Natural Polymers Used in Edible Food Packaging—History, Function and Application Trends as a Sustainable Alternative to Synthetic Plastic
Abstract
:1. Introduction
2. Historical Perspective of Food Packaging
- Improvement of national and international legislation to correctly establish the concepts and required standards related to bioplastics.
- Revision of industrial facilities for the processability of these materials while maintaining optimized and efficient performance.
- Maintaining the adequate quality of products and ensuring their biodegradability and compostability.
- Adjustments of the waste management system of each country/region to the collection of biobased plastics since each city has its own program.
3. Some Natural and Renewable Macromolecules Used to Prepare Edible Films and Coatings
3.1. Alginates
3.2. Carrageenans
3.3. Chitosan
3.4. Starch
3.5. Pea Protein
3.6. Improvements by the Incorporation of Natural Bioactive Substances into Edible Films
| Additives | Biological Properties | Main Results | Reference |
|---|---|---|---|
| Maqui berry extracts | Antimicrobial and antioxidant |
| [145] |
| Grape seed extract and Ziziphora clinopodioides essential oil |
| [146] | |
| Propolis extract |
| [147] | |
| Apple peel polyphenols |
| [148] | |
| Zataria multiflora Boiss essential oil |
| [149] | |
| Zataria multiflora Boiss essential oil and grape seed extract | Antioxidant |
| [150] |
| Green tea and black tea extracts |
| [151] | |
| Carvacrol | Antibacterial activity |
| [5] |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Verma, M.K.; Shakya, S.; Kumar, P.; Madhavi, J.; Murugaiyan, J.; Rao, M.V.R. Trends in Packaging Material for Food Products: Historical Background, Current Scenario, and Future Prospects. J. Food Sci. Technol. 2021, 58, 4069–4082. [Google Scholar] [CrossRef]
- Brody, A.L.; Bugusu, B.; Han, J.H.; Sand, C.K.; McHugh, T.H. Innovative Food Packaging Solutions. J. Food Sci. 2008, 73, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Page, B. Rigid Metal Packaging. In Packaging Technology; Emblem, A., Emblem, H., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 122–162. [Google Scholar] [CrossRef]
- Jia, L.; Evans, S.; van der Linden, S. Motivating Actions to Mitigate Plastic Pollution. Nat. Commun. 2019, 10, 4582. [Google Scholar] [CrossRef] [PubMed]
- Kamdem, D.P.; Shen, Z.; Nabinejad, O. Development of Biodegradable Composite Chitosan-Based Films Incorporated with Xylan and Carvacrol for Food Packaging Application. Food Packag. Shelf Life 2019, 21, 100344. [Google Scholar] [CrossRef]
- Carina, D.; Sharma, S.; Jaiswal, A.K.; Jaiswal, S. Seaweeds Polysaccharides in Active Food Packaging: A Review of Recent Progress. Trends Food Sci. Technol. 2021, 110, 559–572. [Google Scholar] [CrossRef]
- Mohamed, S.A.A.; El-Sakhawy, M.; El-Sakhawy, M.A.-M. Polysaccharides, Protein and Lipid -Based Natural Edible Films in Food Packaging: A Review. Carbohydr. Polym. 2020, 238, 116178. [Google Scholar] [CrossRef] [PubMed]
- Petkoska, A.T.; Daniloski, D.; D’Cunha, N.M.; Naumovski, N.; Broach, A.T. Edible Packaging: Sustainable Solutions and Novel Trends in Food Packaging. Food Res. Int. 2021, 140, 109981. [Google Scholar] [CrossRef]
- Miller, K.S.; Krochta, J.M. Oxygen and Aroma Barrier Properties of Edible Films: A Review. Trends Food Sci. Technol. 1997, 8, 228–237. [Google Scholar] [CrossRef]
- Risch, S.J. Food Packaging History and Innovations. J. Agric. Food Chem. 2009, 57, 8089–8092. [Google Scholar] [CrossRef]
- Twede, D. The Packaging Technology and Science of Ancient Transport Amphoras. Packag. Technol. Sci. 2002, 15, 181–195. [Google Scholar] [CrossRef]
- Barreto, C.; Oliveira, E. Para Além de Potes e Panelas: Cerâmica e Ritual Na Amazônia Antiga. Habitus 2016, 14, 51. [Google Scholar] [CrossRef]
- Berger, K.R.; Welt, B. A Brief History of Packaging. In EDIS-Electronic Data Information Source of University of Florida; Institute of Food and Agricultural Sciences, University of Florida: Gainesville, FL, USA, 2005; pp. 1–5. [Google Scholar] [CrossRef]
- Mkandawire, M.; Aryee, A.N. Resurfacing and Modernization of Edible Packaging Material Technology. Curr. Opin. Food Sci. 2018, 19, 104–112. [Google Scholar] [CrossRef]
- Pavlath, A.E.; Orts, W. Edible Films and Coatings: Why, What, and How? In Edible Films and Coatings for Food Applications; Huber, K.C., Embuscado, M.E., Eds.; Springer: New York, NY, USA, 2009; pp. 1–23. [Google Scholar] [CrossRef]
- Soares, J.; Miguel, I.; Venâncio, C.; Lopes, I.; Oliveira, M. Public Views on Plastic Pollution: Knowledge, Perceived Impacts, and pro-Environmental Behaviours. J. Hazard. Mater. 2021, 412, 125227. [Google Scholar] [CrossRef] [PubMed]
- Groh, K.J.; Backhaus, T.; Carney-Almroth, B.; Geueke, B.; Inostroza, P.A.; Lennquist, A.; Leslie, H.A.; Maffini, M.; Slunge, D.; Trasande, L.; et al. Overview of Known Plastic Packaging-Associated Chemicals and Their Hazards. Sci. Total Environ. 2019, 651, 3253–3268. [Google Scholar] [CrossRef] [PubMed]
- Unilever. Unilever Announces Ambitious New Commitments for a Waste-Free World. 2019. Available online: https://www.unilever.com/news/press-releases/2019/unilever-announces-ambitious-new-commitments-for-a-waste-free-world.html (accessed on 26 April 2021).
- The Coca-Cola Company®. News–DASANI Boots Sustainability Credentials with Launch of Recyclable, Reusable and Package-less Innovations. 2019. Available online: https://www.coca-colacompany.com/news/dasani-boosts-sustainability-credentials (accessed on 26 April 2021).
- The Coca-Cola Company®. Sustainable Business–Sustainable Packaging Design. Make 100% of Our Packaging Recyclable Globally by 2025. 2021. Available online: https://www.coca-colacompany.com/sustainable-business/packaging-sustainability/design (accessed on 26 April 2021).
- AS YOU SOW. After Dialogue with As You Sow, Keurig Dr Pepper Agrees to 20% Cut in Virgin Plastic Use. 2021. Available online: https://www.asyousow.org/press-releases/2021/4/12/after-dialogue-with-as-you-sow-keurig-dr-pepper-agrees-to-20-cut-in-virgin-plastic-use?utm_medium=email&utm_source=rasa_io&PostID=28618185&MessageRunDetailID=4922559192 (accessed on 26 April 2021).
- Campos, C.A.; Gerschenson, L.N.; Flores, S.K. Development of Edible Films and Coatings with Antimicrobial Activity. Food Bioprocess Technol. 2011, 4, 849–875. [Google Scholar] [CrossRef]
- Imre, B.; García, L.; Puglia, D.; Vilaplana, F. Reactive Compatibilization of Plant Polysaccharides and Biobased Polymers: Review on Current Strategies, Expectations and Reality. Carbohydr. Polym. 2019, 209, 20–37. [Google Scholar] [CrossRef]
- ISO-International Organization for Standardization. Standard ISO 16620-1:2015, Plastics—Biobased Content—Part 1: General Principles; International Organization for Standardization: Geneva, Switzerland, 2015. [Google Scholar]
- Dhall, R.K.; Alam, M.S. Biodegradable Packaging. In Encyclopedia of Renewable and Sustainable Materials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 26–43. [Google Scholar] [CrossRef]
- Gross, R.A.; Kalra, B. Biodegradable Polymers for the Enviroment. Science 2002, 297, 803–807. [Google Scholar] [CrossRef] [Green Version]
- Flury, M.; Narayan, R. Biodegradable Plastic as an Integral Part of the Solution to Plastic Waste Pollution of the Environment. Curr. Opin. Green Sustain. Chem. 2021, 30, 100490. [Google Scholar] [CrossRef]
- Siracusa, V.; Lotti, N. Biobased Plastics for Food Packaging. In Reference Module in Food Science; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–19. [Google Scholar] [CrossRef]
- Noori, N.; Khanjari, A.; Rezaeigolestani, M.; Karabagias, I.K.; Mokhtari, S. Development of Antibacterial Biocomposites Based on Poly(Lactic Acid) with Spice Essential Oil (Pimpinella Anisum) for Food Applications. Polymers 2021, 13, 3791. [Google Scholar] [CrossRef]
- van den Oever, M.; Molenveld, K.; van der Zee, M.; Bos, H. Bio-Based and Biodegradable Plastics: Facts and Figures: Focus on Food Packaging in the Netherlands; Springer International Publishing: Cham, Switzerland, 2017; Volume 4. [Google Scholar] [CrossRef] [Green Version]
- Haghighi, H.; Licciardello, F.; Fava, P.; Siesler, H.W.; Pulvirenti, A. Recent Advances on Chitosan-Based Films for Sustainable Food Packaging Applications. Food Packag. Shelf Life 2020, 26, 100551. [Google Scholar] [CrossRef]
- ASTM-American Society for Testing and Materials. Standard D 996–04. In Standard Terminology of Packaging and Distribution Environments; ASTM International: Philadelphia, PA, USA, 2004; pp. 1–12. [Google Scholar]
- Halonen, N.; Pálvölgyi, P.S.; Bassani, A.; Fiorentini, C.; Nair, R.; Spigno, G.; Kordas, K. Bio-Based Smart Materials for Food Packaging and Sensors—A Review. Front. Mater. 2020, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Intrado Globe Newswire. Bioplastic Market Size to Reach $13.1 Billion by 2027. Available online: https://www.globenewswire.com/news-release/2020/11/19/2130218/0/en/Bioplastic-Market-Size-to-Reach-13-1-Billion-by-2027-CAGR-13-8-AMR.html (accessed on 24 April 2021).
- European Bioplastics Org. Applications for Bioplastics. Available online: https://www.european-bioplastics.org/market/applications-sectors/ (accessed on 26 April 2021).
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-Based Films and Coatings for Food Packaging: A Review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Liang, J.; Yan, H.; Zhang, J.; Dai, W.; Gao, X.; Zhou, Y.; Wan, X.; Puligundla, P. Preparation and Characterization of Antioxidant Edible Chitosan Films Incorporated with Epigallocatechin Gallate Nanocapsules. Carbohydr. Polym. 2017, 171, 300–306. [Google Scholar] [CrossRef]
- Quirós-Sauceda, A.E.; Ayala-Zavala, J.F.; Olivas, G.I.; González-Aguilar, G.A. Edible Coatings as Encapsulating Matrices for Bioactive Compounds: A Review. J. Food Sci. Technol. 2014, 51, 1674–1685. [Google Scholar] [CrossRef] [Green Version]
- Burger, T.G.; Zhang, Y. Recent Progress in the Utilization of Pea Protein as an Emulsifier for Food Applications. Trends Food Sci. Technol. 2019, 86, 25–33. [Google Scholar] [CrossRef]
- Assad, I.; Bhat, S.U.; Gani, A.; Shah, A. Protein Based Packaging of Plant Origin: Fabrication, Properties, Recent Advances and Future Perspectives. Int. J. Biol. Macromol. 2020, 164, 707–716. [Google Scholar] [CrossRef]
- Silva-Weiss, A.; Ihl, M.; Sobral, P.J.A.; Gómez-Guillén, M.C.; Bifani, V. Natural Additives in Bioactive Edible Films and Coatings: Functionality and Applications in Foods. Food Eng. Rev. 2013, 5, 200–216. [Google Scholar] [CrossRef]
- Otoni, C.G.; Avena-Bustillos, R.J.; Azeredo, H.M.C.; Lorevice, M.V.; Moura, M.R.; Mattoso, L.H.C.; McHugh, T.H. Recent Advances on Edible Films Based on Fruits and Vegetables-A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1151–1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sousa, A.M.M.; Souza, H.K.S.; Latona, N.; Liu, C.K.; Gonçalves, M.P.; Liu, L. Choline Chloride Based Ionic Liquid Analogues as Tool for the Fabrication of Agar Films with Improved Mechanical Properties. Carbohydr. Polym. 2014, 111, 206–214. [Google Scholar] [CrossRef]
- Vital, A.C.P.; Guerrero, A.; Ornaghi, M.G.; Kempinski, E.M.B.C.; Sary, C.; Monteschio, J.O.; Matumoto-Pintro, P.T.; Ribeiro, R.P.; do Prado, I.N. Quality and Sensory Acceptability of Fish Fillet (Oreochromis Niloticus) with Alginate-Based Coating Containing Essential Oils. J. Food Sci. Technol. 2018, 55, 4945–4955. [Google Scholar] [CrossRef] [PubMed]
- Kowalczyk, D.; Szymanowska, U.; Skrzypek, T.; Basiura-Cembala, M.; Łupina, K.; Biendl, M. Edible Films Based on Gelatin, Carboxymethyl Cellulose, and Their Blends as Carriers of Potassium Salts of Iso-α-Acids: Structural, Physicochemical and Antioxidant Properties. Food Hydrocoll. 2021, 115, 106574. [Google Scholar] [CrossRef]
- Mujtaba, M.; Morsi, R.E.; Kerch, G.; Elsabee, M.Z.; Kaya, M.; Labidi, J.; Khawar, K.M. Current Advancements in Chitosan-Based Film Production for Food Technology; A Review. Int. J. Biol. Macromol. 2019, 121, 889–904. [Google Scholar] [CrossRef]
- Sharma, S.; Rao, T.V.R. Xanthan Gum Based Edible Coating Enriched with Cinnamic Acid Prevents Browning and Extends the Shelf-Life of Fresh-Cut Pears. LWT-Food Sci. Technol. 2015, 62, 791–800. [Google Scholar] [CrossRef]
- Hamzah, H.M.; Osman, A.; Tan, C.P.; Mohamad Ghazali, F. Carrageenan as an Alternative Coating for Papaya (Carica Papaya L. Cv. Eksotika). Postharvest Biol. Technol. 2013, 75, 142–146. [Google Scholar] [CrossRef]
- Tran, T.T.B.; Roach, P.; Nguyen, M.H.; Pristijono, P.; Vuong, Q.V. Development of Biodegradable Films Based on Seaweed Polysaccharides and Gac Pulp (Momordica Cochinchinensis), the Waste Generated from Gac Oil Production. Food Hydrocoll. 2020, 99, 105322. [Google Scholar] [CrossRef]
- Jahromi, M.; Niakousari, M.; Golmakani, M.T.; Mohammadifar, M.A. Physicochemical and Structural Characterization of Sodium Caseinate Based Film-Forming Solutions and Edible Films as Affected by High Methoxyl Pectin. Int. J. Biol. Macromol. 2020, 165, 1949–1959. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; He, Y.; Liu, F.; Liao, L.; Huang, X.; Li, R.; Zou, Y.; Zhou, L.; Zou, L.; Liu, Y.; et al. Carboxymethyl Chitosan-Pullulan Edible Films Enriched with Galangal Essential Oil: Characterization and Application in Mango Preservation. Carbohydr. Polym. 2021, 256, 117579. [Google Scholar] [CrossRef]
- Moreno, O.; Atarés, L.; Chiralt, A. Effect of the Incorporation of Antimicrobial/Antioxidant Proteins on the Properties of Potato Starch Films. Carbohydr. Polym. 2015, 133, 353–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, J.C.M.; Miki, K.S.L.; da Silva Ramos, A.; Teixeira-Costa, B.E. Development of Biodegradable Films Based on Purple Yam Starch/Chitosan for Food Application. Heliyon 2020, 6, 1–10. [Google Scholar] [CrossRef]
- Chevalier, E.; Chaabani, A.; Assezat, G.; Prochazka, F.; Oulahal, N. Casein/Wax Blend Extrusion for Production of Edible Films as Carriers of Potassium Sorbate—A Comparative Study of Waxes and Potassium Sorbate Effect. Food Packag. Shelf Life 2018, 16, 41–50. [Google Scholar] [CrossRef]
- Jiang, Y.; Lan, W.; Sameen, D.E.; Ahmed, S.; Qin, W.; Zhang, Q.; Chen, H.; Dai, J.; He, L.; Liu, Y. Preparation and Characterization of Grass Carp Collagen-Chitosan-Lemon Essential Oil Composite Films for Application as Food Packaging. Int. J. Biol. Macromol. 2020, 160, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Liu, Z.; Zhang, L.; Wang, X.; Li, L. Effects of Plasticizer Type and Concentration on Rheological, Physico-Mechanical and Structural Properties of Chitosan/Zein Film. Int. J. Biol. Macromol. 2020, 143, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Acquah, C.; Zhang, Y.; Dubé, M.A.; Udenigwe, C.C. Formation and Characterization of Protein-Based Films from Yellow Pea (Pisum Sativum) Protein Isolate and Concentrate for Edible Applications. Curr. Res. Food Sci. 2020, 2, 61–69. [Google Scholar] [CrossRef]
- Maryam Adilah, Z.A.; Jamilah, B.; Nur Hanani, Z.A. Functional and Antioxidant Properties of Protein-Based Films Incorporated with Mango Kernel Extract for Active Packaging. Food Hydrocoll. 2018, 74, 207–218. [Google Scholar] [CrossRef]
- Ansorena, M.R.; Zubeldía, F.; Marcovich, N.E. Active Wheat Gluten Films Obtained by Thermoplastic Processing. LWT-Food Sci. Technol. 2016, 69, 47–54. [Google Scholar] [CrossRef]
- Azevedo, V.M.; Borges, S.V.; Marconcini, J.M.; Yoshida, M.I.; Neto, A.R.S.; Pereira, T.C.; Pereira, C.F.G. Effect of Replacement of Corn Starch by Whey Protein Isolate in Biodegradable Film Blends Obtained by Extrusion. Carbohydr. Polym. 2017, 157, 971–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De León-Zapata, M.A.; Sáenz-Galindo, A.; Rojas-Molina, R.; Rodríguez-Herrera, R.; Jasso-Cantú, D.; Aguilar, C.N. Edible Candelilla Wax Coating with Fermented Extract of Tarbush Improves the Shelf Life and Quality of Apples. Food Packag. Shelf Life 2015, 3, 70–75. [Google Scholar] [CrossRef] [Green Version]
- Rocca-Smith, J.R.; Marcuzzo, E.; Karbowiak, T.; Centa, J.; Giacometti, M.; Scapin, F.; Venir, E.; Sensidoni, A.; Debeaufort, F. Effect of Lipid Incorporation on Functional Properties of Wheat Gluten Based Edible Films. J. Cereal Sci. 2016, 69, 275–282. [Google Scholar] [CrossRef]
- Rodrigues, D.C.; Cunha, A.P.; Brito, E.S.; Azeredo, H.M.C.; Gallão, M.I. Mesquite Seed Gum and Palm Fruit Oil Emulsion Edible Films: Influence of Oil Content and Sonication. Food Hydrocoll. 2016, 56, 227–235. [Google Scholar] [CrossRef]
- Garrido, T.; Etxabide, A.; Leceta, I.; Cabezudo, S.; de la Caba, K.; Guerrero, P. Valorization of Soya By-Products for Sustainable Packaging. J. Clean. Prod. 2014, 64, 228–233. [Google Scholar] [CrossRef]
- Ferreira, M.S.L.; Fai, A.E.C.; Andrade, C.T.; Picciani, P.H.; Azero, E.G.; Gonçalves, É.C.B.A. Edible Films and Coatings Based on Biodegradable Residues Applied to Acerolas (Malpighia punicifolia L.). J. Sci. Food Agric. 2016, 96, 1634–1642. [Google Scholar] [CrossRef]
- Munhoz, D.R.; Moreira, F.K.V.; Bresolin, J.D.; Bernardo, M.P.; De Sousa, C.P.; Mattoso, L.H.C. Sustainable Production and in Vitro Biodegradability of Edible Films from Yellow Passion Fruit Coproducts via Continuous Casting. ACS Sustain. Chem. Eng. 2018, 6, 9883–9892. [Google Scholar] [CrossRef]
- Espitia, P.J.P.; Avena-Bustillos, R.J.; Du, W.-X.; Teófilo, R.F.; Soares, N.F.F.; McHugh, T.H. Optimal Antimicrobial Formulation and Physical–Mechanical Properties of Edible Films Based on Açaí and Pectin for Food Preservation. Food Packag. Shelf Life 2014, 2, 38–49. [Google Scholar] [CrossRef]
- Tavassoli-Kafrani, E.; Shekarchizadeh, H.; Masoudpour-Behabadi, M. Development of Edible Films and Coatings from Alginates and Carrageenans. Carbohydr. Polym. 2016, 137, 360–374. [Google Scholar] [CrossRef]
- Liu, K.; Yuan, C.; Chen, Y.; Li, H.; Liu, J. Combined Effects of Ascorbic Acid and Chitosan on the Quality Maintenance and Shelf Life of Plums. Sci. Hortic. Amst. 2014, 176, 45–53. [Google Scholar] [CrossRef]
- Valero, D.; Díaz-Mula, H.M.; Zapata, P.J.; Guillén, F.; Martínez-Romero, D.; Castillo, S.; Serrano, M. Effects of Alginate Edible Coating on Preserving Fruit Quality in Four Plum Cultivars during Postharvest Storage. Postharvest. Biol. Technol. 2013, 77, 1–6. [Google Scholar] [CrossRef]
- Chen, C.; Peng, X.; Zeng, R.; Chen, M.; Wan, C.; Chen, J. Ficus Hirta Fruits Extract Incorporated into an Alginate-Based Edible Coating for Nanfeng Mandarin Preservation. Sci. Hortic. Amst. 2016, 202, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Fakhouri, F.M.; Martelli, S.M.; Caon, T.; Velasco, J.I.; Mei, L.H.I. Edible Films and Coatings Based on Starch/Gelatin: Film Properties and Effect of Coatings on Quality of Refrigerated Red Crimson Grapes. Postharvest. Biol. Technol. 2015, 109, 57–64. [Google Scholar] [CrossRef]
- Bastarrachea, L.; Dhawan, S.; Sablani, S.S. Engineering Properties of Polymeric-Based Antimicrobial Films for Food Packaging: A Review. Food Eng. Rev. 2011, 3, 79–93. [Google Scholar] [CrossRef]
- Chakravartula, S.S.N.; Soccio, M.; Lotti, N.; Balestra, F.; Dalla Rosa, M.; Siracusa, V. Characterization of Composite Edible Films Based on Pectin/Alginate/Whey Protein Concentrate. Materials 2019, 12, 2454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parreidt, T.S.; Müller, K.; Schmid, M. Alginate-Based Edible Films and Coatings for Food Packaging Applications. Foods 2018, 7, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos, Ó.L.; Pereira, R.N.; Cerqueira, M.A.; Martins, J.R.; Teixeira, J.A.; Malcata, F.X.; Vicente, A.A. Bio-Based Nanocomposites for Food Packaging and Their Effect in Food Quality and Safety; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Domene-López, D.; Delgado-Marín, J.J.; Martin-Gullon, I.; García-Quesada, J.C.; Montalbán, M.G. Comparative Study on Properties of Starch Films Obtained from Potato, Corn and Wheat Using 1-Ethyl-3-Methylimidazolium Acetate as Plasticizer. Int. J. Biol. Macromol. 2019, 135, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Maniglia, B.C.; Tessaro, L.; Ramos, A.P.; Tapia-Blácido, D.R. Which Plasticizer Is Suitable for Films Based on Babassu Starch Isolated by Different Methods? Food Hydrocoll. 2019, 89, 143–152. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agüero, L.; Zaldivar-Silva, D.; Peña, L.; Dias, M. Alginate Microparticles as Oral Colon Drug Delivery Device: A Review. Carbohydr. Polym. 2017, 168, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Baybaş, D.; Serdaroğlu, G.; Semerci, B. The Composite Microbeads of Alginate, Carrageenan, Gelatin, and Poly(Lactic-Co-Glycolic Acid): Synthesis, Characterization and Density Functional Theory Calculations. Int. J. Biol. Macromol. 2021, 181, 322–338. [Google Scholar] [CrossRef]
- Goh, C.H.; Heng, P.W.S.; Chan, L.W. Alginates as a Useful Natural Polymer for Microencapsulation and Therapeutic Applications. Carbohydr. Polym. 2012, 88, 1–12. [Google Scholar] [CrossRef]
- Draget, K.I.; Smidsrød, O.; Skjåk-Bræk, G. Alginates from Algae. In Polysaccharides and Polyamides in the Food Industry. Properties, Production and Patents; Steinbunchel, A., Rhee, S.K., Eds.; Wiley-VCH Verlar GmbH & Co.: Trenton, NJ, USA, 2005; pp. 1–30. [Google Scholar]
- Hu, C.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Ions-Induced Gelation of Alginate: Mechanisms and Applications. Int. J. Biol. Macromol. 2021, 177, 578–588. [Google Scholar] [CrossRef]
- Porse, H.; Rudolph, B. The Seaweed Hydrocolloid Industry: 2016 Updates, Requirements, and Outlook. J. Appl. Phycol. 2017, 29, 2187–2200. [Google Scholar] [CrossRef]
- Buschmann, A.H.; Camus, C.; Infante, J.; Neori, A.; Israel, Á.; Hernández-González, M.C.; Pereda, S.V.; Gomez-Pinchetti, J.L.; Golberg, A.; Tadmor-Shalev, N.; et al. Seaweed Production: Overview of the Global State of Exploitation, Farming and Emerging Research Activity. Eur. J. Phycol. 2017, 52, 391–406. [Google Scholar] [CrossRef]
- Qin, Y.; Jiang, J.; Zhao, L.; Zhang, J.; Wang, F. Applications of Alginate as a Functional Food Ingredient. In Biopolymers for Food Design; Grumezescu, A.M., Holban, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 409–429. [Google Scholar] [CrossRef]
- Conzatti, G.; Faucon, D.; Castel, M.; Ayadi, F.; Cavalie, S.; Tourrette, A. Alginate/Chitosan Polyelectrolyte Complexes: A Comparative Study of the Influence of the Drying Step on Physicochemical Properties. Carbohydr. Polym. 2017, 172, 142–151. [Google Scholar] [CrossRef] [Green Version]
- Jurić, S.; Jurić, M.; Režek Jambrak, A.; Vinceković, M. Tailoring Alginate/Chitosan Microparticles Loaded with Chemical and Biological Agents for Agricultural Application and Production of Value-Added Foods. Appl. Sci. 2021, 11, 4061. [Google Scholar] [CrossRef]
- Perera, K.Y.; Sharma, S.; Pradhan, D.; Jaiswal, A.K.; Jaiswal, S. Seaweed Polysaccharide in Food Contact Materials (Active Packaging, Intelligent Packaging, Edible Films, and Coatings). Foods 2021, 10, 2088. [Google Scholar] [CrossRef]
- Song, D.; Hoa, V.B.; Kim, H.W.; Khang, S.M.; Cho, S.; Ham, J.; Seol, K. Edible Films on Meat and Meat Products. Coatings 2021, 11, 1344. [Google Scholar] [CrossRef]
- Hassan, R.A.; Heng, L.Y.; Tan, L.L. Novel DNA Biosensor for Direct Determination of Carrageenan. Sci. Rep. 2019, 9, 6379. [Google Scholar] [CrossRef] [PubMed]
- Jancikova, S.; Dordevic, D.; Jamroz, E.; Behalova, H.; Tremlova, B. Chemical and Physical Characteristics of Edible Films, Based on κ- And ι-Carrageenans with the Addition of Lapacho Tea Extract. Foods 2020, 9, 357. [Google Scholar] [CrossRef] [Green Version]
- Simona, J.; Dani, D.; Petr, S.; Marcela, N.; Jakub, T.; Bohuslava, T. Edible Films from Carrageenan/Orange Essential Oil/Trehalose—Structure, Optical Properties, and Antimicrobial Activity. Polymers 2021, 13, 332. [Google Scholar] [CrossRef] [PubMed]
- Crini, G. Historical Review on Chitin and Chitosan Biopolymers. Environ. Chem. Lett. 2019, 17, 1623–1643. [Google Scholar] [CrossRef]
- Khayrova, A.; Lopatin, S.; Varlamov, V. Obtaining Chitin, Chitosan and Their Melanin Complexes from Insects. Int. J. Biol. Macromol. 2021, 167, 1319–1328. [Google Scholar] [CrossRef]
- Schmitz, C.; Auza, L.G.A.; Koberidze, D.; Rasche, S.; Fischer, R.; Bortesi, B. Conversion of Chitin to Defined Chitosan Oligomers: Current Status and Future Prospects. Mar. Drugs 2019, 17, 452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a Bioactive Polymer: Processing, Properties and Applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Younes, I.; Rinaudo, M. Chitin and Chitosan Preparation from Marine Sources. Structure, Properties and Applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [Green Version]
- González-Reza, R.M.; Hernández-Sánchez, H.; Quintanar-Guerrero, D.; Alamilla-Beltrán, L.; Cruz-Narváez, Y.; Zambrano-Zaragoza, M.L. Synthesis, Controlled Release, and Stability on Storage of Chitosan-Thyme Essential Oil Nanocapsules for Food Applications. Gels 2021, 7, 212. [Google Scholar] [CrossRef] [PubMed]
- Teixeira-Costa, B.E.; Silva Pereira, B.C.; Lopes, G.K.; Tristão Andrade, C. Encapsulation and Antioxidant Activity of Assai Pulp Oil (Euterpe Oleracea) in Chitosan/Alginate Polyelectrolyte Complexes. Food Hydrocoll. 2020, 109, 106097. [Google Scholar] [CrossRef]
- Li, Y.; Song, H.; Xiong, S.; Tian, T.; Liu, T.; Sun, Y. Chitosan-Stablized Bovine Serum Albumin Nanoparticles Having Ability to Control the Release of NELL-1 Protein. Int. J. Biol. Macromol. 2018, 109, 672–680. [Google Scholar] [CrossRef]
- Oberlintner, A.; Bajić, M.; Kalčíková, G.; Likozar, B.; Novak, U. Biodegradability Study of Active Chitosan Biopolymer Films Enriched with Quercus Polyphenol Extract in Different Soil Types. Environ. Technol. Innov. 2021, 21, 101318. [Google Scholar] [CrossRef]
- Teixeira-Costa, B.E.; Andrade, C.T. Chitosan as a Valuable Biomolecule from Seafood Industry Waste in the Design of Green Food Packaging. Biomolecules 2021, 28, 1599. [Google Scholar] [CrossRef]
- Hiremani, V.D.; Khanapure, S.; Gasti, T.; Goudar, N.; Vootla, S.K.; Masti, S.P.; Malabadi, R.B.; Mudigoudra, B.S.; Chougale, R.B. Preparation and Physicochemical Assessment of Bioactive Films Based on Chitosan and Starchy Powder of White Turmeric Rhizomes (Curcuma Zedoaria) for Green Packaging Applications. Int. J. Biol. Macromol. 2021, 11, 50. [Google Scholar] [CrossRef]
- Sady, S.; Błaszczyk, A.; Kozak, W.; Boryło, P.; Szindler, M. Quality Assessment of Innovative Chitosan-Based Biopolymers for Edible Food Packaging Applications. Food Packag. Shelf Life 2021, 30, 100756. [Google Scholar] [CrossRef]
- Pavinatto, A.; de Almeida Mattos, A.V.; Malpass, A.C.G.; Okura, M.H.; Balogh, D.T.; Sanfelice, R.C. Coating with Chitosan-Based Edible Films for Mechanical/Biological Protection of Strawberries. Int. J. Biol. Macromol. 2020, 151, 1004–1011. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Y.; Guo, M.; Jin, T.Z.; Arabi, S.A.; He, Q.; Ismail, B.B.; Hu, Y.; Liu, D. Antimicrobial and UV Blocking Properties of Composite Chitosan Films with Curcumin Grafted Cellulose Nanofiber. Food Hydrocoll. 2021, 112, 106337. [Google Scholar] [CrossRef]
- Qiao, C.; Ma, X.; Wang, X.; Liu, L. Structure and Properties of Chitosan Films: Effect of the Type of Solvent Acid. LWT-Food Sci. Technol. 2021, 135, 109984. [Google Scholar] [CrossRef]
- Jiang, T.; Duan, Q.; Zhu, J.; Liu, H.; Yu, L. Starch-Based Biodegradable Materials: Challenges and Opportunities. Adv. Ind. Eng. Polym. Res. 2020, 3, 8–18. [Google Scholar] [CrossRef]
- Jiménez, A.; Fabra, M.J.; Talens, P.; Chiralt, A. Edible and Biodegradable Starch Films: A Review. Food Bioprocess Technol. 2012, 5, 2058–2076. [Google Scholar] [CrossRef]
- Versino, F.; Lopez, O.V.; Garcia, M.A.; Zaritzky, N.E. Starch-Based Films and Food Coatings: An Overview. Starch-Stärke 2016, 68, 1026–1037. [Google Scholar] [CrossRef]
- Tagliapietra, B.L.; Felisberto, M.H.F.; Sanches, E.A.; Campelo, P.H.; Clerici, M.T.P.S. Non-Conventional Starch Sources. Curr. Opin. Food Sci. 2021, 39, 93–102. [Google Scholar] [CrossRef]
- Tosif, M.M.; Najda, A.; Bains, A.; Zawiślak, G.; Maj, G.; Chawla, P. Starch–Mucilage Composite Films: An Inclusive on Physicochemical and Biological Perspective. Polymers 2021, 13, 2588. [Google Scholar] [CrossRef]
- Vamadevan, V.; Bertoft, E. Structure-Function Relationships of Starch Components. Starch-Stärke 2014, 67, 55–68. [Google Scholar] [CrossRef]
- Cui, C.; Ji, N.; Wang, Y.; Xiong, L.; Sun, Q. Bioactive and Intelligent Starch-Based Films: A Review. Trends Food Sci. Technol. 2021, 116, 854–869. [Google Scholar] [CrossRef]
- Pedreiro, S.; Figueirinha, A.; Silva, A.S.; Ramos, F. Bioactive Edible Films and Coatings Based in Gums and Starch: Phenolic Enrichment and Foods Application. Coatings 2021, 11, 1393. [Google Scholar] [CrossRef]
- Shah, U.; Naqash, F.; Gani, A.; Masoodi, F.A. Art and Science behind Modified Starch Edible Films and Coatings: A Review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 568–580. [Google Scholar] [CrossRef]
- Thakur, R.; Pristijono, P.; Scarlett, C.J.; Bowyer, M.; Singh, S.P.; Vuong, Q.V. Starch-Based Films: Major Factors Affecting Their Properties. Int. J. Biol. Macromol. 2019, 132, 1079–1089. [Google Scholar] [CrossRef] [PubMed]
- Luchese, C.L.; Pavoni, J.M.F.; dos Santos, N.Z.; Quines, L.K.; Pollo, L.D.; Spada, J.C.; Tessaro, I.C. Effect of Chitosan Addition on the Properties of Films Prepared with Corn and Cassava Starches. J. Food Sci. Technol. 2018, 55, 2963–2973. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Lei, Y.; Lu, J.; Zhu, R.; Xiao, D.; Jiao, C.; Xia, R.; Zhang, Z.; Shen, G.; Liu, Y.; et al. Effect of Citric Acid Induced Crosslinking on the Structure and Properties of Potato Starch/Chitosan Composite Films. Food Hydrocoll. 2019, 97, 105208. [Google Scholar] [CrossRef]
- Nogueira, G.F.; Soares, C.T.; Cavasini, R.; Fakhouri, F.M.; de Oliveira, R.A. Bioactive Films of Arrowroot Starch and Blackberry Pulp: Physical, Mechanical and Barrier Properties and Stability to PH and Sterilization. Food Chem. 2019, 275, 417–425. [Google Scholar] [CrossRef]
- Saberi, B.; Vuong, Q.V.; Chockchaisawasdee, S.; Golding, J.B.; Scarlett, C.J.; Stathopoulos, C.E. Physical, Barrier, and Antioxidant Properties of Pea Starch-Guar Gum Biocomposite Edible Films by Incorporation of Natural Plant Extracts. Food Bioprocess Technol. 2017, 10, 2240–2250. [Google Scholar] [CrossRef] [Green Version]
- Farahnaky, A.; Saberi, B.; Majzoobi, M. Effect of Glycerol on Physical and Mechanical Properties of Wheat Starch Edible Films. J. Texture Stud. 2013, 44, 176–186. [Google Scholar] [CrossRef]
- Thirathumthavorn, D.; Thongunruan, W. Incorporation of Rice Starch Affecting on Morphology, Mechanical Properties and Water Vapor Permeability of Glutelin-Based Composite Films. J. Food Process. Preserv. 2014, 38, 1799–1806. [Google Scholar] [CrossRef]
- Adebowale, A.A.; Olatunde, O.O.; Adegunwa, M.O.; Asiru, W.B.; Sanni, L.O. Mechanical and Sensorial Characteristics of Cassava and Yam Composite Starch Films. J. Food Process. Preserv. 2014, 38, 1994–1998. [Google Scholar] [CrossRef]
- Fischer, E.; Cachon, R.; Cayot, N. Pisum Sativum vs Glycine Max, a Comparative Review of Nutritional, Physicochemical, and Sensory Properties for Food Uses. Trends Food Sci. Technol. 2020, 95, 196–204. [Google Scholar] [CrossRef]
- Tulbek, M.C.; Lam, R.S.H.; Wang, Y.C.; Asavajaru, P.; Lam, A. Pea: A Sustainable Vegetable Protein Crop. In Sustainable Protein Sources; Nadathur, S.R., Wanasundara, J.P.D., Scanlin, L., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2017; pp. 145–164. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations-FAO. FAOSTAT-Crops. Available online: http://www.fao.org/faostat/en/#data/QC/visualize (accessed on 25 May 2021).
- McCarthy, N.A.; Kennedy, D.; Hogan, S.A.; Kelly, P.M.; Thapa, K.; Murphy, K.M.; Fenelon, M.A. Emulsification Properties of Pea Protein Isolate Using Homogenization, Microfluidization and Ultrasonication. Food Res. Int. 2016, 89, 415–421. [Google Scholar] [CrossRef]
- Shevkani, K.; Singh, N.; Kaur, A.; Rana, J.C. Structural and Functional Characterization of Kidney Bean and Field Pea Protein Isolates: A Comparative Study. Food Hydrocoll. 2015, 43, 679–689. [Google Scholar] [CrossRef]
- Wei, Y.; Cai, Z.; Wu, M.; Guo, Y.; Tao, R.; Li, R.; Wang, P.; Ma, A.; Zhang, H. Comparative Studies on the Stabilization of Pea Protein Dispersions by Using Various Polysaccharides. Food Hydrocoll. 2020, 98, 105233. [Google Scholar] [CrossRef]
- Djemaoune, Y.; Cases, E.; Saurel, R. The Effect of High-Pressure Microfluidization Treatment on the Foaming Properties of Pea Albumin Aggregates. J. Food Sci. 2019, 84, 2242–2249. [Google Scholar] [CrossRef]
- Barac, M.; Cabrilo, S.; Pesic, M.; Stanojevic, S.; Zilic, S.; Macej, O.; Ristic, N. Profile and Functional Properties of Seed Proteins from Six Pea (Pisum Sativum) Genotypes. Int. J. Mol. Sci. 2010, 11, 4973–4990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmer, C.; Karaca, A.C.; Low, N.H.; Nickerson, M.T. Complex Coacervation in Pea Protein Isolate–Chitosan Mixtures. Food Res. Int. 2011, 44, 1441–1446. [Google Scholar] [CrossRef]
- Kumar, M.; Tomar, M.; Potkule, J.; Verma, R.; Punia, S.; Mahapatra, A.; Belwal, T.; Dahuja, A.; Joshi, S.; Berwal, M.K.; et al. Advances in the Plant Protein Extraction: Mechanism and Recommendations. Food Hydrocoll. 2021, 115, 106595. [Google Scholar] [CrossRef]
- Yang, J.; Zamani, S.; Liang, L.; Chen, L. Extraction Methods Significantly Impact Pea Protein Composition, Structure and Gelling Properties. Food Hydrocoll. 2021, 117, 106678. [Google Scholar] [CrossRef]
- Stone, A.K.; Karalash, A.; Tyler, R.T.; Warkentin, T.D.; Nickerson, M.T. Functional Attributes of Pea Protein Isolates Prepared Using Different Extraction Methods and Cultivars. Food Res. Int. 2015, 76, 31–38. [Google Scholar] [CrossRef]
- Karaca, A.C.; Low, N.; Nickerson, M. Emulsifying Properties of Chickpea, Faba Bean, Lentil and Pea Proteins Produced by Isoelectric Precipitation and Salt Extraction. Food Res. Int. 2011, 44, 2742–2750. [Google Scholar] [CrossRef]
- Dubey, N.K.; Dubey, R. Edible Films and Coatings: An Update on Recent Advances. In Biopolymer-Based Formulations: Biomedical and Food Applications; Pal, K., Banerjee, I., Sarkar, P., Kim, D., Deng, W.-P., Dubey, N.K., Majumder, K., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2020; pp. 675–695. [Google Scholar] [CrossRef]
- Salgado, P.R.; Ortiz, C.M.; Musso, Y.S.; Di Giorgio, L.; Mauri, A.N. Edible Films and Coatings Containing Bioactives. Curr. Opin. Food Sci. 2015, 5, 86–92. [Google Scholar] [CrossRef]
- Domínguez, R.; Barba, F.J.; Gómez, B.; Putnik, P.; Bursać Kovačević, D.; Pateiro, M.; Santos, E.M.; Lorenzo, J.M. Active Packaging Films with Natural Antioxidants to Be Used in Meat Industry: A Review. Food Res. Int. 2018, 113, 93–101. [Google Scholar] [CrossRef]
- Nogueira, G.F.; Oliveira, R.A.; Velasco, J.I.; Fakhouri, F.M. Methods of Incorporating Plant-Derived Bioactive Compounds into Films Made with Agro-Based Polymers for Application as Food Packaging: A Brief Review. Polymers 2020, 12, 2518. [Google Scholar] [CrossRef]
- Xiao, J.; Wang, W.; Huang, Q.; Li, Y. Edible Delivery Systems Based on Favorable Interactions for Encapsulation of Phytochemicals. Ref. Modul. Food Sci. 2018, 1, 1–6. [Google Scholar] [CrossRef]
- Genskowsky, E.; Puente, L.A.; Pérez-Álvarez, J.A.; Fernandez-Lopez, J.; Muñoz, L.A.; Viuda-Martos, M. Assessment of Antibacterial and Antioxidant Properties of Chitosan Edible Films Incorporated with Maqui Berry (Aristotelia Chilensis). LWT-Food Sci. Technol. 2015, 64, 1057–1062. [Google Scholar] [CrossRef]
- Shahbazi, Y. The Properties of Chitosan and Gelatin Films Incorporated with Ethanolic Red Grape Seed Extract and Ziziphora Clinopodioides Essential Oil as Biodegradable Materials for Active Food Packaging. Int. J. Biol. Macromol. 2017, 99, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Siripatrawan, U.; Vitchayakitti, W. Improving Functional Properties of Chitosan Films as Active Food Packaging by Incorporating with Propolis. Food Hydrocoll. 2016, 61, 695–702. [Google Scholar] [CrossRef]
- Riaz, A.; Lei, S.; Akhtar, H.M.S.; Wan, P.; Chen, D.; Jabbar, S.; Abid, M.; Hashim, M.M.; Zeng, X. Preparation and Characterization of Chitosan-Based Antimicrobial Active Food Packaging Film Incorporated with Apple Peel Polyphenols. Int. J. Biol. Macromol. 2018, 114, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Hematizad, I.; Khanjari, A.; Basti, A.A.; Karabagias, I.K.; Noori, N.; Ghadami, F.; Gholami, F.; Teimourifard, R. In Vitro Antibacterial Activity of Gelatin-Nanochitosan Films Incorporated with Zataria Multiflora Boiss Essential Oil and Its Influence on Microbial, Chemical, and Sensorial Properties of Chicken Breast Meat during Refrigerated Storage. Food Packag. Shelf Life 2021, 30, 100751. [Google Scholar] [CrossRef]
- Moradi, M.; Tajik, H.; Razavi Rohani, S.M.; Oromiehie, A.R.; Malekinejad, H.; Aliakbarlu, J.; Hadian, M. Characterization of Antioxidant Chitosan Film Incorporated with Zataria Multiflora Boiss Essential Oil and Grape Seed Extract. LWT-Food Sci. Technol. 2012, 46, 477–484. [Google Scholar] [CrossRef]
- Peng, Y.; Wu, Y.; Li, Y. Development of Tea Extracts and Chitosan Composite Films for Active Packaging Materials. Int. J. Biol. Macromol. 2013, 59, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Lagos, J.B.; Vargas, F.C.; de Oliveira, T.G.; da Aparecida Makishi, G.L.; do Amaral Sobral, P.J. Recent Patents on the Application of Bioactive Compounds in Food: A Short Review. Curr. Opin. Food Sci. 2015, 5, 1–7. [Google Scholar] [CrossRef]
- Devi, N.; Sarmah, M.; Khatun, B.; Maji, T.K. Encapsulation of Active Ingredients in Polysaccharide–Protein Complex Coacervates. Adv. Colloid Interface Sci. 2017, 239, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Eghbal, N.; Choudhary, R. Complex Coacervation: Encapsulation and Controlled Release of Active Agents in Food Systems. LWT-Food Sci. Technol. 2018, 90, 254–264. [Google Scholar] [CrossRef]
- Ma, T.; Zhao, H.; Wang, J.; Sun, B. Effect of Processing Conditions on the Morphology and Oxidative Stability of Lipid Microcapsules during Complex Coacervation. Food Hydrocoll. 2019, 87, 637–643. [Google Scholar] [CrossRef]
- Fakhouri, F.M.; Martelli, S.M.; Caon, T.; Velasco, J.I.; Buontempo, R.C.; Bilck, A.P.; Mei, L.H.I. The Effect of Fatty Acids on the Physicochemical Properties of Edible Films Composed of Gelatin and Gluten Proteins. LWT-Food Sci. Technol. 2018, 87, 293–300. [Google Scholar] [CrossRef]
- Rutz, J.K.; Borges, C.D.; Zambiazi, R.C.; Crizel-Cardozo, M.M.; Kuck, L.S.; Noreña, C.P.Z. Microencapsulation of Palm Oil by Complex Coacervation for Application in Food Systems. Food Chem. 2017, 220, 59–66. [Google Scholar] [CrossRef]
- Rutz, J.K.; Borges, C.D.; Zambiazi, R.C.; Da Rosa, C.G.; Da Silva, M.M. Elaboration of Microparticles of Carotenoids from Natural and Synthetic Sources for Applications in Food. Food Chem. 2016, 202, 324–333. [Google Scholar] [CrossRef]
- Pereda, M.; Amica, G.; Marcovich, N.E. Development and Characterization of Edible Chitosan/Olive Oil Emulsion Films. Carbohydr. Polym. 2012, 87, 1318–1325. [Google Scholar] [CrossRef]
- Comunian, T.A.; Favaro-Trindade, C.S. Microencapsulation Using Biopolymers as an Alternative to Produce Food Enhanced with Phytosterols and Omega-3 Fatty Acids: A Review. Food Hydrocoll. 2016, 61, 442–457. [Google Scholar] [CrossRef]
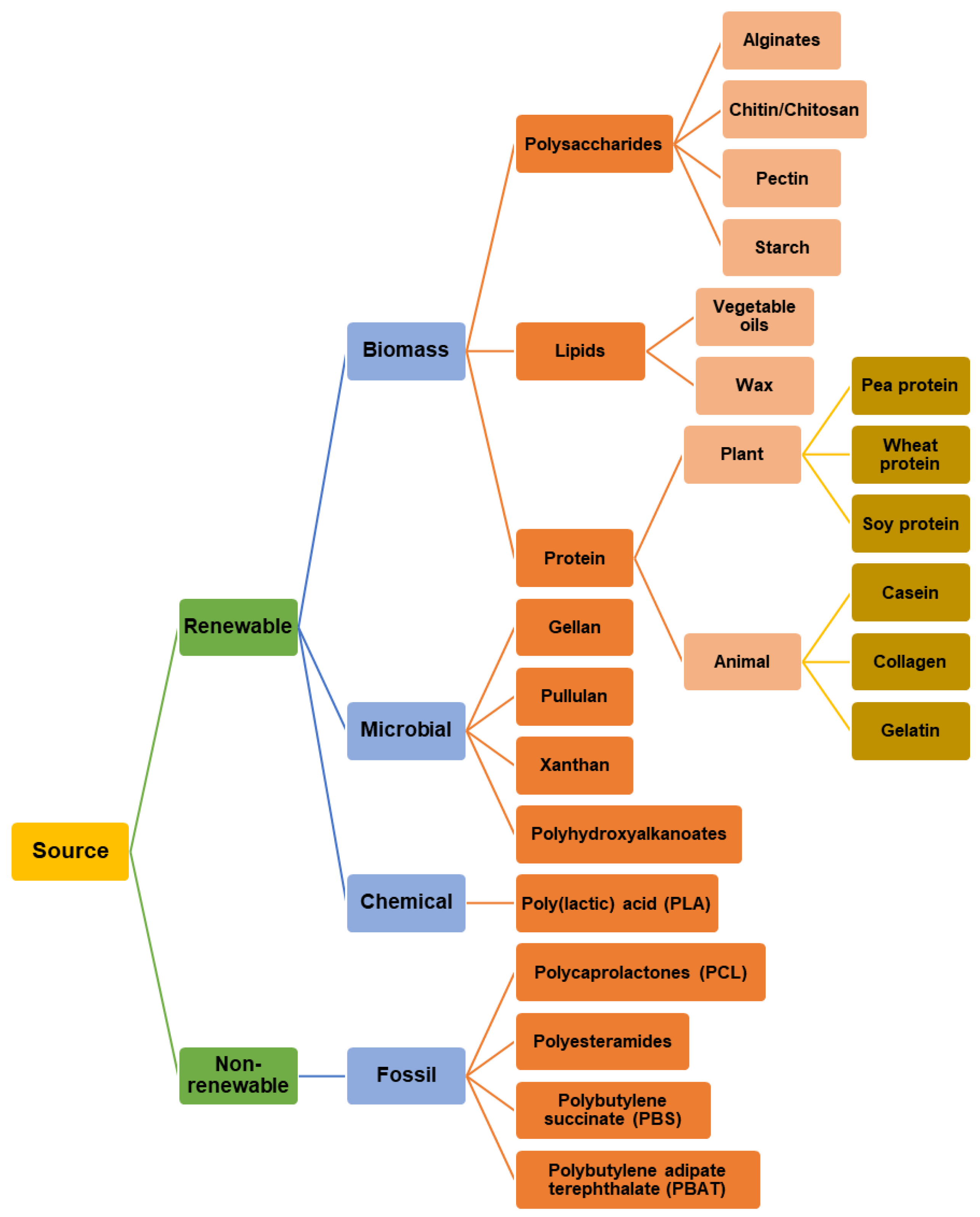








| Biomacromolecules | Raw Materials | References |
|---|---|---|
| Polysaccharides | Agar | [43] |
| Alginate | [44] | |
| Cellulose | [45] | |
| Chitosan | [46] | |
| Gums | [47] | |
| K-carrageenan | [48,49] | |
| Pectin | [50] | |
| Pullulan | [51] | |
| Starch | [52,53] | |
| Proteins | Casein | [54] |
| Collagen | [55] | |
| Corn zein | [56] | |
| Gelatin | [45] | |
| Pea protein | [57] | |
| Sodium caseinate | [50] | |
| Soy protein | [58] | |
| Wheat gluten | [59] | |
| Whey protein | [60] | |
| Lipids | Candelilla wax | [61] |
| Bee wax | [62] | |
| Vegetable oils | [63] | |
| Composites | Blends | [5,51,54,55,60] |
| Residues and by-products from agriculture | [64,65,66] | |
| Fruit pulps | [67] |
| Matrix | Concentration | Additives | Food Product | Reference |
|---|---|---|---|---|
| Carboxymethyl chitosan/pullulan | Different mixing ratio of 5:0, 4:1, 3:2, 2.5:2.5, 2:3 and 1:4 (w/w) | 8% galangal essential oil and 20% glycerol (w/w) | Mangoes | [51] |
| Chitosan | 1% (w/v) | 40 mmol L−1 of ascorbic acid | Plums (Prunus salicina) | [69] |
| Κ-carrageenan | 0.2–0.8% (w/v) | 0–1% glycerol (w/v) | Papaya (Carica papaya) | [48] |
| Sodium alginate | 0, 1 and 3% (w/v) | 20% glycerol (v/v) | Plum (Prunus salicina) | [70] |
| Sodium alginate | 1.5% (w/v) | Ficus hirta extract, 0.7% citric acid and 1.0% sucrose ester (w/v) | Nanfeng mandarin (Citrus reticulata) | [71] |
| Starch/chitosan | 2%/0.5–1.0% (w/v) | 2% glycerol (w/v) | Apples Fuji cultivar | [53] |
| Starch/gelatin | 3–5%/10% (1:1) | 10% sorbitol | Red Crimson grapes | [72] |
| Xantham gum | 2.5 g L−1 | 1.0 g L−1 cinnamic acid | Pears (Pyrus pyrifolia and P. communis) | [47] |
| Matrix | Biopolymer Component | Additives | Reference |
|---|---|---|---|
| Potato, or corn, or wheat starches | - | 1-ethyl-3-methylimidazolium acetate (1.5 wt%) | [77] |
| White turmeric (Curcuma zedoaria) starch | Chitosan | Glycerol | [105] |
| Purple yam starch | Chitosan | Glycerol | [53] |
| Corn or cassava starches | Chitosan | Lactic acid (1%, v/v), Glycerol (0.9 g 100 mL−1) | [120] |
| Potato starch | Chitosan | Citric acid (5–20%) | [121] |
| Arrowroot starch | - | Blackberry pulp (0–40%) | [122] |
| Babassu starch | - | Glycerol, sorbitol, glucose, or urea | [78] |
| Pea starch | Guar gum | Glycerol (25%) | [123] |
| Wheat starch | - | Glycerol (0–50%) | [124] |
| Rice starch | Glutelin | Sorbitol (40%) | [125] |
| Cassava starch | Yam starch | Glycerol (20%) | [126] |
| Hydrocolloids | Lipids | Form | Application | Reference |
|---|---|---|---|---|
| Chitosan and sodium alginate | Açaí pulp oil | Microcapsules by complex formation | Potential application to biobased packaging | [101] |
| Gelatin and gum Arabic | Methyl oleate | Microcapsules by complex coacervation | Functional lipids and lipophilic food ingredients | [155] |
| Gluten and gelatin plasticized with glycerol or sorbitol | Fatty acids | Incorporated in edible films | Biobased packaging | [156] |
| Mesquite seed gum | Palm fruit oil | Emulsion edible film | Biobased packaging | [63] |
| Chitosan/xanthan or chitosan/pectin | Palm oil | Microcapsules by complex formation | Yogurt preparation | [157] |
| Chitosan/sodium tripolyphosphate or chitosan/carboxymethyl cellulose | Palm oil and β-carotene | Microcapsules by complex formation | Food systems under gastrointestinal simulant conditions | [158] |
| Chitosan plasticized with glycerol | Olive oil | Emulsion film | Biobased packaging | [159] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixeira-Costa, B.E.; Andrade, C.T. Natural Polymers Used in Edible Food Packaging—History, Function and Application Trends as a Sustainable Alternative to Synthetic Plastic. Polysaccharides 2022, 3, 32-58. https://doi.org/10.3390/polysaccharides3010002
Teixeira-Costa BE, Andrade CT. Natural Polymers Used in Edible Food Packaging—History, Function and Application Trends as a Sustainable Alternative to Synthetic Plastic. Polysaccharides. 2022; 3(1):32-58. https://doi.org/10.3390/polysaccharides3010002
Chicago/Turabian StyleTeixeira-Costa, Barbara E., and Cristina T. Andrade. 2022. "Natural Polymers Used in Edible Food Packaging—History, Function and Application Trends as a Sustainable Alternative to Synthetic Plastic" Polysaccharides 3, no. 1: 32-58. https://doi.org/10.3390/polysaccharides3010002
APA StyleTeixeira-Costa, B. E., & Andrade, C. T. (2022). Natural Polymers Used in Edible Food Packaging—History, Function and Application Trends as a Sustainable Alternative to Synthetic Plastic. Polysaccharides, 3(1), 32-58. https://doi.org/10.3390/polysaccharides3010002







