Polysaccharide Stalks in Didymosphenia geminata Diatom: Real World Applications and Strategies to Combat Its Spread
Abstract
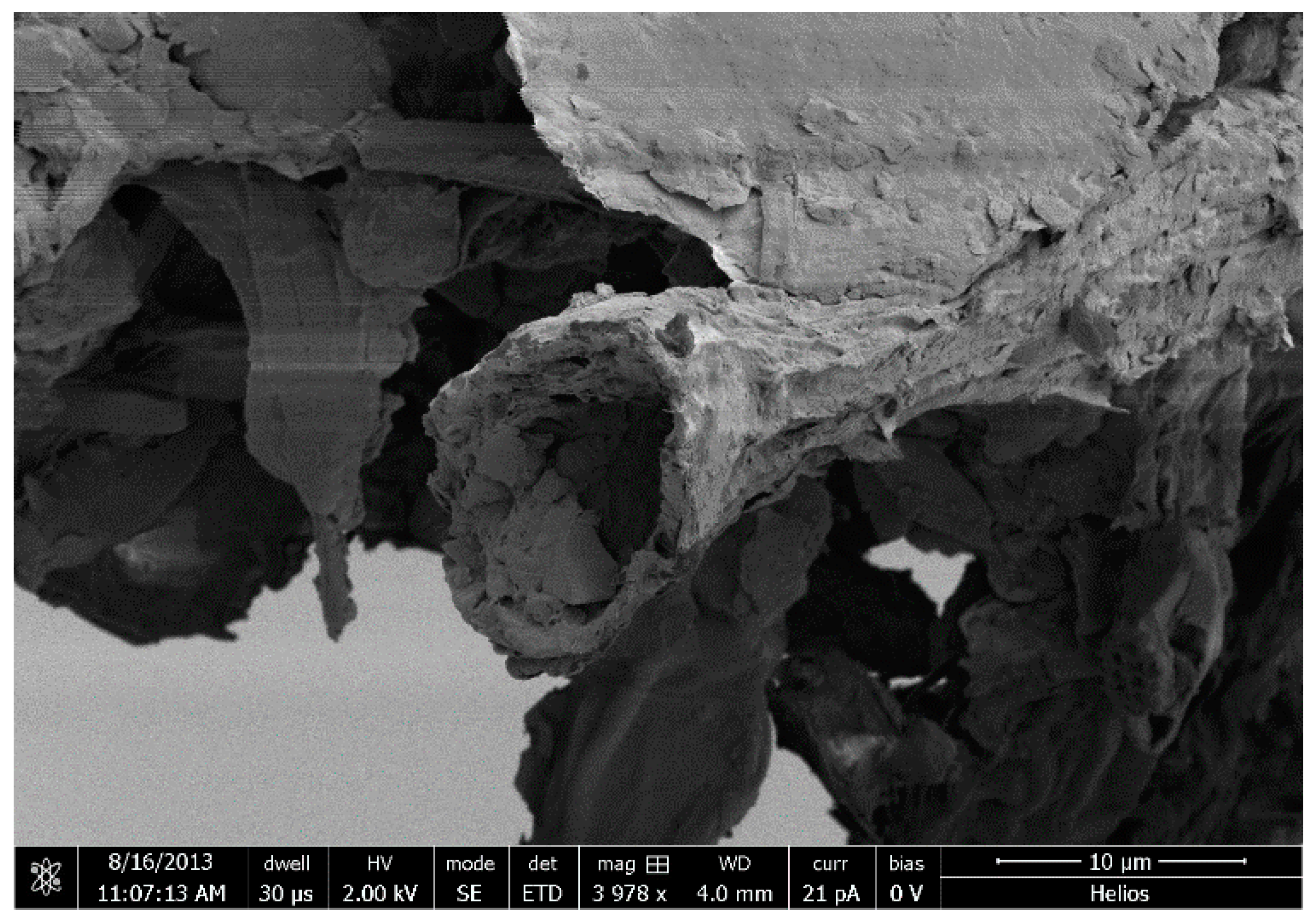
:1. Introduction
2. Applications of D. geminata as a Biomaterial: Cell Adhesion, Proliferation, and Drug Delivery
3. D. geminata Applications in Wastewater Treatment
4. Environmental Regulations and Policies Pertaining to D. geminata
5. D. geminata’s Economic Impacts
6. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Whitton, B.A.; Ellwood, N.T.W.; Kawecka, B. Biology of the Freshwater Diatom Didymosphenia: A Review. Hydrobiologia 2009, 630, 1–37. [Google Scholar] [CrossRef] [Green Version]
- Hussein, H.A.; Abdullah, M.A. Anticancer Compounds Derived from Marine Diatoms. Mar. Drugs 2020, 18, 356. [Google Scholar] [CrossRef]
- Sundareshwar, P.V.; Upadhayay, S.; Abessa, M.; Honomichl, S.; Berdanier, B.; Spaulding, S.A.; Sandvik, C.; Trennepohl, A. Didymosphenia geminata: Algal Blooms in Oligotrophic Streams and Rivers. Geophys. Res. Lett. 2011, 38. [Google Scholar] [CrossRef] [Green Version]
- Wysokowski, M.; Bartczak, P.; Żółtowska-Aksamitowska, S.; Chudzińska, A.; Piasecki, A.; Langer, E.; Bazhenov, V.V.; Petrenko, I.; Noga, T.; Stelling, A.L.; et al. Adhesive Stalks of Diatom Didymosphenia geminata as a Novel Biological Adsorbent for Hazardous Metals Removal. CLEAN-Soil Air Water 2017, 45, 1600678. [Google Scholar] [CrossRef]
- Ladrera, R.; Gomà, J.; Prat, N. Effects of Didymosphenia geminata Massive Growth on Stream Communities: Smaller Organisms and Simplified Food Web Structure. PLoS ONE 2018, 13, e0193545. [Google Scholar] [CrossRef]
- Ejaz, H.; Somanader, E.; Dave, U.; Ehrlich, H.; Rahman, M.A. Didymo and Its Polysaccharide Stalks: Beneficial to the Environment or Not? Polysaccharides 2021, 2, 69–79. [Google Scholar] [CrossRef]
- Elwell, L.C.; Gillis, C.-A.; Kunza, L.A.; Modley, M.D. Management Challenges of Didymosphenia geminata. Diatom Res. 2014, 29, 303–305. [Google Scholar] [CrossRef]
- Taylor, B.W.; Bothwell, M.L. The Origin of Invasive Microorganisms Matters for Science, Policy, and Management: The Case of Didymosphenia geminata. Bioscience 2014, 64, 531–538. [Google Scholar] [CrossRef] [Green Version]
- Wustmann, M.; Poulsen, N.; Kröger, N.; van Pée, K.-H. Chitin Synthase Localization in the Diatom Thalassiosira Pseudonana. BMC Mater. 2020, 2, 10. [Google Scholar] [CrossRef]
- Brunner, E.; Richthammer, P.; Ehrlich, H.; Paasch, S.; Simon, P.; Ueberlein, S.; van Pée, K.-H. Chitin-Based Organic Networks: An Integral Part of Cell Wall Biosilica in the Diatom Thalassiosira Pseudonana. Angew. Chem. Int. Ed. 2009, 48, 9724–9727. [Google Scholar] [CrossRef]
- Zgłobicka, I.; Li, Q.; Gluch, J.; Płocińska, M.; Noga, T.; Dobosz, R.; Szoszkiewicz, R.; Witkowski, A.; Zschech, E.; Kurzydłowski, K.J. Visualization of the Internal Structure of Didymosphenia geminata Frustules Using Nano X-ray Tomography. Sci. Rep. 2017, 7, 9086. [Google Scholar] [CrossRef] [Green Version]
- Brand, C.; Grech, M. Recent Invasion of Didymosphenia geminata (Lyngbye) M. Schmidt in a Patagonian Regulated River Promotes Changes in Composition and Density of Macroinvertebrate Community. Biol. Invasions 2020, 22, 1903–1915. [Google Scholar] [CrossRef]
- Jellyman, P.G.; Harding, J.S. Disentangling the Stream Community Impacts of Didymosphenia geminata: How Are Higher Trophic Levels Affected? Biol. Invasions 2016, 18, 3419–3435. [Google Scholar] [CrossRef]
- Jones, L.R.; Manrique, J.M.; Uyua, N.M.; Whitton, B.A. Genetic Analysis of the Invasive Alga Didymosphenia geminata in Southern Argentina: Evidence of a Pleistocene Origin of Local Lineages. Sci. Rep. 2019, 9, 18706. [Google Scholar] [CrossRef] [PubMed]
- Bothwell, M.L.; Taylor, B.W.; Kilroy, C. The Didymo Story: The Role of Low Dissolved Phosphorus in the Formation of Didymosphenia geminata Blooms. Diatom Res. 2014, 29, 229–236. [Google Scholar] [CrossRef]
- Kilroy, C.; Bothwell, M. Environmental Control of Stalk Length in the Bloom-Forming, Freshwater Benthic Diatom Didymosphenia geminata (Bacillariophyceae). J. Phycol. 2011, 47, 981–989. [Google Scholar] [CrossRef]
- Reinoso-Guerra, E.; Aristizabal, J.; Arce, B.; Zurob, E.; Dennett, G.; Fuentes, R.; Suescún, A.V.; Cárdenas, L.; da Cunha, T.H.R.; Cabezas, R.; et al. Nanostructured Didymosphenia geminata-Based Membrane for Efficient Lead Adsorption from Aqueous Solution. J. Environ. Chem. Eng. 2021, 9, 105269. [Google Scholar] [CrossRef]
- Wahab, I.F.; Razak, S.I. Polysaccharides as Composite Biomaterials. In Composites from Renewable and Sustainable Materials; InteahOpen: London, UK, 2016; pp. 65–84. [Google Scholar] [CrossRef] [Green Version]
- Figueroa, F.A.; Abdala-Díaz, R.; Hernández, V.; Pedreros, P.; Aranda, M.; Cabrera-Pardo, J.R.; Pérez, C.; Becerra, J.; Urrutia, R. Invasive Diatom Didymosphenia geminata as a Source of Polysaccharides with Antioxidant and Immunomodulatory Effects on Macrophage Cell Lines. J. Appl. Phycol. 2020, 32, 93–102. [Google Scholar] [CrossRef]
- Williams, D.F. On the Nature of Biomaterials. Biomaterials 2009, 30, 5897–5909. [Google Scholar] [CrossRef] [PubMed]
- Bothwell, M.L.; Spaulding, S.A. Synopsis of the 2007 International Workshop on Didymosphenia geminate. In Proceedings of the 2007 International Workshop on Didymosphenia geminate; Fisheries and Oceans Canada: Ottawa, ON, Canada, 2008; 96p. [Google Scholar]
- Zgłobicka, I. Aspects of Structural Biology of Didymosphenia geminata (Lyngb.) M. Schmidt (Bacillariophyta). IJA 2013, 15, 291–310. [Google Scholar] [CrossRef]
- Zglobicka, I. Frustules of Didymosphenia geminata as a Modifier of Resins. Mater. Eng. 2018, 1, 10–16. [Google Scholar] [CrossRef]
- Arora, M.; Arora, E. The Promise of Silicon: Bone Regeneration and Increased Bone Density. J. Arthrosc. Jt. Surg. 2017, 4, 103–105. [Google Scholar] [CrossRef]
- Zgłobicka, I. Exploratory Study of the Use of Didymosphenia geminata Stalks as a Functional Biomaterial. Ph.D. Thesis, Division of Materials Design, The Warsaw University of Technology, Warszawa, Poland, 2015. [Google Scholar]
- Wang, N.; Cheng, X.; Li, N.; Wang, H.; Chen, H. Nanocarriers and their loading strategies. Adv. Healthc. Mater. 2019, 8, 1801002. [Google Scholar] [CrossRef] [PubMed]
- Przekora, A. The Summary of the Most Important Cell-Biomaterial Interactions That Need to Be Considered during in Vitro Biocompatibility Testing of Bone Scaffolds for Tissue Engineering Applications. Mater. Sci. Eng. C 2019, 97, 1036–1051. [Google Scholar] [CrossRef]
- Andryukov, B.G.; Besednova, N.N.; Kuznetsova, T.A.; Zaporozhets, T.S.; Ermakova, S.P.; Zvyagintseva, T.N.; Chingizova, E.A.; Gazha, A.K.; Smolina, T.P. Sulfated Polysaccharides from Marine Algae as a Basis of Modern Biotechnologies for Creating Wound Dressings: Current Achievements and Future Prospects. Biomedicines 2020, 8, 301. [Google Scholar] [CrossRef]
- Kuznetsova, T.A.; Andryukov, B.G.; Besednova, N.N.; Zaporozhets, T.S.; Kalinin, A.V. Marine Algae Polysaccharides as Basis for Wound Dressings, Drug Delivery, and Tissue Engineering: A Review. J. Mar. Sci. Eng. 2020, 8, 481. [Google Scholar] [CrossRef]
- Medarević, Đ.; Losić, D.; Ibrić, S. Diatoms—Nature materials with great potential for bioapplications. Hem. Ind. 2016, 70, 613–627. [Google Scholar] [CrossRef] [Green Version]
- Marreco, P.R.; Moreira, P.D.L.; Genari, S.C.; Moraes, Â.M. Effects of different sterilization methods on the morphology, mechanical properties, and cytotoxicity of chitosan membranes used as wound dressings. J. Biomed. Mater. Res. 2004, 71, 268–277. [Google Scholar] [CrossRef]
- Ebi, K.L.; Hess, J.J. Health Risks Due to Climate Change: Inequity in Causes and Consequences. Health Aff. 2020, 39, 2056–2062. [Google Scholar] [CrossRef]
- Ehrlich, H.; Motylenko, M.; Sundareshwar, P.V.; Ereskovsky, A.; Zgłobicka, I.; Noga, T.; Płociński, T.; Tsurkan, M.V.; Wyroba, E.; Suski, S.; et al. Multiphase Biomineralization: Enigmatic Invasive Siliceous Diatoms Produce Crystalline Calcite. Adv. Funct. Mater. 2016, 26, 2503–2510. [Google Scholar] [CrossRef]
- Na, Y.; Lee, J.; Lee, S.H.; Kumar, P.; Kim, J.H.; Patel, R. Removal of Heavy Metals by Polysaccharide: A Review. Polym.-Plast. Technol. Mater. 2020, 59, 1770–1790. [Google Scholar] [CrossRef]
- Zeraatkar, A.K.; Ahmadzadeh, H.; Talebi, A.F.; Moheimani, N.R.; McHenry, M.P. Potential Use of Algae for Heavy Metal Bioremediation, a Critical Review. J. Environ. Manag. 2016, 181, 817–831. [Google Scholar] [CrossRef]
- Ubando, A.T.; Africa, A.D.M.; Maniquiz-Redillas, M.C.; Culaba, A.B.; Chen, W.-H.; Chang, J.-S. Microalgal Biosorption of Heavy Metals: A Comprehensive Bibliometric Review. J. Hazard. Mater. 2021, 402, 123431. [Google Scholar] [CrossRef] [PubMed]
- Arbabi, M.; Hemati, S.; Amiri, M. Removal of Lead Ions from Industrial Wastewater: A Review of Removal Methods. Int. J. Epidemiol. Res. 2015, 2, 105–109. [Google Scholar]
- Molazadeh, P.; Khanjani, N.; Rahimi, M.; Nasiri, A. Adsorption of lead by microalgae Chaetoceros sp. and Chlorella sp. from aqueous solution. J. Community Health Res. 2015, 4, 114–127. [Google Scholar]
- Das, D.; Chakraborty, S.; Bhattacharjee, C.; Chowdhury, R. Biosorption of Lead Ions (Pb2+) from Simulated Wastewater Using Residual Biomass of Microalgae. Desalination Water Treat. 2016, 57, 4576–4586. [Google Scholar] [CrossRef]
- Suganya, S.; Saravanan, A.; Senthil Kumar, P.; Yashwanthraj, M.; Sundar Rajan, P.; Kayalvizhi, K. Sequestration of Pb (II) and Ni (II) Ions from Aqueous Solution Using Microalga Rhizoclonium hookeri: Adsorption Thermodynamics, Kinetics, and Equilibrium Studies. J. Water Reuse Desalination 2016, 7, 214–227. [Google Scholar] [CrossRef] [Green Version]
- Gupta, V.K.; Rastogi, A. Biosorption of Lead from Aqueous Solutions by Green Algae Spirogyra Species: Kinetics and Equilibrium Studies. J. Hazard. Mater. 2008, 152, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Su, Y.; Su, H.; Wang, X.; Zhu, X. Sorption and Desorption of Lead (II) from Wastewater by Green Algae Cladophora fascicularis. J. Hazard. Mater. 2007, 143, 220–225. [Google Scholar] [CrossRef]
- Romera, E.; González, F.; Ballester, A.; Blázquez, M.L.; Muñoz, J.A. Comparative Study of Biosorption of Heavy Metals Using Different Types of Algae. Bioresour. Technol. 2007, 98, 3344–3353. [Google Scholar] [CrossRef]
- Rincón, J.; González, F.; Ballester, A.; Blázquez, M.; Muñoz, J. Biosorption of Heavy Metals by Chemically Activated Alga Fucus vesiculosus. J. Chem. Technol. Biotechnol. 2005, 80, 1403–1407. [Google Scholar] [CrossRef]
- Pavithra, K.G.; Kumar, P.S.; Jaikumar, V.; Vardhan, K.H.; SundarRajan, P. Microalgae for Biofuel Production and Removal of Heavy Metals: A Review. Environ. Chem. Lett. 2020, 18, 1905–1923. [Google Scholar] [CrossRef]
- Zgłobicka, I.; Chlanda, A.; Woźniak, M.; Łojkowski, M.; Szoszkiewicz, R.; Mazurkiewicz-Pawlicka, M.; Święszkowski, W.; Wyroba, E.; Kurzydłowski, K.J. Microstructure and Nanomechanical Properties of Single Stalks from Diatom Didymosphenia geminata and Their Change Due to Adsorption of Selected Metal Ions. J. Phycol. 2017, 53, 880–888. [Google Scholar] [CrossRef] [PubMed]
- Davis, M.A.; Chew, M.K.; Hobbs, R.J.; Lugo, A.E.; Ewel, J.J.; Vermeij, G.J.; Brown, J.H.; Rosenzweig, M.L.; Gardener, M.R.; Carroll, S.P.; et al. Do not Judge Species on Their Origins. Nature 2011, 474, 153–154. [Google Scholar] [CrossRef] [PubMed]
- Ministère du Développement Durable, de L’environnement et des Parcs; Ministère des Ressources Naturelles et de la Faune. What Is Didymo and How Can We Prevent It from Spreading in Our Rivers? Ministère du Développement Durable, de l’Environnement et des Parcs: Rouyn-Noranda, QC, Canada, 2008; 13p.
- Ministry of Primary Industries. Check, Clean, Dry: Preventing Didymo and Other Pests. Available online: https://www.mpi.govt.nz/outdoor-activities/boating-and-watersports-tips-to-prevent-spread-of-pests/check-clean-dry/ (accessed on 27 October 2021).
- Kilroy, C.; Bothwell, M.L. Didymosphenia geminata Growth Rates and Bloom Formation in Relation to Ambient Dissolved Phosphorus Concentration. Freshw. Biol. 2012, 57, 641–653. [Google Scholar] [CrossRef]
- Credit Valley Conservation. CVC Priority Aquatic Invasive Species & Fish Diseases. 2009. Available online: https://cvc.ca/wp-content/uploads/2011/02/CVC_AquaticInvasives_FishDiseases1.pdf (accessed on 15 August 2021).
- Government of New Brunswick Canada. Didymosphenia geminate. Available online: https://www2.gnb.ca/content/gnb/en/departments/erd/natural_resources/content/fish/content/Didymo.html (accessed on 24 October 2021).
- Government of Saskatchewan. Didymo Rock Snot Fact Sheet. Available online: http://www.environment.gov.sk.ca/Default.aspx?DN=e1c161fd-0a5c-4804-8296-0709df01300d (accessed on 27 October 2021).
- Rosaen, A.L.; Grover, E.A.; Spencer, C.W. The Costs of Aquatic Invasive Species to Great Lakes States; Anderson Economic Group: East Lansing, MI, USA, 2016; 51p. [Google Scholar]
- Beville, S.T.; Kerr, G.N.; Hughey, K.F.D. Valuing Impacts of the Invasive Alga Didymosphenia geminata on Recreational Angling. Ecol. Econ. 2012, 82, 1–10. [Google Scholar] [CrossRef]
- Bergey, E.A.; Cooper, J.T.; Phillips, B.C. Substrate Characteristics Affect Colonization by the Bloom-Forming Diatom Didymosphenia geminata. Aquat. Ecol. 2010, 44, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Government of Canada, Fisheries and Oceans Canada. A Canadian Action Plan to Address the Threat of Aquatic Invasive Species. Available online: https://www.dfo-mpo.gc.ca/species-especes/publications/ais-eae/plan/page01-eng.html (accessed on 24 October 2021).



| Applications of D. geminata as a Biomaterial | References |
|---|---|
| Cell Adhesion | [18,22,24,29] |
| Cell Proliferation | [18,24,29] |
| Drug Delivery | [2,6,18,19,23,25,29,30] |
| Wound Dressing | [18,28,29] |
| Algae Species | Pb(II) Sorption Capacity (mg g−1) | Original Reference | |
|---|---|---|---|
| Microalgae | Didymosphenia geminata | 129–175.48 | [4,17] |
| Chaetoceros sp. | 8 | [38] | |
| Chlorella sp. | 10.4 | [38] | |
| Phormidium sp. | 2.3 | [39] | |
| Rhizoclonium hookeri | 81.7 | [40] | |
| Macroalgae | Spirogyra sp. | 140 | [41] |
| Cladophora fascicularis | 198.5 | [42] | |
| Ascophyllum nodosum | 178.6 | [43] | |
| Fucus vesiculosus | 215.5–259 | [44] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Somanader, E.; Sreenivas, R.; Siavash, G.; Rodriguez, N.; Gao, T.; Ehrlich, H.; Rahman, M.A. Polysaccharide Stalks in Didymosphenia geminata Diatom: Real World Applications and Strategies to Combat Its Spread. Polysaccharides 2022, 3, 83-94. https://doi.org/10.3390/polysaccharides3010004
Somanader E, Sreenivas R, Siavash G, Rodriguez N, Gao T, Ehrlich H, Rahman MA. Polysaccharide Stalks in Didymosphenia geminata Diatom: Real World Applications and Strategies to Combat Its Spread. Polysaccharides. 2022; 3(1):83-94. https://doi.org/10.3390/polysaccharides3010004
Chicago/Turabian StyleSomanader, Esther, Roshini Sreenivas, Golnoosh Siavash, Nicole Rodriguez, Tingxiao Gao, Hermann Ehrlich, and M. Azizur Rahman. 2022. "Polysaccharide Stalks in Didymosphenia geminata Diatom: Real World Applications and Strategies to Combat Its Spread" Polysaccharides 3, no. 1: 83-94. https://doi.org/10.3390/polysaccharides3010004
APA StyleSomanader, E., Sreenivas, R., Siavash, G., Rodriguez, N., Gao, T., Ehrlich, H., & Rahman, M. A. (2022). Polysaccharide Stalks in Didymosphenia geminata Diatom: Real World Applications and Strategies to Combat Its Spread. Polysaccharides, 3(1), 83-94. https://doi.org/10.3390/polysaccharides3010004









