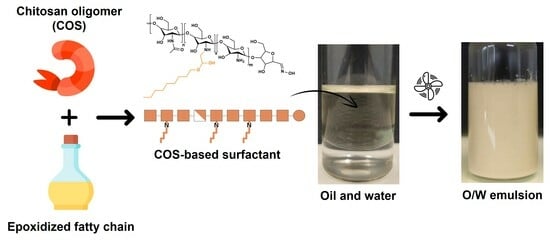
Concentrated O/W Emulsion Stability of Non-Ionic Chitosan Oligomer Surfactants Modified by Epoxidized Fatty Chains at pH7: Influence of Emulsification Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Chitooligosaccharides (COS)
2.2. Functionalization of COS with C9 or C16 Epoxy Fatty Chains
2.3. Functionalization of COS with Epoxidized Cardanol
2.4. Calculation of the Grafting Efficiency
2.5. HLB Calculation
2.6. Zeta Potential
2.7. CMC and Surface Tension
2.8. Preparation of the Emulsions
2.9. Droplet Size
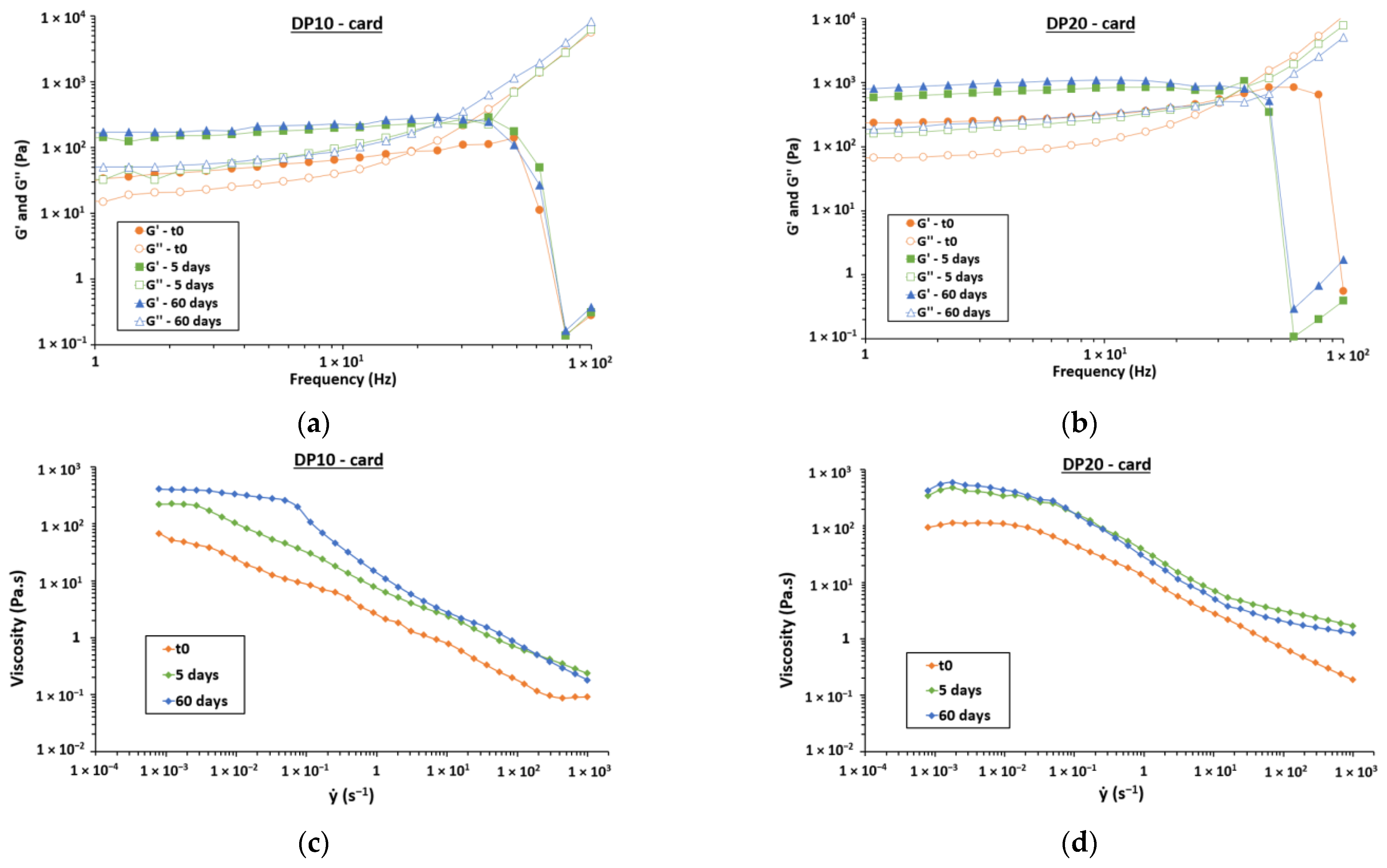
2.10. Rheological Behavior
2.11. Turbiscan
2.12. Microscopy Measurements
3. Results and Discussion
3.1. Synthesis and Characterization of COS-Based Surfactants
3.2. Optimization of Emulsification Protocol
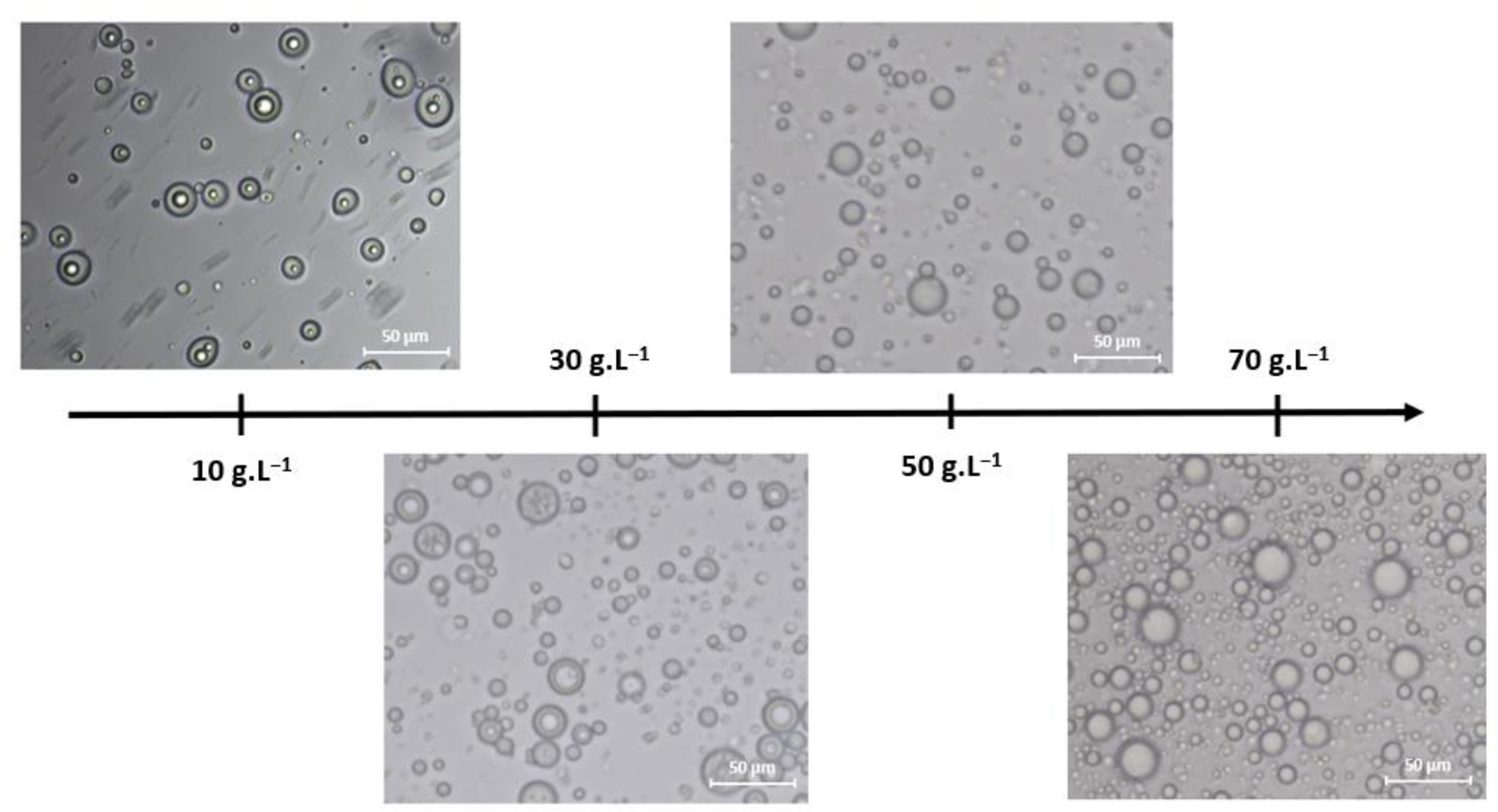
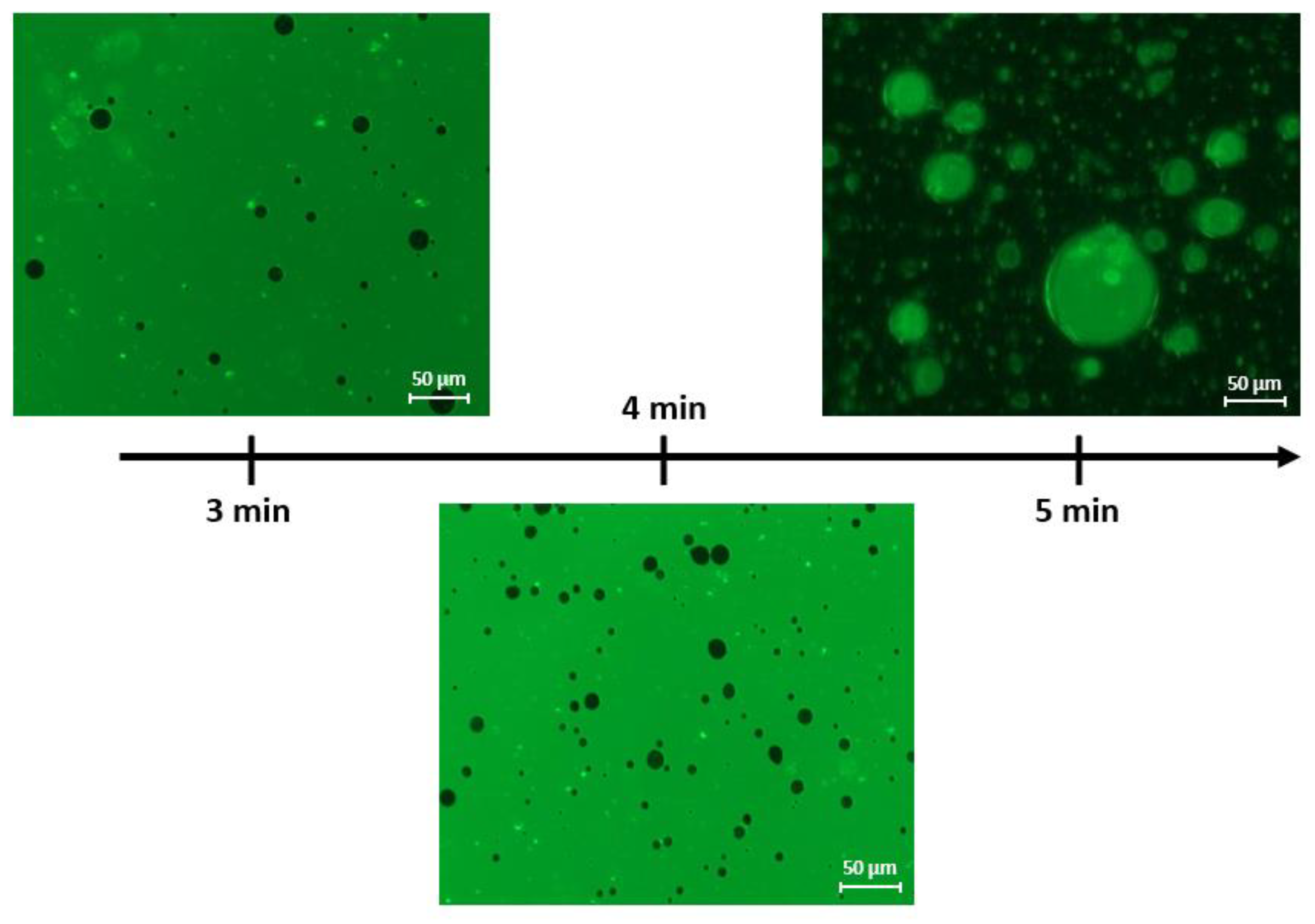
3.2.1. Stirring Time
3.2.2. Stirring Speed
3.2.3. Surfactant Structure
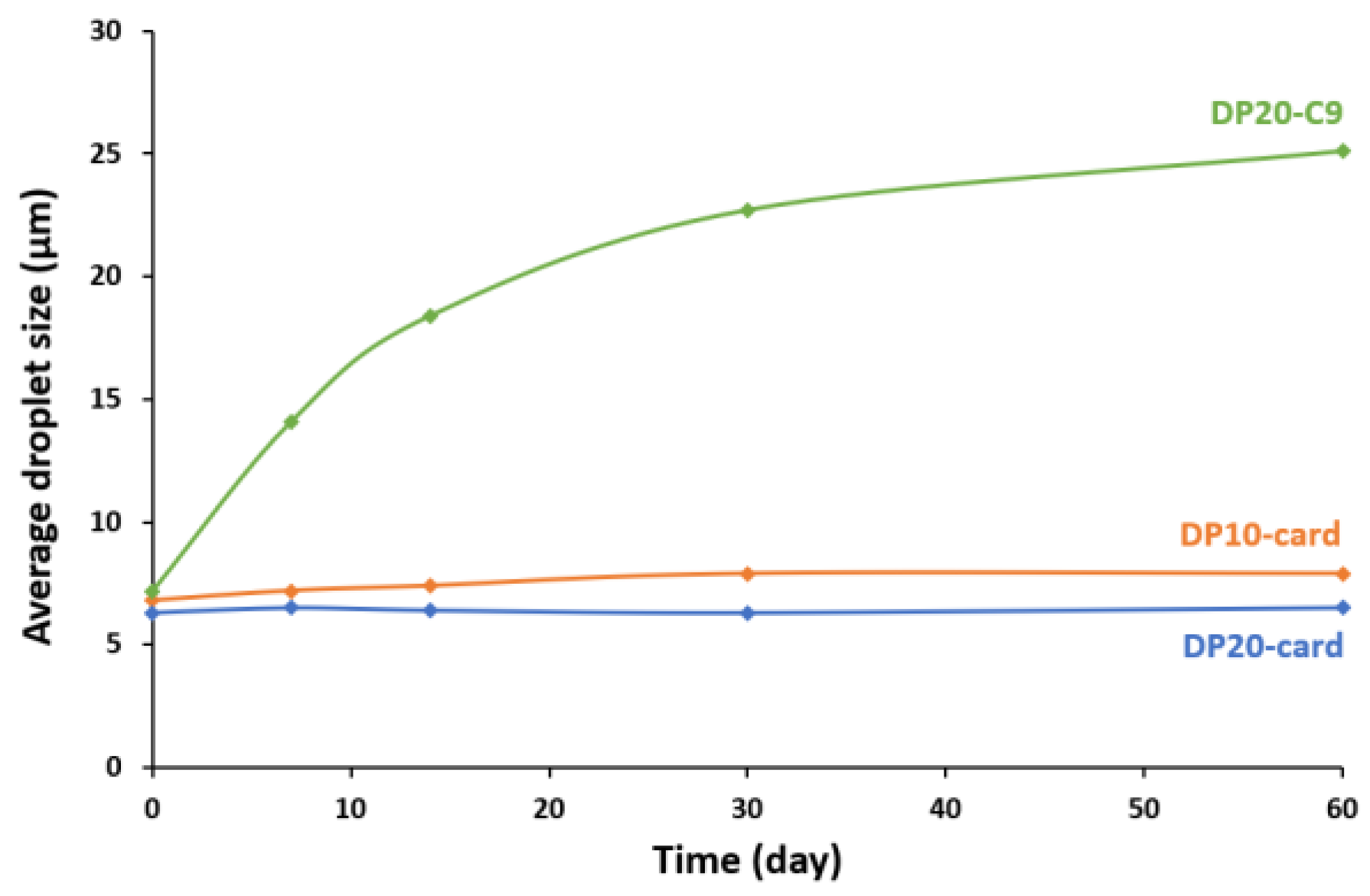
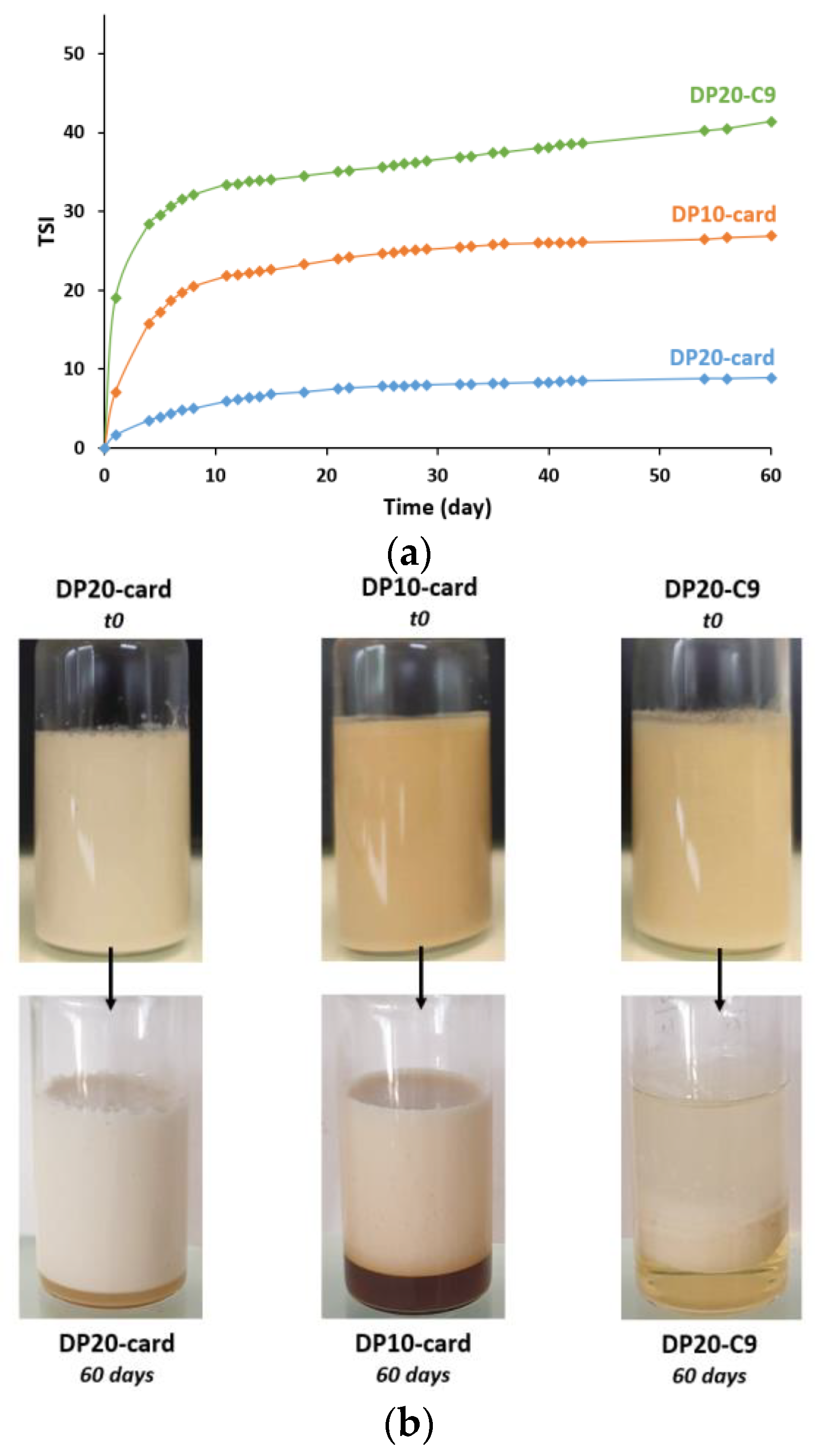
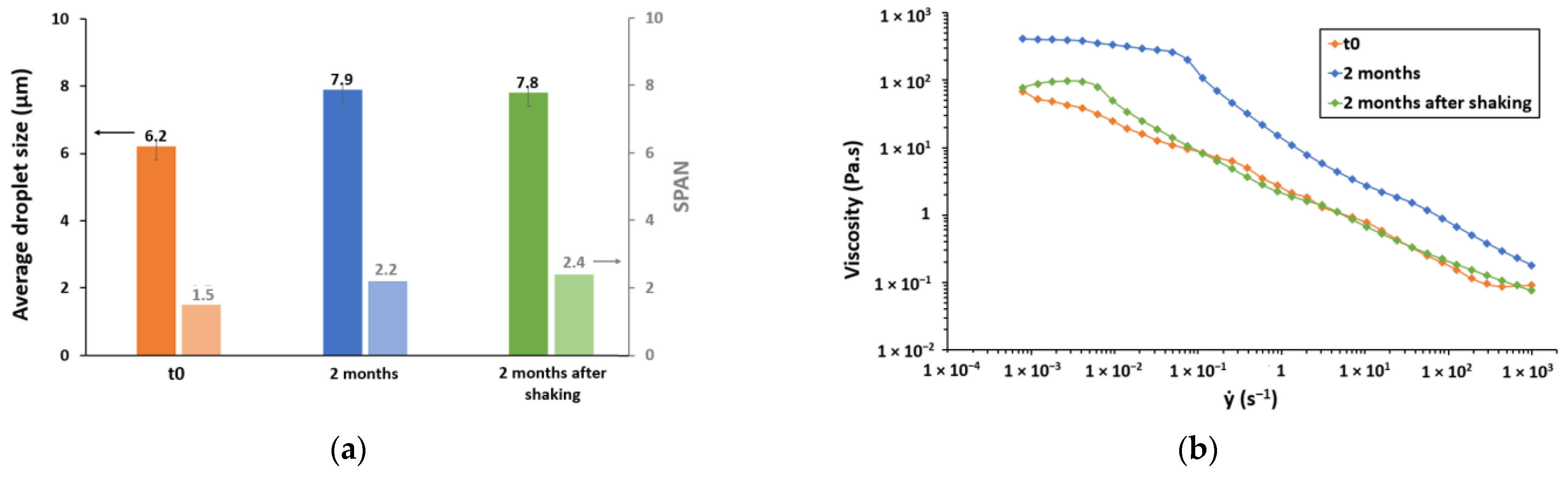
3.3. Stability over 2 Months
3.3.1. Aggregation and Fusion of Droplets
3.3.2. Gravitational Separation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pradilla, D.; Vargas, W.; Alvarez, O. The Application of a Multi-scale Approach to the Manufacture of Concentrated and Highly Concentrated Emulsion. Chem. Eng. Res. Des. 2015, 95, 162–172. [Google Scholar] [CrossRef]
- Briceno, M.; Salager, J.L.; Bertrand, J. Influence of Dispersed Phase Content and Viscosity on the Mixing of Concentrated Oil-in-Water Emulsion in the Transition Flow Regime. Chem. Eng. Res. Des. 2001, 79, 943–948. [Google Scholar] [CrossRef]
- Nor Hayati, I.; Bin Che Man, Y.; Ping Tan, C.; Nor Aini, I. Stability and Rheology of Concentrated O/W Emulsions Based on Soybean Oil/palm Kernel Olein Blends. Food Res. Int. 2007, 40, 1051–1061. [Google Scholar] [CrossRef]
- Atkin, R.; Craig, V.S.J.; Wanless, E.J.; Biggs, S. Mechanism of Cationic Surfactant Adsorption at the Solid–Aqueous Interface. Adv. Colloid Interface Sci. 2003, 103, 219–304. [Google Scholar] [CrossRef] [PubMed]
- Rebello, S.; Asok, A.K.; Mundayoor, S.; Jisha, M.S. Surfactants: Toxicity, Remediation and Green Surfactants. Environ. Chem. Lett. 2014, 12, 275–287. [Google Scholar] [CrossRef]
- Debois, D.; Fernandez, O.; Franzil, L.; Jourdan, E.; De Brogniez, A.; Willems, L.; Clément, C.; Dorey, S.; De Pauw, E.; Ongena, M. Plant Polysaccharides Initiate Underground Crosstalk with Bacilli by Inducing Synthesis of the Immunogenic Lipopeptide Surfactin: Bacillus Lipopeptides Induced by Plant Polymers. Environ. Microbiol. Rep. 2015, 7, 570–582. [Google Scholar] [CrossRef] [PubMed]
- Delbeke, E.; Van Geem, K. Sophorolipid Modification: The Power of Yeasts and Enzymes. In Lipid Modification by Enzymes and Engineered Microbes; Bornscheuer, U.T., Ed.; AOCS Press: Urbana, IL, USA, 2018; Chapter 14; pp. 315–341. [Google Scholar] [CrossRef]
- Bouyer, E.; Ghozlene, M.; Rosilio, V.; Grossiord, J.L.; Agnely, F. Proteins, Polysaccharides, and Their Complexes Used as Stabilizers for Emulsions: Alternatives to Synthetic Surfactants in the Pharmaceutical Field. Int. J. Pharm. 2012, 436, 359–378. [Google Scholar] [CrossRef] [PubMed]
- Adewuyi, A.; Göpfert, A.; Wolff, T. Properties of Sodium Phosphate-Hydroxy Ethanolamide Gemini Surfactant Synthesized from the Seed Oil of Luffa Cylindrical. Open Chem. 2013, 11, 1368–1380. [Google Scholar] [CrossRef]
- Farias, C.B.B.; Almeida, F.C.; Silva, I.A.; Souza, T.C.; Meira, H.M.; Rita de Cássia, F.; Luna, J.M.; Santos, V.A.; Converti, A.; Banat, I.M.; et al. Production of Green Surfactants: Market Prospects. Electron. J. Biotechnol. 2021, 51, 28–39. [Google Scholar] [CrossRef]
- Ribeiro, B.G.; Guerra, J.M.C.; Sarubbo, L.A. Biosurfactants: Production and application prospects in the food industry. Biotechnol. Progress 2020, 36, 5. [Google Scholar] [CrossRef]
- Satpute, S.K.; Kulkarni, G.R.; Banpurkar, A.G.; Banat, I.M.; Mone, N.S.; Patil, R.H.; Cameotra, S.S. Biosurfactant/s from Lactobacilli species: Properties, challenges and potential biomedical applications. J. Basic Microbiol. 2016, 56, 1140–1158. [Google Scholar] [CrossRef] [PubMed]
- Lsabee, M.Z.; Morsi, R.E.; Al-Sabagh, A.M. Surface active properties of chitosan and its derivatives. Colloids Surf. B Biointerfaces 2009, 74, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kou, S.; Peters, L.M.; Mucalo, M.R. Chitosan: A review of sources and preparation methods. Int. J. Biol. Macromol. 2021, 169, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, I.; Alcantara, A.R.; Civera, M.C.; Aria, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Majeti, N.V.; Kumar, R. A Review of Chitin and Chitosan Applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Luengo, G.S.; Leonforte, F.; Greaves, A.; Rubio, R.G.; Guzman, E. Phisico-chemical challenges on the self-assembly of natural and bio-based ingredients on hair surfaces: Towards sustainable haircare formulations. Green Chem. 2023, 25, 7863–7882. [Google Scholar] [CrossRef]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Elsaber Batiha, G.; Khafaga, A.F.; Abd El-Hakim, Y.M.; Al-Sagheer, A.A. Antimicrobial and Antioxidant Properties of Chitosan and Its Derivatives and Their Applications: A Review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef]
- Pestman, J.M. Carbohydrate-Derived Surfactants Containing an N-acylated Amine Functionality: Fundamental Aspects and Practical Applications. Ph.D. Thesis, University of Groningen, Groningen, The Netherlands, 1998. [Google Scholar]
- Rodriguez, M.S.; Albertengo, L.A.; Agullo, E. Emulsification Capacity of Chitosan. Carbohydr. Polym. 2002, 48, 271–276. [Google Scholar] [CrossRef]
- Oh, B.H.L.; Bismarck, A.; Chan-Park, M.B. Modified Chitosan Emulsifiers: Small Compositional Changes Produce Vastly Different High Internal Phase Emulsion Types. J. Mater. Chem. B 2015, 3, 4118–4122. [Google Scholar] [CrossRef]
- Chapelle, C.; David, G.; Caillol, S.; Negrell, C.; Durand, G.; Desroches Le Foll, M.; Trombotto, S. Water-Soluble 2,5-Anhydro-d-Mannofuranose Chain End Chitosan Oligomers of a Very Low Molecular Weight: Synthesis and Characterization. Biomacromolecules 2019, 20, 4353–4360. [Google Scholar] [CrossRef]
- Naveed, M.; Phil, L.; Sohail, M.; Hasnat, M.; Baig, M.M.F.A.; Ihsan, A.U.; Shumzaid, M.; Kakar, M.U.; Khan, T.M.; Akabar, M.D.; et al. Chitosan Oligosaccharide (COS): An Overview. Int. J. Biol. Macromol. 2019, 129, 827–843. [Google Scholar] [CrossRef] [PubMed]
- Qinghong, Z.; Li, Y.; Song, Y.; Li, J.; Wang, Z. Properties of Branched Alcohol Polyoxyethylene Ether Carboxylates. J. Mol. Liq. 2018, 258, 34–39. [Google Scholar] [CrossRef]
- Adkins, S.S.; Chen, X.; Nguyen, Q.P.; Sanders, A.W.; Johnston, K.P. Effect of Branching on the Interfacial Properties of Nonionic Hydrocarbon Surfactants at the Air–Water and Carbon Dioxide–Water Interfaces. J. Colloid Interface Sci. 2010, 346, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Wen, Y.; Deng, P.; Teng, F.; Zhou, F.; Xu, H.; Feng, S.; Zhu, L.; Feng, R. Linolenic Acid-Modified Methoxy Poly (Ethylene Glycol)-Oligochitosan Conjugate Micelles for Encapsulation of Amphotericin B. Carbohydr. Polym. 2019, 205, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Chapelle, C.; David, G.; Caillol, S.; Negrell, C.; Catrouillet, S.; Desroches Le Foll, M.; Azéma, N. Surfactant Properties of Chemically Modified Chitooligosaccharides and Their Potential Application in Bitumen Emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127327. [Google Scholar] [CrossRef]
- Chen, G.; Tao, D. An Experimental Study of Stability of Oil–Water Emulsion. Fuel Process Technol. 2005, 86, 499–508. [Google Scholar] [CrossRef]
- Thakur, R.K.; Villette, C.; Aubry, J.M.; Delaplace, G. Dynamic Emulsification and Catastrophic Phase Inversion of Lecithin-Based Emulsions. Colloids Surf. A Physicochem. Eng. Asp. 2008, 315, 285–293. [Google Scholar] [CrossRef]
- Gao, H.; Ma, L.; Cheng, C.; Liu, J.; Liang, R.; Zou, L.; Liu, W.; McClements, D.J. Review of Recent Advances in the Preparation, Properties, and Applications of High Internal Phase Emulsions. Trends Food Sci. Technol. 2021, 112, 36–49. [Google Scholar] [CrossRef]
- Grant, J.; Cho, J.; Allen, C. Self-Assembly and Physicochemical and Rheological Properties of a Polysaccharide−Surfactant System Formed from the Cationic Biopolymer Chitosan and Nonionic Sorbitan Esters. Langmuir 2006, 22, 4327–4335. [Google Scholar] [CrossRef]
- Long, X.; Qiu, Z.; Gong, H.; Zhu, C.; Li, Z.; Li, Y.; Dong, M. Rheological Behaviors of Microbial Polysaccharides with Different Substituents in Aqueous Solutions: Effects of Concentration, Temperature, Inorganic Salt and Surfactant. Carbohydr. Polym. 2019, 219, 162–171. [Google Scholar] [CrossRef]
- Chapelle, C.; David, G.; Caillol, S.; Negrell, C.; Durand, G.; Desroches Le Foll, M. Functionalization of Chitosan Oligomers: From Aliphatic Epoxide to Cardanol-Grafted Oligomers for Oil-in-Water Emulsions. Biomacromolecules 2021, 22, 846–854. [Google Scholar] [CrossRef] [PubMed]
- Mengual, O.; Meunier, G.; Cayre, I.; Puech, K.; Snabre, P. Characterisation of instability of concentrated dispersions by a new optical analyser: The TURBISCAN MA 1000. Colloids Surf. 1999, 152, 111–123. [Google Scholar] [CrossRef]
- Blazi, M.; Algieri, C. Application of Turbiscan Stability Index for the Preparation of Alumina Photocatalytic Membranes for Dye Removal. Membranes 2023, 13, 400. [Google Scholar] [CrossRef]
- Tesch, S.; Schubert, H. Influence of Increasing Viscosity of the Aqueous Phase on the Short-Term Stability of Protein Stabilized Emulsions. J. Food Eng. 2002, 52, 305–312. [Google Scholar] [CrossRef]
- Wang, Z.; Hui, L.; Zhennan, Z.; Baojiang, S.; Jianbo, Z.; Wenqiang, L. Research on the Effects of Liquid Viscosity on Droplet Size in Vertical Gas–Liquid Annular Flows. Chem. Eng. Sci. 2020, 220, 115621. [Google Scholar] [CrossRef]
- Kundu, P.; Kuma, V.; Mishra, I.M. Modeling the Steady-Shear Rheological Behavior of Dilute to Highly Concentrated Oil-in-Water (o/w) Emulsions: Effect of Temperature, Oil Volume Fraction and Anionic Surfactant Concentration. J. Pet. Sci. Eng. 2015, 129, 189–204. [Google Scholar] [CrossRef]
- Pal, R. Novel Viscosity Equations for Emulsions of Two Immiscible Liquids. J. Rheol. 2001, 45, 509–520. [Google Scholar] [CrossRef]
- Romero, A.; Perez-Puyana, V.; Marchal, P.; Choplin, L.; Guerrero, A. Emulsification Process Controlled by a Mixer Type Rheometer in O/W Protein-Based Emulsions. LWT-Food Sci. Technol. 2017, 76, 26–32. [Google Scholar] [CrossRef]
- Fontenot, K.; Schork, F.J. Sensitivities of Droplet Size and Stability in Monomeric Emulsions. Ind. Eng. Chem. Res. 1993, 32, 373–385. [Google Scholar] [CrossRef]
- Meinders, M.B.J.; Kloek, W.; van Vliet, T. Effect of Surface Elasticity on Ostwald Ripening in Emulsions. Langmuir 2001, 17, 3923–3929. [Google Scholar] [CrossRef]
- Ilia, A.; Abdurahman, N. Effect of Viscosity and Droplet Diameter on water-in-oil (w/o) Emulsion: An Experimental Study. World Acad. Sci. Eng. Technol. 2010, 62, 595–598. [Google Scholar] [CrossRef]
- Caubet, S.; Le Guer, Y.; Grassl, B.; El Omari, K.; Normandin, E. A Low-energy Emulsification Batch Mixer for Concentrated Oil-in-water Emulsions. AIChE J. 2011, 57, 27–39. [Google Scholar] [CrossRef]
- Feng, J.; Chen, Q.; Wu, X.; Jafari, S.M.; McClements, D.J. Formulation of Oil-in-water Emulsions for Pesticide Applications: Impact of Surfactant Type and Concentration on Physical Stability. Environ. Sci. Pollut. Res. 2018, 25, 21742–21751. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Sun, D.; Li, C.; Liu, Q.; Xu, J. Formation and Stability of Paraffin Oil-in-water Nano-emulsion Prepared by the Emulsion Inversion Point Method. J. Colloid Interface Sci. 2006, 303, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Sajjadi, S. Formation of Fine Emulsions by Emulsification at High Viscosity or Low Interfacial Tension: A Comparative Study. Colloids Surf. A Physicochem. Eng. Asp. 2007, 299, 73–78. [Google Scholar] [CrossRef]
- Pichot, R.; Spyropoulos, F.; Norton, I.T. Competitive adsorption of surfactants and hydrophilic silica particles at the oil-water interface: Interfacial tension and contact angle studies. J. Colloid Interface Sci. 2012, 377, 396–405. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, M.; Howe, A.J.; Clarke, S.M.; Routh, A.F. Heteroaggregation of water-in-oil droplets: The effects of relative internal solute concentrations and size. Soft Matter 2011, 7, 11216–11221. [Google Scholar] [CrossRef]
- Ghannam, M.T. Investigation of Thixotropy Behavior of Crude Oil-Polyacrylamide Emulsions. J. Appl. Polym. Sci. 2008, 12, 867–875. [Google Scholar] [CrossRef]
- Pal, R. Rheology of high internal phase ratio emulsions. Food Hydrocoll. 2006, 20, 997–1005. [Google Scholar] [CrossRef]
- Tyrode, E.; Allouche, J.; Choplin, L.; Salager, J.L. Emulsion catatrophic inversion from abnormal to normal morphology. Following the emulsion viscosity during three inversion protocols and extending the critical dispersed-phase concept. Ind. Eng. Chem. Res. 2005, 44, 67–74. [Google Scholar] [CrossRef]
- Subeen, K.; KyuHan, K.; Siyoung, Q.C. Controllable one-step double emulsion formation via phase inversion. Soft Matter 2018, 14, 1094–1099. [Google Scholar] [CrossRef]
- Mehrina, M.A.; Jafari, S.M.; Makhamal-Zadeh, B.S.; Maghsoudlou, Y. Crocin loaded nano-emulsion: Factors affecting emulsion properties in spontaneous emulsification. Int. J. Biol. Macromol. 2016, 84, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Brooks, B.W.; Richmond, H.N. Phase inversion in non-ionic surfactant-oil-water systems. Drop size studies in catastrophic inversion with turbulent mixing. Chem. Eng. Sci. 1994, 49, 1065–1075. [Google Scholar] [CrossRef]
- Terreros, A.; Gomez, P.G.; Lopez-Cabarcos, E. Influence of the surfactant chain length and the molecular weight of poly(oxyethylene) on the stability of oil-in-water concentrated emulsion. Prog. Colloid Polym. Sci. 2000, 115, 50–54. [Google Scholar] [CrossRef]
- Niraula, B.; Cok King, T.; Kok Chun, T.; Misran, M. Rheology Properties of Glucopyranoside Stabilized Oil-Water Emulsions: Effect of Alkyl Chain Length and Bulk Concentration of the Surfactant. Colloids Surf. A Physicochem. Eng. Asp. 2004, 251, 117–132. [Google Scholar] [CrossRef]
- Tadros, T. Application of rheology for assessment and prediction of the long-term physical stability of emulsions. Adv. Colloids Interface Sci. 2004, 108, 227–258. [Google Scholar] [CrossRef]


| Surfactant | DS (%) | HLB | Zeta Potential at pH7 (mV) | CMC (g·L−1) | Surface Tension at 1 g·L−1 |
|---|---|---|---|---|---|
| DP10-C9 | 29.4 | 14.8 | +3.2 ± 1.56 | 0.204 | 28.6 |
| DP10-C16 | 6.6 | 17.8 | +2.7 ± 1.37 | 0.756 | 51.8 |
| DP10-card | 23 | 13.4 | +2.9 ± 1.46 | 0.411 | 39.5 |
| DP20-C9 | 23.8 | 15.5 | +2.3 ± 1.67 | 0.602 | 42.2 |
| DP20-C16 | 1.2 | 19.5 | +4.2 ± 2.17 | 0.782 | 52.9 |
| DP20-card | 8.6 | 16.9 | +2.6 ± 1.62 | 0.706 | 43.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berthalon, S.; Frugier, J.; Azema, N.; Negrell, C.; David, G. Concentrated O/W Emulsion Stability of Non-Ionic Chitosan Oligomer Surfactants Modified by Epoxidized Fatty Chains at pH7: Influence of Emulsification Conditions. Polysaccharides 2024, 5, 67-84. https://doi.org/10.3390/polysaccharides5020005
Berthalon S, Frugier J, Azema N, Negrell C, David G. Concentrated O/W Emulsion Stability of Non-Ionic Chitosan Oligomer Surfactants Modified by Epoxidized Fatty Chains at pH7: Influence of Emulsification Conditions. Polysaccharides. 2024; 5(2):67-84. https://doi.org/10.3390/polysaccharides5020005
Chicago/Turabian StyleBerthalon, Steve, Jérémy Frugier, Nathalie Azema, Claire Negrell, and Ghislain David. 2024. "Concentrated O/W Emulsion Stability of Non-Ionic Chitosan Oligomer Surfactants Modified by Epoxidized Fatty Chains at pH7: Influence of Emulsification Conditions" Polysaccharides 5, no. 2: 67-84. https://doi.org/10.3390/polysaccharides5020005