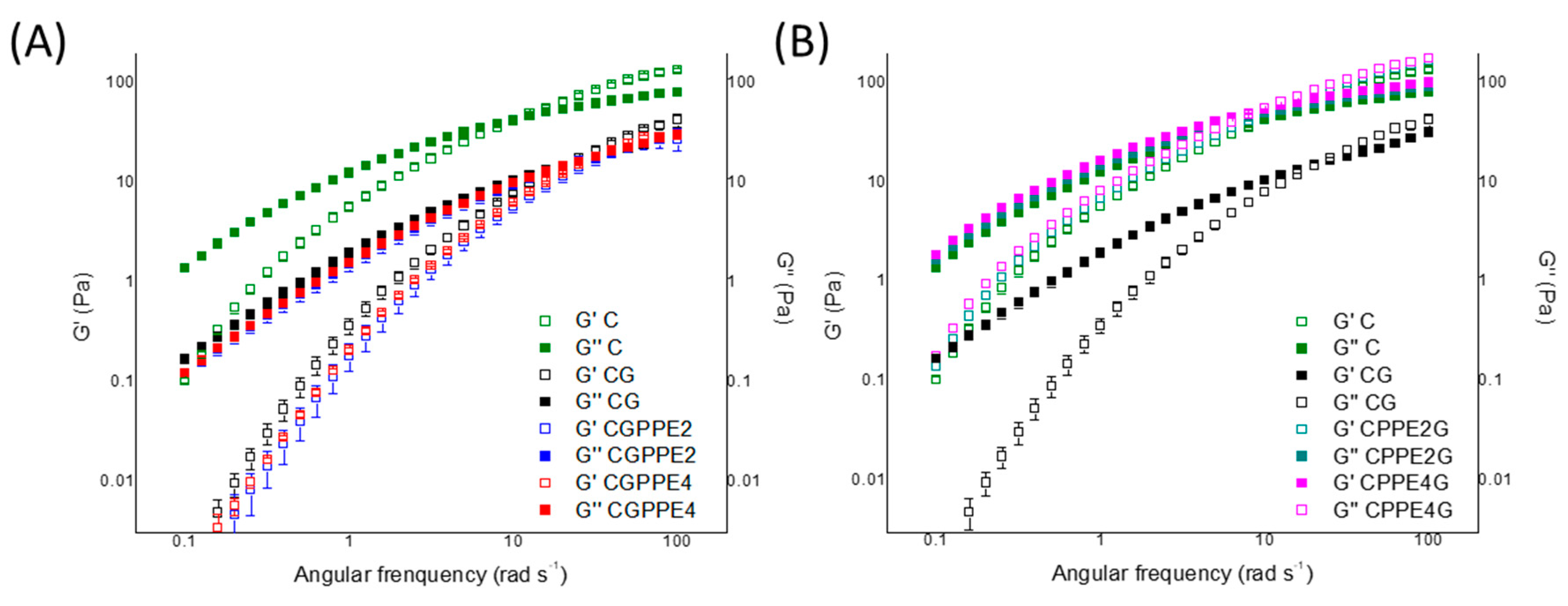
4.3. Frequency Sweep Measurements
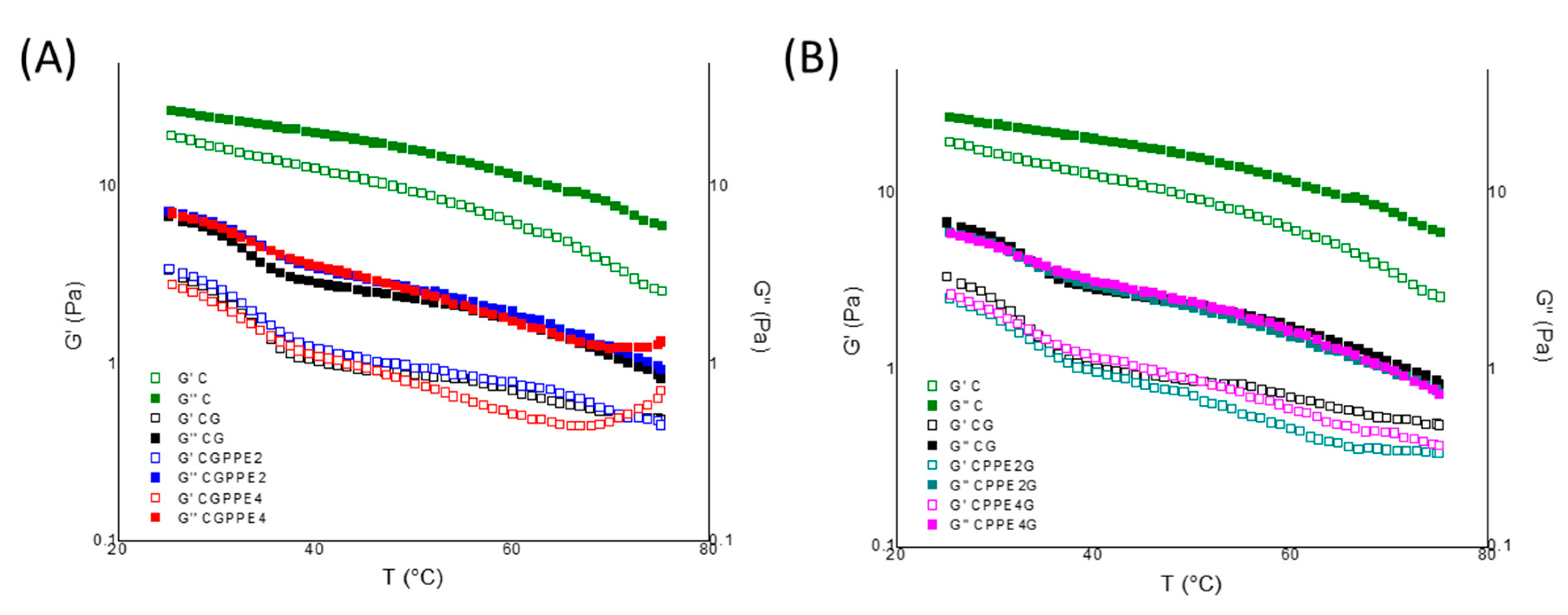
4.4. Temperature Sweep Measurements
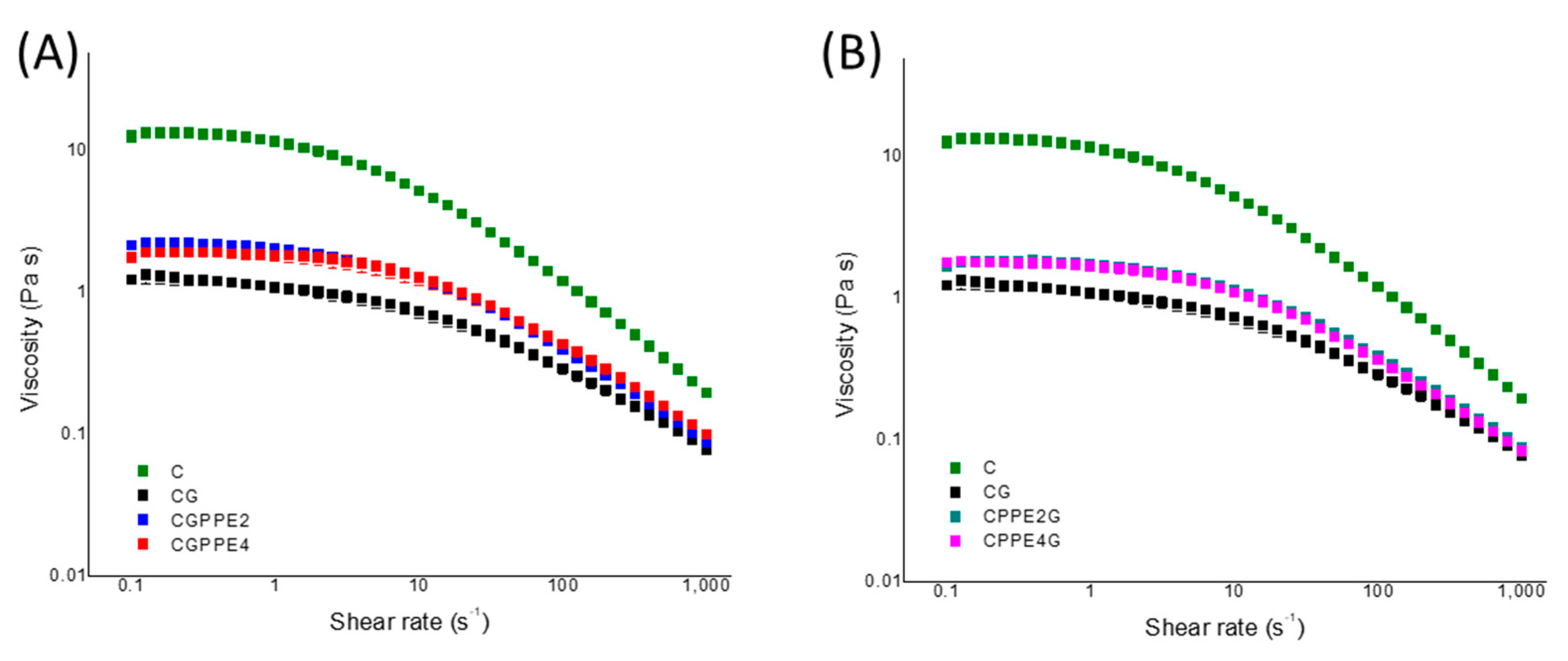
4.5. Steady Shear Measurements
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lei, Y.; Wu, H.; Jiao, C.; Jiang, Y.; Liu, R.; Xiao, D.; Lu, J.; Zhang, Z.; Shen, G.; Li, S. Investigation of the Structural and Physical Properties, Antioxidant and Antimicrobial Activity of Pectin-Konjac Glucomannan Composite Edible Films Incorporated With Tea Polyphenol. Food Hydrocoll. 2019, 94, 128–135. [Google Scholar] [CrossRef]
- Sun, J.; Jiang, H.; Li, M.; Lu, Y.; Du, Y.; Tong, C.; Pang, J.; Wu, C. Preparation and Characterization of Multifunctional Konjac Glucoman-nan/Carboxymethyl Chitosan Biocomposite Films Incorporated With Epigallocatechin Gallate. Food Hydrocoll. 2020, 105, 105756. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Ghaderi, J.; Gómez-Guillén, M.C. Trans-Cinnamaldehyde-Doped Quadripartite Biopolymeric Films: Rheological Behavior of Film-Forming Solutions and Biofunctional Performance of Films. Food Hydrocoll. 2021, 112, 106339. [Google Scholar] [CrossRef]
- Nair, M.S.; Saxena, A.; Kaur, C. Effect of Chitosan and Alginate Based Coatings Enriched With Pomegranate Peel Extract to Extend the Postharvest Quality of Guava (Psidium Guajava L.). Food Chem. 2018, 240, 245–252. [Google Scholar] [CrossRef]
- Ngo, D.-H.; Vo, T.-S.; Kang, K.-H.; Je, J.-Y.; Pham, H.N.-D.; Byun, H.-G.; Kim, S.-K. Biological Effects of Chitosan and Its Derivatives. Food Hydrocoll. 2015, 51, 200–216. [Google Scholar] [CrossRef]
- Bonilla, J.; Sobral, P.J. Investigation of the Physicochemical, Antimicrobial and Antioxidant Properties of Gelatin-Chitosan Edible Film Mixed With Plant Ethanolic Extracts. Food Biosci. 2016, 16, 17–25. [Google Scholar] [CrossRef]
- Bertolo, M.R.V.; Martins, V.D.C.A.; Plepis, A.M.D.G.; Junior, S.B. Rheological Study of the Incorporation of Grape Seed Extract in Chitosan and Gelatin Coatings. J. Appl. Polym. Sci. 2021, 138, 50052. [Google Scholar] [CrossRef]
- Nowzari, F.; Shábanpour, B.; Ojagh, S.M. Comparison of chitosan–gelatin Composite and Bilayer Coating and Film Effect on the Quality of Refrigerated Rainbow Trout. Food Chem. 2013, 141, 1667–1672. [Google Scholar] [CrossRef] [PubMed]
- Bertolo, M.R.; Martins, V.; Horn, M.M.; Brenelli, L.B.; Plepis, A. Rheological and Antioxidant Properties of chitosan/Gelatin-Based Materials Functionalized by Pomegranate Peel Extract. Carbohydr. Polym. 2020, 228, 115386. [Google Scholar] [CrossRef]
- Rodrigues, M.; Álison, V.; Bertolo, M.R.V.; Marangon, C.; Martins, V.; Plepis, A.M.D.G. Chitosan and Gelatin Materials Incorporated With Phenolic Extracts of Grape Seed and Jabuticaba Peel: Rheological, Physicochemical, Antioxidant, Antimicrobial and Barrier Properties. Int. J. Biol. Macromol. 2020, 160, 769–779. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, G.P.; Dutra, M.P.; Fontes, P.R.; Ramos, A.D.L.S.; Gomide, L.A.D.M.; Ramos, E.M. Selection of a Chitosan Gelatin-Based Edible Coating for Color Preservation of Beef in Retail Display. Meat Sci. 2016, 114, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.H.; Li, Y.Z.; Yu, D.; Lin, W.; Cailing, T.; Hu, Y.; Pang, J.; Yan, Z. Preparation and Characterization of Konjac Glucomannan-Based Bionanocompo-Site Film for Active Food Packaging. Food Hydrocoll. 2019, 89, 682–690. [Google Scholar] [CrossRef]
- Silva-Weiss, A.; Bifani, V.; Ihl, M.; Sobral, P.; Gómez-Guillén, M.C. Structural Properties of Films and Rheology of Film-Forming Solutions Based on Chitosan and Chitosan-Starch Blend Enriched With Murta Leaf Extract. Food Hydrocoll. 2013, 31, 458–466. [Google Scholar] [CrossRef] [Green Version]
- Steffe, J.F. Rheological Methods in Food Process Engineering; Freeman Press: East Lansing, MI, USA, 1996. [Google Scholar]
- Horn, M.M.; Martins, V.; Plepis, A.M.D.G. Interaction of Anionic Collagen With Chitosan: Effect on Thermal and Morphological Characteristics. Carbohydr. Polym. 2009, 77, 239–243. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and Chitosan: Properties and Applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Lavertu, M.; Xia, Z.; Serreqi, A.; Berrada, M.; Rodrigues, A.G.; Wang, D.; Buschmann, M.; Gupta, A. A Validated 1H NMR Method for the Determination of the Degree of Deacetylation of Chitosan. J. Pharm. Biomed. Anal. 2003, 32, 1149–1158. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. [14] Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Derakhshan, Z.; Ferrante, M.; Tadi, M.; Ansari, F.; Heydari, A.; Hosseini, M.S.; Conti, G.O.; Sadrabad, E.K. Antioxidant Activity and Total Phenolic Content of Ethanolic Extract of Pomegranate Peels, Juice and Seeds. Food Chem. Toxicol. 2018, 114, 108–111. [Google Scholar] [CrossRef]
- Razmkhah, S.; Mohammad, S.; Razavi, A. Dilute Solution, Flow Behavior, Thixotropy and Viscoelastic Characterization of Cress Seed (Lepidium Sativum) Gum Fractions. Food Hydrocoll. 2017, 63, 404–413. [Google Scholar] [CrossRef]
- Luciano, C.G.; Tessaro, L.; Lourenço, R.V.; Barbosa Bittante, A.M.Q.; Fernandes, A.M.; Freitas Moraes, I.C.; do Amaral Sobral, P.J. Effects of Nisin Concentration on Properties of Gelatin Film Forming so-Lutions and Their Films. Int. J. Food Sci. Technol. 2021, 56, 587–599. [Google Scholar] [CrossRef]
- Mandala, I.; Savvas, T.; Kostaropoulos, A. Xanthan and Locust Bean Gum Influence on the Rheology and Structure of a White Model-Sauce. J. Food Eng. 2004, 64, 335–342. [Google Scholar] [CrossRef]
- Nair, S.B.; Jyothi, A.N.; Sajeev, M.S.; Misra, R. Rheological, Mechanical and Moisture Sorption Charac-Teristics of Cassava starch-konjac Glucomannan Blend Films. Starch 2011, 63, 728. [Google Scholar] [CrossRef]
- Tudorache, M.; Bordenave, N. Phenolic Compounds Mediate Aggregation of Water-Soluble Polysaccha-Rides and Change Their Rheological Properties: Effect of Different Phenolic Compounds. Food Hydrocoll. 2019, 97, 105193. [Google Scholar] [CrossRef]
- Phillips, G.O.; Williams, P.A. Handbook of Hydrocolloids; Elsevier: Amsterdam, The Netherlands, 2009. [Google Scholar] [CrossRef]
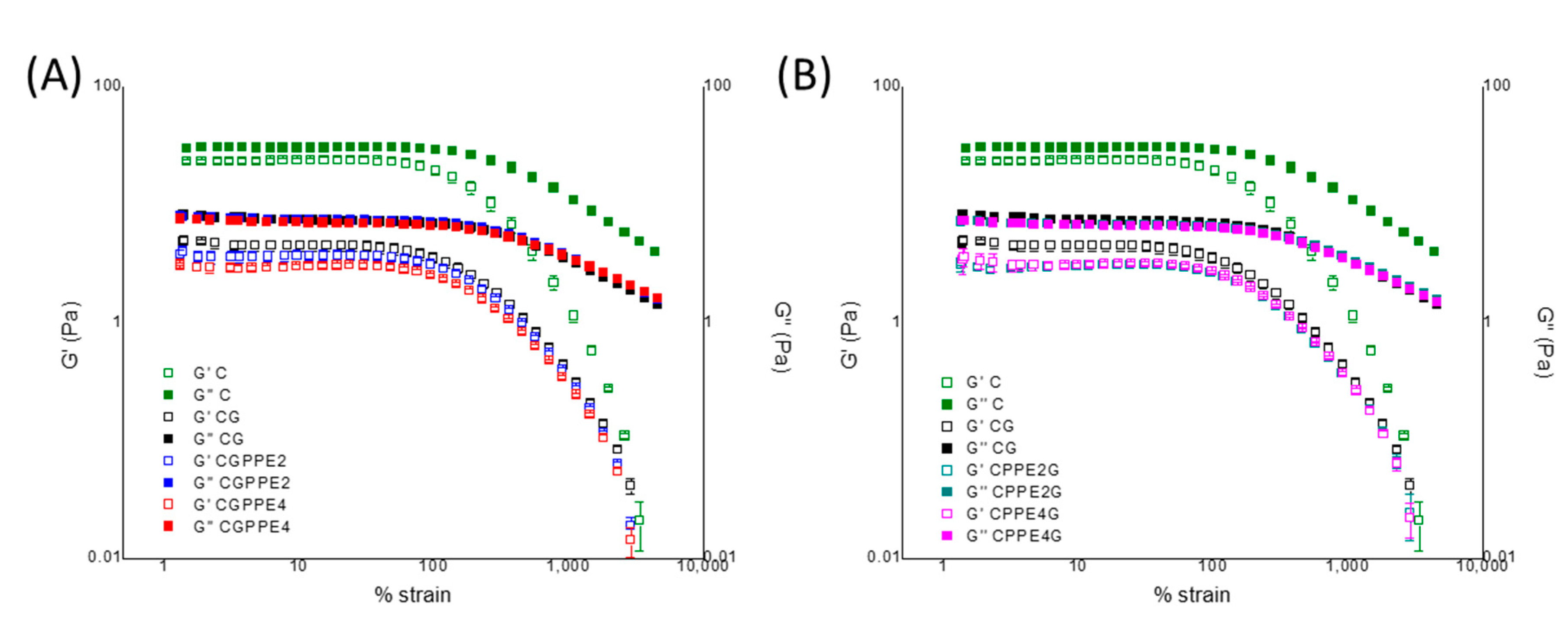
| Sample | γL (%) | G′LVR (Pa) | tanδ | G″–G′ (Pa) |
|---|---|---|---|---|
| C | 48.87 ± 3.24 a | 22.80 ± 1.49 a | 1.32 ± 0.02 e | 6.51 ± 0.27 a |
| CG | 40.78 ± 0.32 b | 4.27 ± 0.45 b | 1.68 ± 0.06 d | 2.94 ± 0.06 d |
| CGPPE2 | 48.93 ± 0.30 a | 3.56 ± 0.28 b | 2.05 ± 0.11 c | 3.74 ± 0.07 b, c |
| CGPPE4 | 48.26 ± 0.40 a | 2.92 ± 0.16 b | 2.33 ± 0.06 a | 4.04 ± 0.14 b |
| CPPE2 G | 49.01 ± 0.47 a | 2.97 ± 0.12 b | 2.23 ± 0.01 a, b | 3.77 ± 0.18 b, c |
| CPPE4 G | 49.76 ± 0.33 a | 3.04 ± 0.11 b | 2.12 ± 0.02 b, c | 3.57 ± 0.10 c |
| Solution | ωcrossover (rad s−1) | G′crossover (Pa) |
|---|---|---|
| C | 9.99 ± 0.01 d | 41.54 ± 2.97 a |
| CG | 19.98 ± 0.01 c, d | 14.65 ± 0.78 d |
| CGPPE2 | 25.13 ± 0.01 b, c | 18.81 ± 1.33 c |
| CGPPE4 | 37.04 ± 4.64 a | 22.01 ± 1.51 a, b |
| CPPE2 G | 37.04 ± 4.64 a | 19.63 ± 0.75 b, c |
| CPPE4G | 31.69 ± 0.01 a, b | 17.85 ± 0.26 c, d |
| Solution | η0 (Pa s) | k (s) | n |
|---|---|---|---|
| C | 14.90 ± 1.27 a | 0.212 ± 0.020 a | 0.800 ± 0.002 a |
| CG | 2.85 ± 0.14 b | 0.119 ± 0.004 b | 0.720 ± 0.006 c |
| CGPPE2 | 2.37 ± 0.01 b | 0.086 ± 0.004 c | 0.744 ± 0.011 b, c |
| CGPPE4 | 2.05 ± 0.18 b | 0.056 ± 0.001 d | 0.771 ± 0.031 a, b |
| CPPE2G | 1.97 ± 0.11 b | 0.066 ± 0.002 c, d | 0.744 ± 0.006 b, c |
| CPPE4G | 1.92 ± 0.14 b | 0.069 ± 0.003 c, d | 0.759 ± 0.009 b, c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romanelli Vicente Bertolo, M.; Leme, R.; da Conceição Amaro Martins, V.; de Guzzi Plepis, A.M.; Bogusz Junior, S. Rheological Characterization of the Influence of Pomegranate Peel Extract Addition and Concentration in Chitosan and Gelatin Coatings. Polysaccharides 2021, 2, 648-660. https://doi.org/10.3390/polysaccharides2030039
Romanelli Vicente Bertolo M, Leme R, da Conceição Amaro Martins V, de Guzzi Plepis AM, Bogusz Junior S. Rheological Characterization of the Influence of Pomegranate Peel Extract Addition and Concentration in Chitosan and Gelatin Coatings. Polysaccharides. 2021; 2(3):648-660. https://doi.org/10.3390/polysaccharides2030039
Chicago/Turabian StyleRomanelli Vicente Bertolo, Mirella, Rafael Leme, Virginia da Conceição Amaro Martins, Ana Maria de Guzzi Plepis, and Stanislau Bogusz Junior. 2021. "Rheological Characterization of the Influence of Pomegranate Peel Extract Addition and Concentration in Chitosan and Gelatin Coatings" Polysaccharides 2, no. 3: 648-660. https://doi.org/10.3390/polysaccharides2030039
APA StyleRomanelli Vicente Bertolo, M., Leme, R., da Conceição Amaro Martins, V., de Guzzi Plepis, A. M., & Bogusz Junior, S. (2021). Rheological Characterization of the Influence of Pomegranate Peel Extract Addition and Concentration in Chitosan and Gelatin Coatings. Polysaccharides, 2(3), 648-660. https://doi.org/10.3390/polysaccharides2030039