Algal Polysaccharides-Based Nanomaterials: General Aspects and Potential Applications in Food and Biomedical Fields
Abstract
:1. Introduction
2. Algae as Sources of Polysaccharides
3. Algal Polysaccharides in Food and Biomedical Context
4. Algal Polysaccharide-Based Nanomaterials
5. Nano-Formulations Added Algal Polysaccharides
5.1. Application in Food Science
5.2. Applications in Biomedical Science
6. Industrial Potential of Algal Polysaccharides
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mohan, A.A.; Antony, A.R.; Greeshma, K.; Yun, J.-H.; Ramanan, R.; Kim, H.-S. Algal Biopolymers as Sustainable Resources for a Net-Zero Carbon Bioeconomy. Bioresour. Technol. 2022, 344, 126397. [Google Scholar] [CrossRef]
- Ścieszka, S.; Klewicka, E. Algae in Food: A General Review. Crit. Rev. Food Sci. Nutr. 2019, 59, 3538–3547. [Google Scholar] [CrossRef] [PubMed]
- Seidi, F.; Yazdi, M.K.; Jouyandeh, M.; Habibzadeh, S.; Munir, M.T.; Vahabi, H.; Bagheri, B.; Rabiee, N.; Zarrintaj, P.; Saeb, M.R. Crystalline Polysaccharides: A Review. Carbohydr. Polym. 2022, 275, 118624. [Google Scholar] [CrossRef] [PubMed]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Health Benefits of Dietary Fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef]
- Ahmad, A.; Gulraiz, Y.; Ilyas, S.; Bashir, S. Polysaccharide Based Nano Materials: Health Implications. Food Hydrocoll. Health 2022, 2, 100075. [Google Scholar] [CrossRef]
- Meng, Q.; Zhong, S.; Gao, Y.; Cui, X. Advances in Polysaccharide-Based Nano/Microcapsules for Biomedical Applications: A Review. Int. J. Biol. Macromol. 2022, 220, 878–891. [Google Scholar] [CrossRef]
- Mandal, S.; Nagi, G.K.; Corcoran, A.A.; Agrawal, R.; Dubey, M.; Hunt, R.W. Algal Polysaccharides for 3D Printing: A Review. Carbohydr. Polym. 2023, 300, 120267. [Google Scholar] [CrossRef]
- Morais, M.G.; Santos, T.D.; Moraes, L.; Vaz, B.S.; Morais, E.G.; Costa, J.A.V. Exopolysaccharides from Microalgae: Production in a Biorefinery Framework and Potential Applications. Bioresour. Technol. Rep. 2022, 18, 101006. [Google Scholar] [CrossRef]
- Moreira, J.B.; Vaz, B.S.; Cardias, B.B.; Cruz, C.G.; Almeida, A.C.A.; Costa, J.A.V.; Morais, M.G. Microalgae Polysaccharides: An Alternative Source for Food Production and Sustainable Agriculture. Polysaccharides 2022, 3, 441–457. [Google Scholar] [CrossRef]
- Morais, M.G.; Rosa, G.M.; Moraes, L.; Alvarenga, A.G.P.; Silva, J.L.V.; Costa, J.A.V. Microalgae Polysaccharides with Potential Biomedical Application. In Polysaccharides of Microbial Origin; Oliveira, J., Radhouani, H.R.R.L., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–19. ISBN 9783030422141. [Google Scholar]
- Saratale, R.G.; Karuppusamy, I.; Saratale, G.D.; Pugazhendhi, A.; Kumar, G.; Park, Y.; Ghodake, G.S.; Bharagava, R.N.; Banu, J.R.; Shin, H.S. A Comprehensive Review on Green Nanomaterials Using Biological Systems: Recent Perception and Their Future Applications. Colloids Surf. B Biointerfaces 2018, 170, 20–35. [Google Scholar] [CrossRef]
- Pandey, G.; Jain, P. Assessing the Nanotechnology on the Grounds of Costs, Benefits, and Risks. Beni-Suef Univ. J. Basic Appl. Sci. 2020, 9, 63. [Google Scholar] [CrossRef]
- Poornima, V.B.; Katiyar, R.; Banerjee, S.; Patti, A.; Arora, A. Polysaccharide-Based Nanostructures as Nutraceutical Carriers. In Handbook of Nanotechnology in Nutraceuticals; Ahmed, S., Bhattacharya, T., Annu Ali, A., Eds.; CRC Press: Boca Raton, FL, USA, 2022; pp. 263–300. ISBN 9781032127910. [Google Scholar]
- Ahmad, K.; Khan, S.; Afridi, M.; Hassan, A.; Shah, M.M.; Rasheed, H.; Ahmad, R.; Ifqir, H. Marine Macroalgae Polysaccharides-Based Nanomaterials: An Overview with Respect to Nanoscience Applications. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 156. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, X.; Hu, H. Marine Polysaccharides as a Versatile Biomass for the Construction of Nano Drug Delivery Systems. Mar. Drugs 2021, 19, 345. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.K.; Vadrale, A.P.; Singhania, R.R.; Michaud, P.; Pandey, A.; Chen, S.J.; Chen, C.W.; Dong, C. Di Algal Polysaccharides: Current Status and Future Prospects. Phytochem. Rev. 2022, 22, 1167–1196. [Google Scholar] [CrossRef]
- Chevenier, A.; Jouanneau, D.; Ficko-Blean, E. Carrageenan Biosynthesis in Red Algae: A Review. Cell Surf. 2023, 9, 100097. [Google Scholar] [CrossRef] [PubMed]
- Perumal, P.K.; Dong, C.-D.; Chauhan, A.S.; Anisha, G.S.; Kadri, M.S.; Chen, C.-W.; Singhania, R.R.; Patel, A.K. Advances in Oligosaccharides Production from Algal Sources and Potential Applications. Biotechnol. Adv. 2023, 67, 108195. [Google Scholar] [CrossRef]
- Zanchetta, E.; Damergi, E.; Patel, B.; Borgmeyer, T.; Pick, H.; Pulgarin, A.; Ludwig, C. Algal Cellulose, Production and Potential Use in Plastics: Challenges and Opportunities. Algal Res. 2021, 56, 102288. [Google Scholar] [CrossRef]
- Nøkling-Eide, K.; Tan, F.; Wang, S.; Zhou, Q.; Gravdahl, M.; Langeng, A.-M.; Bulone, V.; Aachmann, F.L.; Sletta, H.; Arlov, Ø. Acid Preservation of Cultivated Brown Algae Saccharina latissima and Alaria esculenta and Characterization of Extracted Alginate and Cellulose. Algal Res. 2023, 71, 103057. [Google Scholar] [CrossRef]
- Nøkling-Eide, K.; Langeng, A.-M.; Åslund, A.; Aachmann, F.L.; Sletta, H.; Arlov, Ø. An Assessment of Physical and Chemical Conditions in Alginate Extraction from Two Cultivated Brown Algal Species in Norway: Alaria esculenta and Saccharina latissima. Algal Res. 2023, 69, 102951. [Google Scholar] [CrossRef]
- Birgersson, P.S.; Oftebro, M.; Strand, W.I.; Aarstad, O.A.; Sætrom, G.I.; Sletta, H.; Arlov, Ø.; Aachmann, F.L. Sequential Extraction and Fractionation of Four Polysaccharides from Cultivated Brown Algae Saccharina latissima and Alaria esculenta. Algal Res. 2023, 69, 102928. [Google Scholar] [CrossRef]
- Álvarez-Viñas, M.; González-Ballesteros, N.; Torres, M.D.; López-Hortas, L.; Vanini, C.; Domingo, G.; Rodríguez-Argüelles, M.C.; Domínguez, H. Efficient Extraction of Carrageenans from Chondrus crispus for the Green Synthesis of Gold Nanoparticles and Formulation of Printable Hydrogels. Int. J. Biol. Macromol. 2022, 206, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Song, H.-I.; Han, S.; Park, J.-S.; Kim, Y.-J.; Jeong, C.-B.; Yarish, C.; Kim, J.K. Dark Treatment Effect on the Carrageenan Characterization in a Red Alga, Chondrus crispus. Algal Res. 2022, 68, 102889. [Google Scholar] [CrossRef]
- Bahari, A.; Moelants, K.; Wallecan, J.; Mangiante, G.; Mazoyer, J.; Hendrickx, M.; Grauwet, T. Understanding the Effect of Time, Temperature and Salts on Carrageenan Extraction from Chondrus crispus. Algal Res. 2021, 58, 102371. [Google Scholar] [CrossRef]
- Yin, D.; Sun, X.; Li, N.; Guo, Y.; Tian, Y.; Wang, L. Structural Properties and Antioxidant Activity of Polysaccharides Extracted from Laminaria japonica Using Various Methods. Process Biochem. 2021, 111, 201–209. [Google Scholar] [CrossRef]
- Sun, Y.; Hou, S.; Song, S.; Zhang, B.; Ai, C.; Chen, X.; Liu, N. Impact of Acidic, Water and Alkaline Extraction on Structural Features, Antioxidant Activities of Laminaria japonica Polysaccharides. Int. J. Biol. Macromol. 2018, 112, 985–995. [Google Scholar] [CrossRef]
- Ren, Z.; Zhao, J.S.; Zheng, J.Y.; Wu, W.J. Process Optimum for Extracting Polysaccharide from Laminaria japonica by Ultrasonic-Enzyme Synergistic Method and Preparation of Laminaria japonica Beverage. Xian Dai Hua Gong 2018, 38, 182–186. [Google Scholar] [CrossRef]
- Hans, N.; Pattnaik, F.; Malik, A.; Naik, S. Comparison of Different Green Extraction Techniques and Their Influence on Chemical Characteristics of Sulfated Polysaccharide (Fucoidan) from Padina tetrastromatica and Turbinaria conoides. Algal Res. 2023, 74, 103199. [Google Scholar] [CrossRef]
- Rani, V. Influence of Species, Geographic Location, Seasonal Variation and Extraction Method on the Fucoidan Yield of the Brown Seaweeds of Gulf of Mannar, India. Indian J. Pharm. Sci. 2017, 79, 65–71. [Google Scholar] [CrossRef]
- Sharma, P.P.; Baskaran, V. Polysaccharide (Laminaran and Fucoidan), Fucoxanthin and Lipids as Functional Components from Brown Algae (Padina tetrastromatica) Modulates Adipogenesis and Thermogenesis in Diet-Induced Obesity in C57BL6 Mice. Algal Res. 2021, 54, 102187. [Google Scholar] [CrossRef]
- Cebrián-Lloret, V.; Metz, M.; Martínez-Abad, A.; Knutsen, S.H.; Ballance, S.; López-Rubio, A.; Martínez-Sanz, M. Valorization of Alginate-Extracted Seaweed Biomass for the Development of Cellulose-Based Packaging Films. Algal Res. 2022, 61, 102576. [Google Scholar] [CrossRef]
- Manikandan, N.A.; Lens, P.N.L. Green Extraction and Esterification of Marine Polysaccharide (Ulvan) from Green Macroalgae Ulva sp. Using Citric Acid for Hydrogel Preparation. J. Clean. Prod. 2022, 366, 132952. [Google Scholar] [CrossRef]
- Ben Amor, C.; Jmel, M.A.; Chevallier, P.; Mantovani, D.; Smaali, I. Efficient Extraction of a High Molecular Weight Ulvan from Stranded Ulva sp. Biomass: Application on the Active Biomembrane Synthesis. Biomass Convers. Biorefin. 2023, 13, 3975–3985. [Google Scholar] [CrossRef]
- André, J.; Flórez-Fernández, N.; Domínguez, H.; Torres, M.D. Microwave-Assisted Extraction of Ulva spp. Including a Stage of Selective Coagulation of Ulvan Stimulated by a Bio-Ionic Liquid. Int. J. Biol. Macromol. 2023, 225, 952–963. [Google Scholar] [CrossRef] [PubMed]
- Deamici, K.M.; Morais, M.G.; Santos, L.O.; Muylaert, K.; Gardarin, C.; Costa, J.A.V.; Laroche, C. Static Magnetic Fields Effects on Polysaccharides Production by Different Microalgae Strains. Appl. Sci. 2021, 11, 5299. [Google Scholar] [CrossRef]
- Gui, J.; Tong, W.; Huang, S.; Liang, X.; Fang, Z.; Wang, W.; Zhang, Y. Effects of Chlorella vulgaris Polysaccharides Accumulation on Growth Characteristics of Trachemys scripta elegans. Int. J. Biol. Macromol. 2019, 141, 1304–1313. [Google Scholar] [CrossRef]
- Ben Hlima, H.; Karray, A.; Dammak, M.; Elleuch, F.; Michaud, P.; Fendri, I.; Abdelkafi, S. Production and Structure Prediction of Amylases from Chlorella vulgaris. Environ. Sci. Pollut. Res. 2021, 28, 51046–51059. [Google Scholar] [CrossRef]
- Lv, J.; Zhao, F.; Feng, J.; Liu, Q.; Nan, F.; Liu, X.; Xie, S. The Impact of Particulate and Soluble Organic Matter on Physicochemical Properties of Extracellular Polymeric Substances in a Microalga Neocystis mucosa SX. Algal Res. 2020, 51, 102064. [Google Scholar] [CrossRef]
- Liu, S.; Wang, S.; Han, N.; Li, M.; Li, J.; Li, Y.; Jia, S.; Han, P. Effects of H2O2 Acclimation on the Growth, Polysaccharide Production and Tolerance Performance of Nostoc flagelliforme. Algal Res. 2023, 70, 102968. [Google Scholar] [CrossRef]
- Lee, M.-C.; Chen, Y.-C.; Peng, T.-C. Two-Stage Culture Method for Optimized Polysaccharide Production in Spirulina platensis. J. Sci. Food Agric. 2012, 92, 1562–1569. [Google Scholar] [CrossRef]
- Chen, Y.; Wan, X.; Wu, D.; Ouyang, Y.; Gao, L.; Chen, Z.; El-Seedi, H.R.; Wang, M.; Chen, X.; Zhao, C. Characterization of the Structure and Analysis of the Anti-Oxidant Effect of Microalga Spirulina platensis Polysaccharide on Caenorhabditis Elegans Mediated by Modulating MicroRNAs and Gut Microbiota. Int. J. Biol. Macromol. 2020, 163, 2295–2305. [Google Scholar] [CrossRef]
- Wang, B.; Liu, Q.; Huang, Y.; Yuan, Y.; Ma, Q.; Du, M.; Cai, T.; Cai, Y. Extraction of Polysaccharide from Spirulina and Evaluation of Its Activities. Evid.-Based Complement. Altern. Med. 2018, 2018, 3425615. [Google Scholar] [CrossRef]
- Yang, W.; Huang, G. Extraction Methods and Activities of Natural Glucans. Trends Food Sci. Technol. 2021, 112, 50–57. [Google Scholar] [CrossRef]
- Tanoeiro, J.R.; Fortunato, D.; Cotas, J.; Morais, T.; Afonso, C.; Pereira, L. Different Chondrus Crispus Aquaculture Methods and Carrageenan Extraction. Appl. Sci. 2023, 13, 5466. [Google Scholar] [CrossRef]
- Costa, J.A.V.; Lucas, B.F.; Alvarenga, A.G.P.; Moreira, J.B.; Morais, M.G. Microalgae Polysaccharides: An Overview of Production, Characterization, and Potential Applications. Polysaccharides 2021, 2, 759–772. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.; Liu, H.; Su, J.; Lan, C.Q.; Zhong, M.; Hu, X. Production, Isolation and Bioactive Estimation of Extracellular Polysaccharides of Green Microalga Neochloris oleoabundans. Algal Res. 2020, 48, 101883. [Google Scholar] [CrossRef]
- Bezerra, P.Q.M.; Moraes, L.; Cardoso, L.G.; Druzian, J.I.; Morais, M.G.; Nunes, I.L.; Costa, J.A.V. Spirulina sp. LEB 18 Cultivation in Seawater and Reduced Nutrients: Bioprocess Strategy for Increasing Carbohydrates in Biomass. Bioresour. Technol. 2020, 316, 123883. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, P.Q.M.; Moraes, L.; Silva, T.N.M.; Cardoso, L.G.; Druzian, J.I.; Morais, M.G.; Nunes, I.L.; Costa, J.A.V. Innovative Application of Brackish Groundwater without the Addition of Nutrients in the Cultivation of Spirulina and Chlorella for Carbohydrate and Lipid Production. Bioresour. Technol. 2021, 345, 126543. [Google Scholar] [CrossRef]
- Costa, J.A.V.; Cruz, C.G.; Rosa, A.P.C. Insights into the Technology Utilized to Cultivate Microalgae in Dairy Effluents. Biocatal. Agric. Biotechnol. 2021, 35, 102106. [Google Scholar] [CrossRef]
- Braga, V.S.; Moreira, J.B.; Costa, J.A.V.; Morais, M.G. Potential of Chlorella fusca LEB 111 Cultivated with Thermoelectric Fly Ashes, Carbon Dioxide and Reduced Supply of Nitrogen to Produce Macromolecules. Bioresour. Technol. 2019, 277, 55–61. [Google Scholar] [CrossRef]
- Silveira, J.T.; Rosa, A.P.C.; Morais, M.G.; Costa, J.A.V. Cost Reduction in the Production of Spirulina Biomass and Biomolecules from Indole-3-Acetic Acid Supplementation in Different Growth Phases. Appl. Biochem. Biotechnol. 2023, 195, 2882–2892. [Google Scholar] [CrossRef]
- Kocira, A.; Kozłowicz, K.; Panasiewicz, K.; Staniak, M.; Szpunar-Krok, E.; Hortyńska, P. Polysaccharides as Edible Films and Coatings: Characteristics and Influence on Fruit and Vegetable Quality—A Review. Agronomy 2021, 11, 813. [Google Scholar] [CrossRef]
- Silvello, M.A.C.; Gonçalves, I.S.; Azambuja, S.P.H.; Costa, S.S.; Silva, P.G.P.; Santos, L.O.; Goldbeck, R. Microalgae-Based Carbohydrates: A Green Innovative Source of Bioenergy. Bioresour. Technol. 2022, 344, 126304. [Google Scholar] [CrossRef]
- Caetano, P.A.; Nascimento, T.C.; Fernandes, A.S.; Nass, P.P.; Vieira, K.R.; Maróstica Junior, M.R.; Jacob-Lopes, E.; Zepka, L.Q. Microalgae-Based Polysaccharides: Insights on Production, Applications, Analysis, and Future Challenges. Biocatal. Agric. Biotechnol. 2022, 45, 102491. [Google Scholar] [CrossRef]
- Lafarga, T.; Gallagher, E.; Bademunt, A.; Bobo, G.; Echeverria, G.; Viñas, I.; Aguiló-Aguayo, I. Physiochemical and Nutritional Characteristics, Bioaccessibility and Sensory Acceptance of Baked Crackers Containing Broccoli Co-products. Int. J. Food. Sci. Technol. 2019, 54, 634–640. [Google Scholar] [CrossRef]
- Lucas, B.F.; Morais, M.G.; Santos, T.D.; Costa, J.A.V. Spirulina for Snack Enrichment: Nutritional, Physical and Sensory Evaluations. LWT-Food Sci. Technol. 2018, 90, 270–276. [Google Scholar] [CrossRef]
- Gomes-Dias, J.S.; Teixeira, J.A.; Rocha, C.M.R. Recent Advances in the Valorization of Algae Polysaccharides for Food and Nutraceutical Applications: A Review on the Role of Green Processing Technologies. Food Bioprocess Technol. 2022, 15, 1948–1976. [Google Scholar] [CrossRef]
- Begum, H.; Yusoff, F.; Banerjee, S.; Khatoon, H.; Shariff, M. Availability and Utilization of Pigments from Microalgae. Crit. Rev. Food Sci. Nutr. 2016, 56, 2209–2222. [Google Scholar] [CrossRef]
- Muthukumar, J.; Chidambaram, R.; Sukumaran, S. Sulfated Polysaccharides and Its Commercial Applications in Food Industries—A Review. J. Food. Sci. Technol. 2021, 58, 2453–2466. [Google Scholar] [CrossRef]
- Pérez, M.; Falqué, E.; Domínguez, H. Antimicrobial Action of Compounds from Marine Seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar] [CrossRef]
- Khalil, H.P.S.A.; Lai, T.K.; Tye, Y.Y.; Rizal, S.; Chong, E.W.N.; Yap, S.W.; Hamzah, A.A.; Nurul Fazita, M.R.; Paridah, M.T. A Review of Extractions of Seaweed Hydrocolloids: Properties and Applications. Express Polym. Lett. 2018, 12, 296–317. [Google Scholar] [CrossRef]
- Krempel, M.; Griffin, K.; Khouryieh, H. Hydrocolloids as Emulsifiers and Stabilizers in Beverage Preservation. In Preservatives and Preservation Approaches in Beverages; Grumezescu, A.M., Holban, A.M., Eds.; Elsevier: Duxford, UK, 2019; pp. 427–465. ISBN 978-0-12-816685-7. [Google Scholar]
- Zhang, J.; Liu, L.; Ren, Y.; Chen, F. Characterization of Exopolysaccharides Produced by Microalgae with Antitumor Activity on Human Colon Cancer Cells. Int. J. Biol. Macromol. 2019, 128, 761–767. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.X.; Liu, X.Y.; Xiao, Z.; Huang, Y.F.; Liu, B. Antioxidant Activities of Polysaccharides Obtained from Chlorella pyrenoidosa via Different Ethanol Concentrations. Int. J. Biol. Macromol. 2016, 91, 505–509. [Google Scholar] [CrossRef]
- Ziyaei, K.; Ataie, Z.; Mokhtari, M.; Adrah, K.; Daneshmehr, M.A. An Insight to the Therapeutic Potential of Algae-Derived Sulfated Polysaccharides and Polyunsaturated Fatty Acids: Focusing on the COVID-19. Int. J. Biol. Macromol. 2022, 209, 244–257. [Google Scholar] [CrossRef] [PubMed]
- Belhaj, D.; Frikha, D.; Athmouni, K.; Jerbi, B.; Ahmed, M.B.; Bouallagui, Z.; Kallel, M.; Maalej, S.; Zhou, J.; Ayadi, H. Box-Behnken Design for Extraction Optimization of Crude Polysaccharides from Tunisian Phormidium versicolor Cyanobacteria (NCC 466): Partial Characterization, in vitro Antioxidant and Antimicrobial Activities. Int. J. Biol. Macromol. 2017, 105, 1501–1510. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S.; Varma, R.S. Important Roles of Oligo- and Polysaccharides against SARS-CoV-2: Recent Advances. Appl. Sci. 2021, 11, 3512. [Google Scholar] [CrossRef]
- Yuan, Q.; Li, H.; Wei, Z.; Lv, K.; Gao, C.; Liu, Y.; Zhao, L. Isolation, Structures and Biological Activities of Polysaccharides from Chlorella: A Review. Int. J. Biol. Macromol. 2020, 163, 2199–2209. [Google Scholar] [CrossRef]
- Shen, J.; Lu, Z.; Wang, J.; Zhang, T.; Yang, J.; Li, Y.; Liu, G.; Zhang, X. Advances of Nanoparticles for Leukemia Treatment. ACS Biomater. Sci. Eng. 2020, 6, 6478–6489. [Google Scholar] [CrossRef]
- Miao, T.; Wang, J.; Zeng, Y.; Liu, G.; Chen, X. Polysaccharide-Based Controlled Release Systems for Therapeutics Delivery and Tissue Engineering: From Bench to Bedside. Adv. Sci. 2018, 5, 1700513. [Google Scholar] [CrossRef]
- Yosri, N.; Khalifa, S.A.M.; Guo, Z.; Xu, B.; Zou, X.; El-Seedi, H.R. Marine Organisms: Pioneer Natural Sources of Polysaccharides/Proteins for Green Synthesis of Nanoparticles and Their Potential Applications. Int. J. Biol. Macromol. 2021, 193, 1767–1798. [Google Scholar] [CrossRef]
- Qiu, A.; Wang, Y.; Zhang, G.; Wang, H. Natural Polysaccharide-Based Nanodrug Delivery Systems for Treatment of Diabetes. Polymers 2022, 14, 3217. [Google Scholar] [CrossRef]
- Kanaoujiya, R.; Saroj, S.K.; Srivastava, S.; Chaudhary, M.K. Renewable Polysaccharide and Biomedical Application of Nanomaterials. J. Nanomater. 2022, 2022, 1050211. [Google Scholar] [CrossRef]
- Nakamichi, A.; Kadokawa, J. Fabrication of Chitosan-Based Network Polysaccharide Nanogels. Molecules 2022, 27, 8384. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, A.; Lima, S.C.; Reis, S. Application of pH-Responsive Fucoidan/Chitosan Nanoparticles to Improve Oral Quercetin Delivery. Molecules 2019, 24, 346. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Lu, Y.; Ouyang, X.K.; Ling, J. Development and Characterization of Soybean Protein Isolate and Fucoidan Nanoparticles for Curcumin Encapsulation. Int. J. Biol. Macromol. 2021, 169, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Qin, Y.; Jiang, B.; Chen, J.; Zhang, T. Development of Self-Assembled Zein-Fucoidan Complex Nanoparticles as a Delivery System for Resveratrol. Colloids Surf. B Biointerfaces 2022, 216, 112529. [Google Scholar] [CrossRef]
- Roy, V.C.; Razzak, M.A.; Ho, T.C.; Surendhiran, D.; Park, J.S.; Chun, B.S. Fabrication of Zein and κ-Carrageenan Colloidal Particles for Encapsulation of Quercetin: In vitro Digestibility and Bio-Potential Activities. J. Ind. Eng. Chem. 2022, 111, 272–280. [Google Scholar] [CrossRef]
- Oliyaei, N.; Moosavi-Nasab, M.; Tanideh, N. Preparation of Fucoxanthin Nanoemulsion Stabilized by Natural Emulsifiers: Fucoidan, Sodium Caseinate, and Gum Arabic. Molecules 2022, 27, 6713. [Google Scholar] [CrossRef]
- Malagurski, I.; Levic, S.; Nesic, A.; Mitric, M.; Pavlovic, V.; Dimitrijevic-Brankovic, S. Mineralized Agar-Based Nanocomposite Films: Potential Food Packaging Materials with Antimicrobial Properties. Carbohydr. Polym. 2017, 175, 55–62. [Google Scholar] [CrossRef]
- Duan, N.; Li, Q.; Meng, X.; Wang, Z.; Wu, S. Preparation and Characterization of K-Carrageenan/Konjac Glucomannan/TiO2 Nanocomposite Film with Efficient Anti-Fungal Activity and Its Application in Strawberry Preservation. Food Chem. 2021, 364, 130441. [Google Scholar] [CrossRef]
- Chandrarathna, H.P.S.U.; Liyanage, T.D.; Edirisinghe, S.L.; Dananjaya, S.H.S.; Thulshan, E.H.T.; Nikapitiya, C.; Oh, C.; Kang, D.H.; Zoysa, M. Marine Microalgae, Spirulina maxima-Derived Modified Pectin and Modified Pectin Nanoparticles Modulate the Gut Microbiota and Trigger Immune Responses in Mice. Mar. Drugs 2020, 18, 175. [Google Scholar] [CrossRef]
- Yang, F.; Tang, Q.; Zhong, X.; Bai, Y.; Zhang, Y.; Li, Y.; Zheng, W. Surface Decoration by Spirulina Polysaccharide Enhances the Cellular Uptake and Anticancer Efficacy of Selenium Nanoparticles. Int. J. Nanomed. 2012, 7, 835. [Google Scholar] [CrossRef]
- Liberman, G.N.; Ochbaum, G.; Bitton, R.; Arad, S. Antimicrobial Hydrogels Composed of Chitosan and Sulfated Polysaccharides of Red Microalgae. Polymer 2021, 215, 123353. [Google Scholar] [CrossRef]
- Ebrahiminezhad, A.; Bagheri, M.; Taghizadeh, S.M.; Berenjian, A.; Ghasemi, Y. Biomimetic Synthesis of Silver Nanoparticles Using Microalgal Secretory Carbohydrates as a Novel Anticancer and Antimicrobial. Adv. Nat. Sci. Nanosci. Nanotechnol. 2016, 7, 015018. [Google Scholar] [CrossRef]
- Madany, M.A.; Abdel-Kareem, M.S.; Al-Oufy, A.K.; Haroun, M.; Sheweita, S.A. The Biopolymer Ulvan from Ulva fasciata: Extraction towards Nanofibers Fabrication. Int. J. Biol. Macromol. 2021, 177, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Anil, S.; Kim, S.K.; Shim, M. Seaweed Polysaccharide-Based Nanoparticles: Preparation and Applications for Drug Delivery. Polymers 2016, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Moreira, J.B.; Morais, M.G.; Morais, E.G.; Vaz, B.S.; Costa, J.A.V. Electrospun Polymeric Nanofibers in Food Packaging. In Impact of Nanoscience in the Food Industry; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: London, UK, 2018; pp. 387–417. ISBN 5509780128114933. [Google Scholar]
- Moreira, J.B.; Kuntzler, S.G.; Terra, A.L.M.; Costa, J.A.V.; Morais, M.G. Electrospun Nanofbers Fundamentals, Food Packaging Technology, and Safety. In Food Packaging; Rangappa, S.M., Parameswaranpillai, J., Thiagamani, S.M.K., Krishnasamy, S., Siengchin, S., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 223–254. ISBN 9780429322129. [Google Scholar]
- Zhang, J.; Zhan, P.; Tian, H. Recent Updates in the Polysaccharides-Based Nano-Biocarriers for Drugs Delivery and Its Application in Diseases Treatment: A Review. Int. J. Biol. Macromol. 2021, 182, 115–128. [Google Scholar] [CrossRef]
- Mauri, E.; Giannitelli, S.M.; Trombetta, M.; Rainer, A. Synthesis of Nanogels: Current Trends and Future Outlook. Gels 2021, 7, 36. [Google Scholar] [CrossRef] [PubMed]
- Pathak, C.; Vaidya, F.U.; Pandey, S.M. Mechanism for Development of Nanobased Drug Delivery System. In Applications of Targeted Nano Drugs and Delivery Systems; Mohapatra, S.S., Ranjan, S., Dasgupta, N., Mishra, R.K., Thomas, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 35–67. ISBN 9780128140291. [Google Scholar]
- Lin, J.; Jiao, G.; Kermanshahi-pour, A. Algal Polysaccharides-Based Hydrogels: Extraction, Synthesis, Characterization, and Applications. Mar. Drugs 2022, 20, 306. [Google Scholar] [CrossRef]
- Torres, F.G.; Troncoso, O.P.; Pisani, A.; Gatto, F.; Bardi, G. Natural Polysaccharide Nanomaterials: An Overview of Their Immunological Properties. Int. J. Mol. Sci. 2019, 20, 5092. [Google Scholar] [CrossRef]
- Karimzadeh, M.; Eslampanah-Seyyedi, E.; Behniafar, H. Poly(Tetramethylene Oxide)-Coated Silica Nanoparticles Incorporated into Poly(4,4′-Oxydiphenylene-Pyromellitimide) Matrix. Mater. Manuf. Process. 2018, 33, 1093–1099. [Google Scholar] [CrossRef]
- Li, Q.; Liu, S. Applications of Nanocellulose in the Food Industry. In Nanocellulose; Yang, G., Ullah, M.W., Shi, Z., Eds.; World Scientific Publishing Europe: Tokyo, Japan, 2021; pp. 257–285. ISBN 9781786349477. [Google Scholar]
- Perumal, A.B.; Nambiar, R.B.; Sellamuthu, P.S.; Sadiku, E.R. Application of Biosynthesized Nanoparticles in Food, Food Packaging and Dairy Industries. In Biological Synthesis of Nanoparticles and Their Applications; Karthik, L., Kirthi, A.V., Ranjan, S., Srinivasan, V.M., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 145–158. ISBN 9780429265235. [Google Scholar]
- Rabadan-Chavez, G.; Lugo-Cervantes, E. Phenolic Compounds in Cocoa and Chocolate. In Phenolic Compounds in Food; Nollet, L.M.L., Gutierrez-Uribe, J.A., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 375–394. ISBN 9781315120157. [Google Scholar]
- Zahariev, N.; Katsarov, P.; Lukova, P.; Pilicheva, B. Novel Fucoidan Pharmaceutical Formulations and Their Potential Application in Oncology—A Review. Polymers 2023, 15, 3242. [Google Scholar] [CrossRef] [PubMed]
- Karelakis, C.; Zevgitis, P.; Galanopoulos, K.; Mattas, K. Consumer Trends and Attitudes to Functional Foods. J. Int. Food. Agribus. Mark. 2020, 32, 266–294. [Google Scholar] [CrossRef]
- He, X.; Hwang, H.M. Nanotechnology in Food Science: Functionality, Applicability, and Safety Assessment. J. Food Drug Anal. 2016, 24, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Pateiro, M.; Gómez, B.; Munekata, P.E.S.; Barba, F.J.; Putnik, P.; Kovačević, D.B.; Lorenzo, J.M. Nanoencapsulation of Promising Bioactive Compounds to Improve Their Absorption, Stability, Functionality and the Appearance of the Final Food Products. Molecules 2021, 26, 1547. [Google Scholar] [CrossRef] [PubMed]
- Zavareze, E.R.; Kringel, D.H.; Dias, A.R.G. Nano-Scale Polysaccharide Materials in Food and Agricultural Applications. In Advances in Food and Nutrition Research; Lim, L.T., Rogers, M., Eds.; Academic Press: Cambridge, MA, USA, 2019; Volume 88, pp. 85–128. ISBN 9780128160732. [Google Scholar]
- Mensah, E.O.; Kanwugu, O.N.; Panda, P.K.; Adadi, P. Marine Fucoidans: Structural, Extraction, Biological Activities and Their Applications in the Food Industry. Food Hydrocoll. 2023, 142, 108784. [Google Scholar] [CrossRef]
- Roy, S.; Shankar, S.; Rhim, J.-W. Melanin-Mediated Synthesis of Silver Nanoparticle and Its Use for the Preparation of Carrageenan-Based Antibacterial Films. Food Hydrocoll. 2019, 88, 237–246. [Google Scholar] [CrossRef]
- Gallón, S.M.N.; Alpaslan, E.; Wang, M.; Larese-Casanova, P.; Londoño, M.E.; Atehortúa, L.; Pavón, J.J.; Webster, T.J. Characterization and Study of the Antibacterial Mechanisms of Silver Nanoparticles Prepared with Microalgal Exopolysaccharides. Mater. Sci. Eng. C 2019, 99, 685–695. [Google Scholar] [CrossRef]
- Richa, R.; Choudhury, A.R. Exploration of Polysaccharide Based Nanoemulsions for Stabilization and Entrapment of Curcumin. Int. J. Biol. Macromol. 2020, 156, 1287–1296. [Google Scholar] [CrossRef]
- Nešić, A.; Cabrera-Barjas, G.; Dimitrijević-Branković, S.; Davidović, S.; Radovanović, N.; Delattre, C. Prospect of Polysaccharide-Based Materials as Advanced Food Packaging. Molecules 2019, 25, 135. [Google Scholar] [CrossRef]
- Sarfraz, J.; Gulin-Sarfraz, T.; Nilsen-Nygaard, J.; Pettersen, M.K. Nanocomposites for Food Packaging Applications: An Overview. Nanomaterials 2020, 11, 10. [Google Scholar] [CrossRef]
- Franco-Morgado, M.; Amador-Espejo, G.G.; Pérez-Cortés, M.; Gutiérrez-Uribe, J.A. Microalgae and Cyanobacteria Polysaccharides: Important Link for Nutrient Recycling and Revalorization of Agro-Industrial Wastewater. Appl. Food Res. 2023, 3, 100296. [Google Scholar] [CrossRef]
- Raveendran, S.; Yoshida, Y.; Maekawa, T.; Kumar, D.S. Pharmaceutically Versatile Sulfated Polysaccharide Based Bionano Platforms. Nanomedicine 2013, 9, 605–626. [Google Scholar] [CrossRef] [PubMed]
- Ko, S.W.; Lee, J.Y.; Aguilar, L.E.; Oh, Y.M.; Park, C.H.; Kim, C.S. Fabrication of a Micro/Nano-Net Membrane Using Cellulose Nanocrystals Derived from Seaweed. J. Nanosci. Nanotechnol. 2019, 19, 2232–2235. [Google Scholar] [CrossRef] [PubMed]
- Marine Algae Polysaccharides Market: Global Analysis Report 2033. Available online: https://www.factmr.com/report/marine-algae-polysaccharides-market (accessed on 23 July 2023).
- Algal Polysaccharides Market by Share, Size and Growth 2030. Available online: https://www.credenceresearch.com/report/algal-polysaccharides-market (accessed on 23 July 2023).
- Carrageenan Manufacturer, Purchase Kappa Carrageenan—Foodchem®. Available online: https://www.foodchem.com/product/carrageenan-for-meat-application/ (accessed on 23 July 2023).
- Carrageenan|Cargill Food Ingredients EMEA|Cargill. Available online: https://www.cargill.com/food-bev/emea/texturizers/carrageenan (accessed on 23 July 2023).
- Carrageenan—CP Kelco, a Provider of Nature-Based Hydrocolloid Ingredients. Available online: https://www.cpkelco.com/products/carrageenan/ (accessed on 23 July 2023).
- Products—Gelymar. Available online: https://www.gelymar.com/products/ (accessed on 23 July 2023).
- Maritech Fucoidan|Organic Fucoidan|Innovative Australian Biotechnology. Available online: https://maritechfucoidan.com.au/ (accessed on 23 July 2023).
- Hydrocolloid—Qingdao Gather Great Ocean Algae Industry Group Co., Ltd. Available online: https://en.judayang.com/product/c1.html (accessed on 23 July 2023).
- Laroche, C. Exopolysaccharides from Microalgae and Cyanobacteria: Diversity of Strains, Production Strategies, and Applications. Mar. Drugs 2022, 20, 336. [Google Scholar] [CrossRef] [PubMed]
- Barciela, P.; Carpena, M.; Li, N.-Y.; Liu, C.; Jafari, S.M.; Simal-Gandara, J.; Prieto, M.A. Macroalgae as Biofactories of Metal Nanoparticles; Biosynthesis and Food Applications. Adv. Colloid Interface Sci. 2023, 311, 102829. [Google Scholar] [CrossRef]
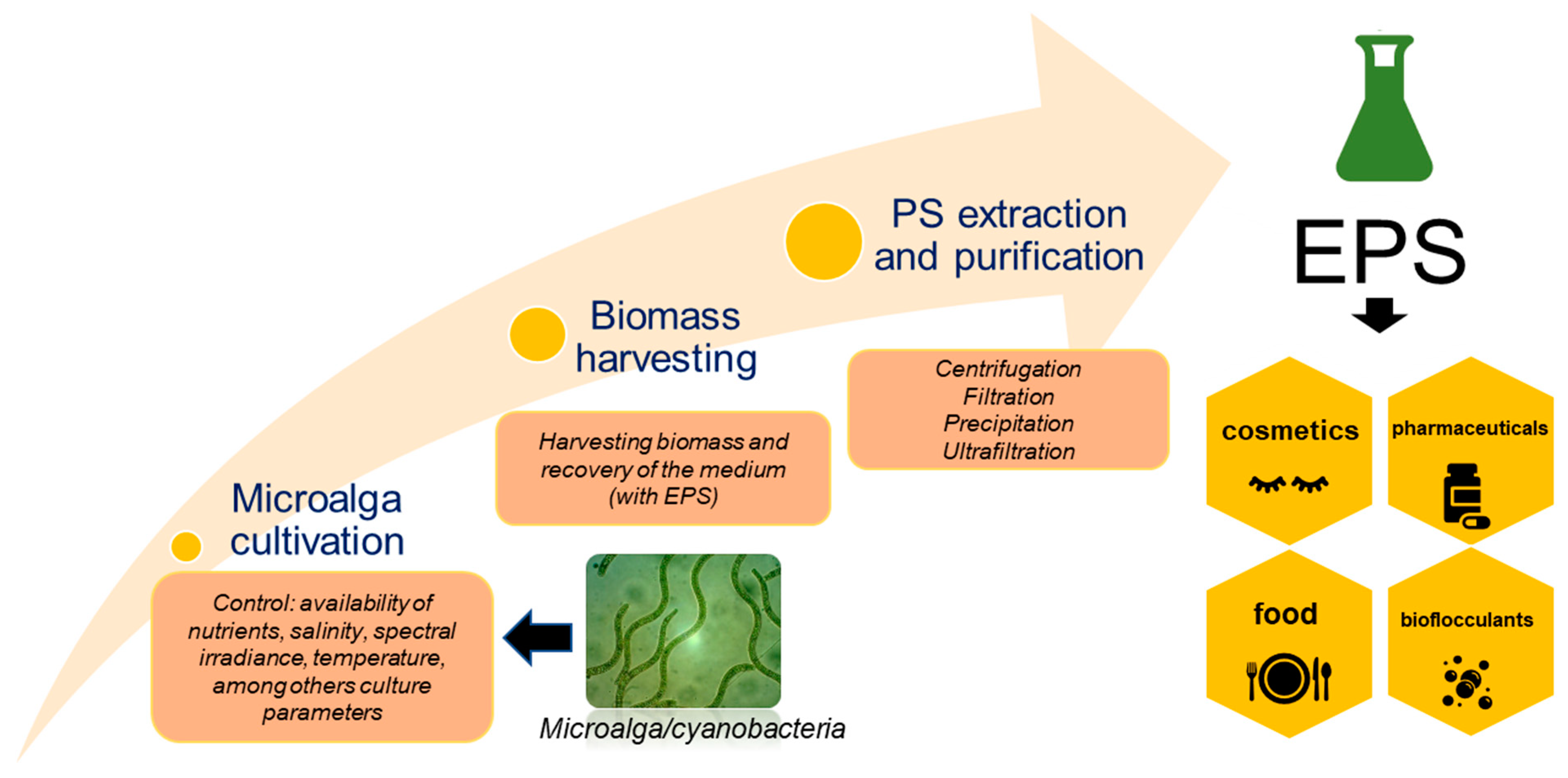
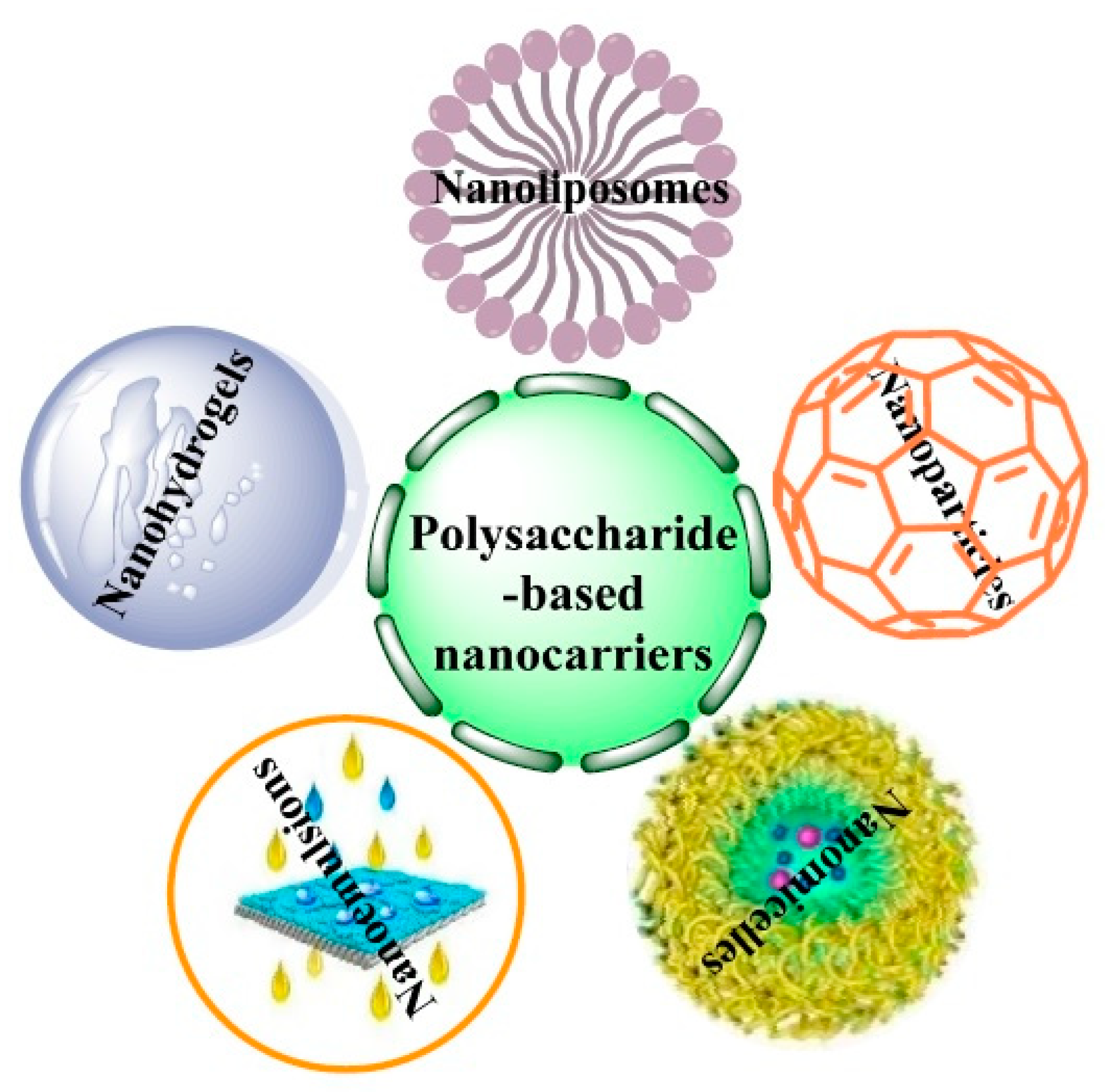
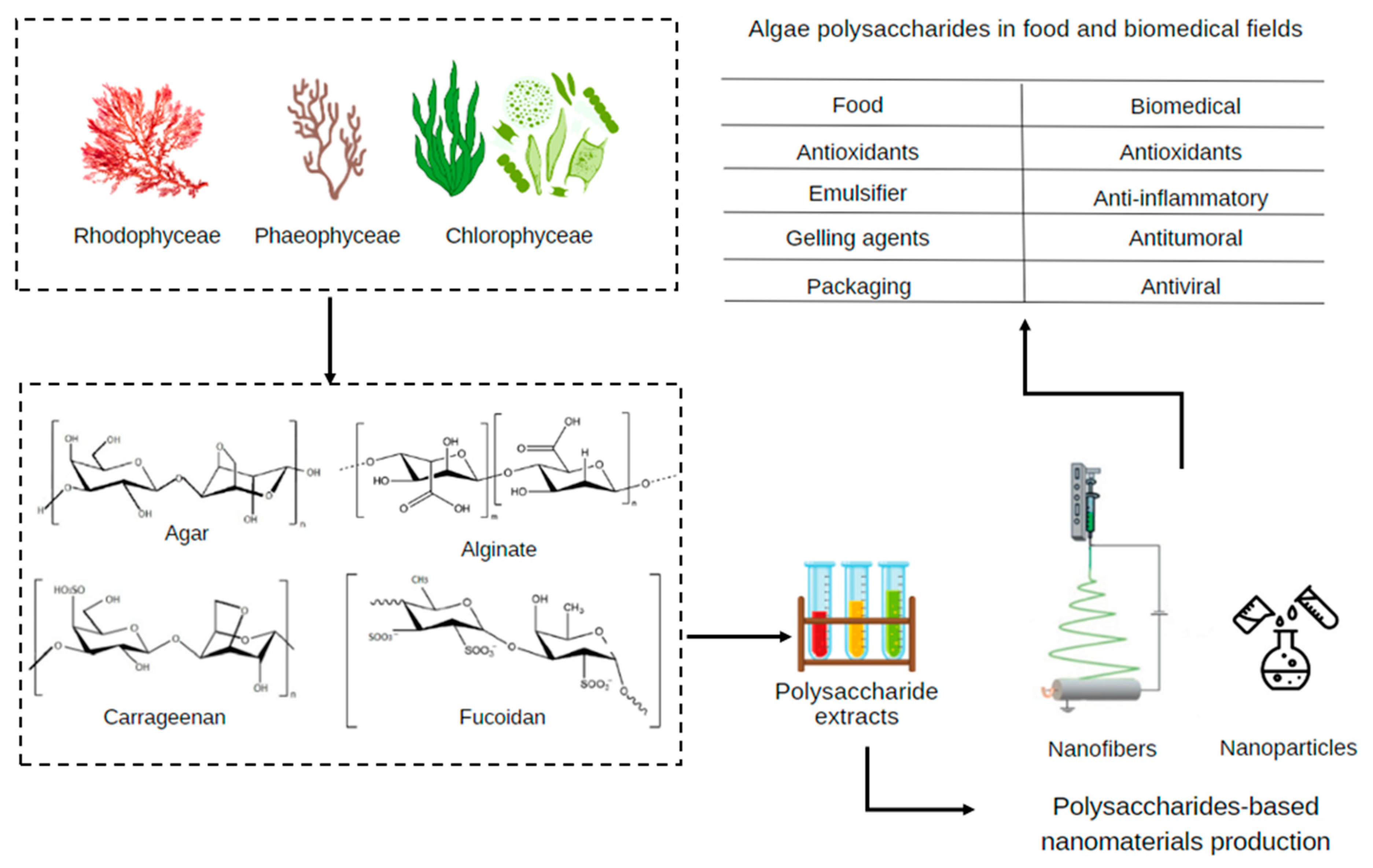
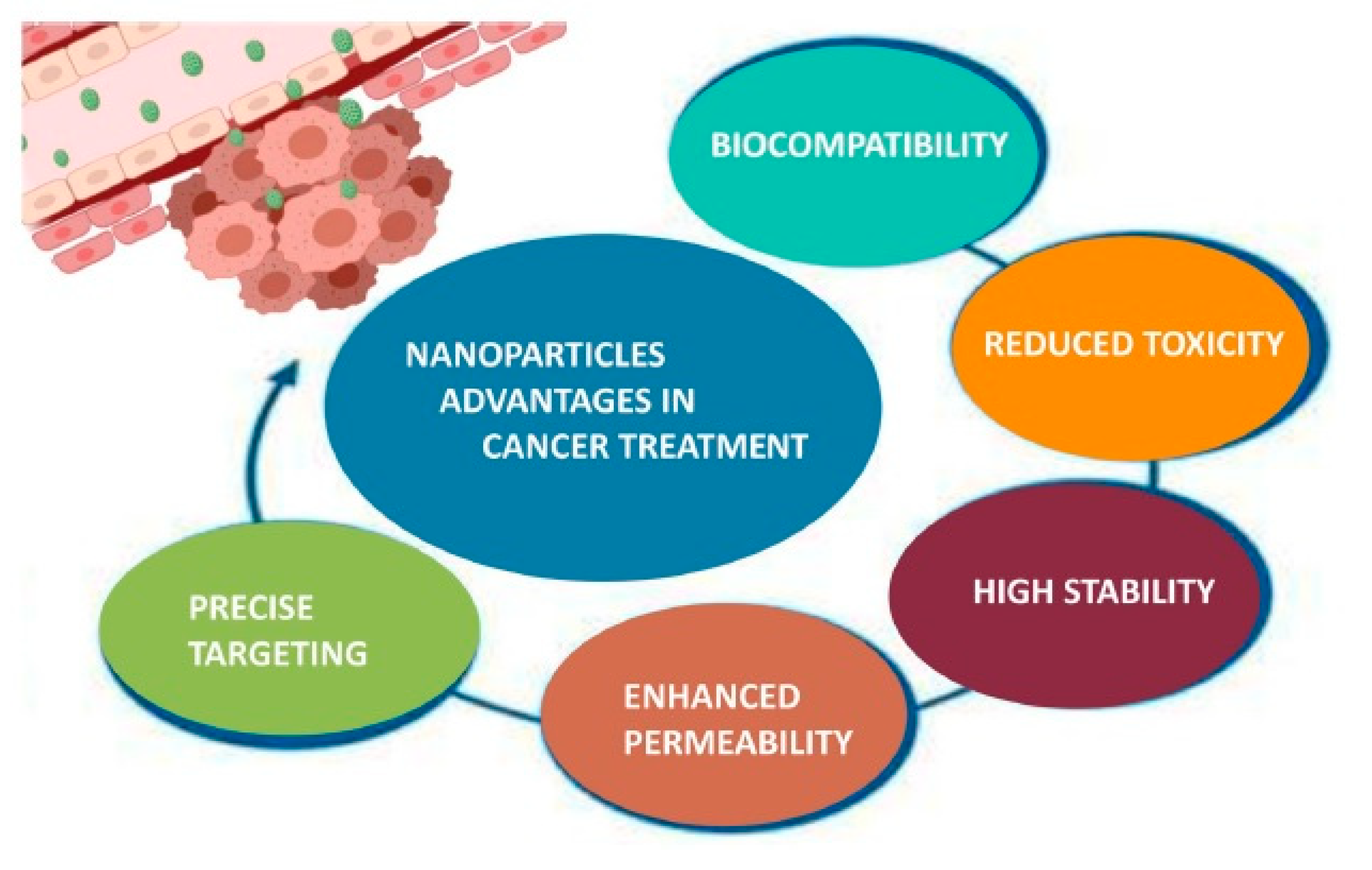
| Macroalga/Microalga | Macroalgae Extraction Method/ Microalgae Cultivation Strategy | Polysaccharides Type | Polysaccharides Yield (% w/w) | Reference |
|---|---|---|---|---|
| Alaria esculenta | Preservation with formic acid (4 weeks; 20 °C) | Alginates | 32.5 * | [20] |
| Short alkaline extraction (1–5 h), pH 9, 20 °C | 24.0 | [21] | ||
| Sequential extraction of fucoidan/laminarin, alginate, and cellulose using mild chemical methods | 7.1 | [22] | ||
| Chondrus crispus | Hydrothermal processing with subcritical water during non-isothermal heating up to 140 °C | Carrageenans | 75.5 | [23] |
| Dark cultivation for 21 days | 44.3 | [24] | ||
| Dispersion with demineralized water stirred with a magnetic rod at 500 rpm for 8 h at 90 °C | 39.2 | [25] | ||
| Laminaria japonica | Ultrasound-assisted Extraction (195 W for 30 min at 60 °C). | Laminaria japonica polysaccharides | 9.7 | [26] |
| Alkaline extraction (NaOH solution of pH 10.0 for 4 h at 80 °C) | 44.6 | [27] | ||
| Ultrasonic-enzyme synergistic method (0.3% cellulase, 0.7% pectinase, and 1.5% papain; 30 min 55 °C) | 19.4 | [28] | ||
| Padina tetrastromatica | Subcritical water extraction (150 °C, 5 MPa, 15 min) | Fucoidans | 14.0 | [29] |
| Water extraction (12 h at room temperature) | 9.5 | [30] | ||
| Treatment with selective solvents (EtOH, CaCl2, HCl, Na2CO3) | 9.4 | [31] | ||
| Saccharina latissima | Preservation with formic acid (16 weeks; 20 °C) | Cellulose | 18.0 | [20] |
| Typical extraction (acidification—HCl, alkaline extraction—Na2CO3), solid/liquid separation, precipitation, and drying) | 26.0 | [32] | ||
| Sequential extraction of fucoidan/ laminarin, alginate, and cellulose using mild chemical methods | 6.9 | [22] | ||
| Ulva sp. | Foliose citric acid-based extraction | Ulvans | 41.0 | [33] |
| Soxhlet extraction with methanol and 5% of ammonium oxalate | 13.8 | [34] | ||
| Microwave-assisted hydrothermal (liquid phase/EtOH, 1:1.5, choline chloride 1%, 120 °C) | 32.5 | [35] | ||
| Arthrospira platensis SAG 21.99 | Indoor cultures; static magnetic fields application for 24 h d−1 | Exopolysaccharides | 34.8 | [36] |
| Chlorella fusca LEB 111 | Outdoor cultures; static magnetic fields application for 1 h d−1 | Starch | 10.9 | [36] |
| Chlorella vulgaris | BG 11 medium, light intensity 65 μmol photons m−2 s−1 and temperature 28 °C | Heteropolysaccharides | 32.7 | [37] |
| Three-stage process with stressed conditions applied in the second stage (light intensity of 360 μmol photons m−2 s−1 and nitrogen starvation (F/2 medium deprived of NaNO3) | Starch | 21.0 | [38] | |
| Neocystis mucosa SX | Cation exchange resin method was used to extract polysaccharides | Exopolysaccharides | 6.2 | [39] |
| Nostoc flagelliforme | H2O2 acclimation method | Exopolysaccharides | 4.7 | [40] |
| Spirulina platensis | Two stage culture: (1) 96 µmol photons m−2 s−1 at 28 °C; (2) light intensity 192 µmol photons m−2 s−1 and 38 °C of temperature for 3 days | Polysaccharide of Spirulina platensis | 27.3 | [41] |
| Commercial microalgal powder was extracted with ultrapure water and ultrasonic treatment (45 kHz, 300 w) at 60 °C for 1 h. | 16.7 | [42] | ||
| Commercial powder and alkaline extraction | 10.8 | [43] | ||
| Spirulina sp. | Outdoor cultures | Exopolysaccharides | 49.3 | [36] |
| Alga | Polysaccharide | Manufacturing Methodology | Outcomes | Reference |
|---|---|---|---|---|
| Fucus vesiculosus | Fucoidan | The polyelectrolyte self-assembly method was used to obtain fucoidan/chitosan nanoparticles to encapsulate quercetin. | Improved physicochemical properties; controlled release under simulated gastrointestinal conditions; encapsulation efficiency from 97% to 99%. | [76] |
| Brown algae | Fucoidan | Nanoparticles prepared via electrostatic interaction using fucoidan and soybean protein isolated to encapsulate curcumin. | Salt tolerance, heat resistance, and storage stability; encapsulation efficiency of >95%. | [77] |
| Laminaria japonica | Fucoidan | The antisolvent precipitation method was used to produce nanoparticles based on zein and fucoidan to encapsulate resveratrol. | Photostability; ionic, pH, and storage stabilities; controlled release under in vitro digestion conditions; encapsulation efficiency of 95.4%. | [78] |
| Red algae | K-carrageenan | The antisolvent precipitation method was used to produce zein-K-carrageenan nanoparticles to encapsulate quercetin. | Improved water dispersibility, thermal stability, and controlled release under in vitro digestion conditions; encapsulation efficiency of 62%. | [79] |
| Sargassum angustifolium | Fucoidan | Ultrasonic treatment was used to prepare nanoemulsions for fucoxanthin encapsulation. | Controlled release under gastrointestinal conditions; encapsulation efficiency of 79%. | [80] |
| Red algae | Agar | Precipitation and solvent-casting methods were used to prepare mineralized agar-based nanocomposite films. | Mechanical and light barrier properties, antimicrobial activity against Staphylococcus aureus. | [81] |
| Red algae | K-carrageenan | Solvent-casting method was used to prepare nanocomposite films from k-carrageenan, konjac glucomannan, and TiO2 nanoparticles. | Thermal stability; mechanical and UV barrier properties; antimicrobial and fresh-keeping properties in strawberry preservation. | [82] |
| Spirulina maxima | Pectin | Pectin was extracted from microalga and then modified using high temperature and pressure for a specific duration. Subsequently, pectin nanoparticles were created through sonication of the modified pectin. | Potential to modulate gut microbial community, enhance the expression of immune-related genes, and improve gut morphology. | [83] |
| Spirulina platensis | Spirulina polysaccharides | Selenium nanoparticles with Spirulina polysaccharides have been developed with a solution-phase method. Microalgal polysaccharides were extracted with hot water. | Enhanced cellular uptake and anticancer efficacy, potential candidate for further evaluation as a chemopreventive and chemotherapeutic agent against human cancers. | [84] |
| Red microalgae | Sulfated polysaccharides | Hydrogels were developed using sulfated polysaccharides, chitosan, and zinc. | A broad spectrum of antimicrobial activities, potential use as wound dressings. | [85] |
| Chlorella vulgaris | Carbohydrates containing polysaccharide | Secreted carbohydrates by microalgal cells were used for reducing and capping silver nanoparticles. | Anticancer and antimicrobial applications. | [86] |
| Ulva fasciata | Ulvan | Deionized water was used to produce ulvan/polyvinyl alcohol (ulvan/PVA) nanofibers. | Desirable thermal stability and mechanical properties for tissue engineering. | [87] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreira, J.B.; Santos, T.D.; Cruz, C.G.; Silveira, J.T.d.; Carvalho, L.F.d.; Morais, M.G.d.; Costa, J.A.V. Algal Polysaccharides-Based Nanomaterials: General Aspects and Potential Applications in Food and Biomedical Fields. Polysaccharides 2023, 4, 371-389. https://doi.org/10.3390/polysaccharides4040022
Moreira JB, Santos TD, Cruz CG, Silveira JTd, Carvalho LFd, Morais MGd, Costa JAV. Algal Polysaccharides-Based Nanomaterials: General Aspects and Potential Applications in Food and Biomedical Fields. Polysaccharides. 2023; 4(4):371-389. https://doi.org/10.3390/polysaccharides4040022
Chicago/Turabian StyleMoreira, Juliana Botelho, Thaisa Duarte Santos, Camila Gonzales Cruz, Jéssica Teixeira da Silveira, Lisiane Fernandes de Carvalho, Michele Greque de Morais, and Jorge Alberto Vieira Costa. 2023. "Algal Polysaccharides-Based Nanomaterials: General Aspects and Potential Applications in Food and Biomedical Fields" Polysaccharides 4, no. 4: 371-389. https://doi.org/10.3390/polysaccharides4040022
APA StyleMoreira, J. B., Santos, T. D., Cruz, C. G., Silveira, J. T. d., Carvalho, L. F. d., Morais, M. G. d., & Costa, J. A. V. (2023). Algal Polysaccharides-Based Nanomaterials: General Aspects and Potential Applications in Food and Biomedical Fields. Polysaccharides, 4(4), 371-389. https://doi.org/10.3390/polysaccharides4040022








