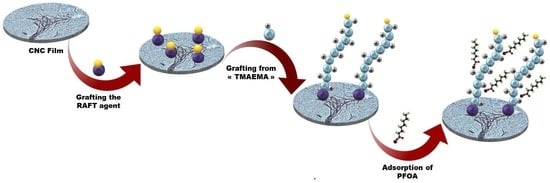
Surface Modification of Cellulose Nanocrystal Films via RAFT Polymerization for Adsorption of PFAS
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CNC Film
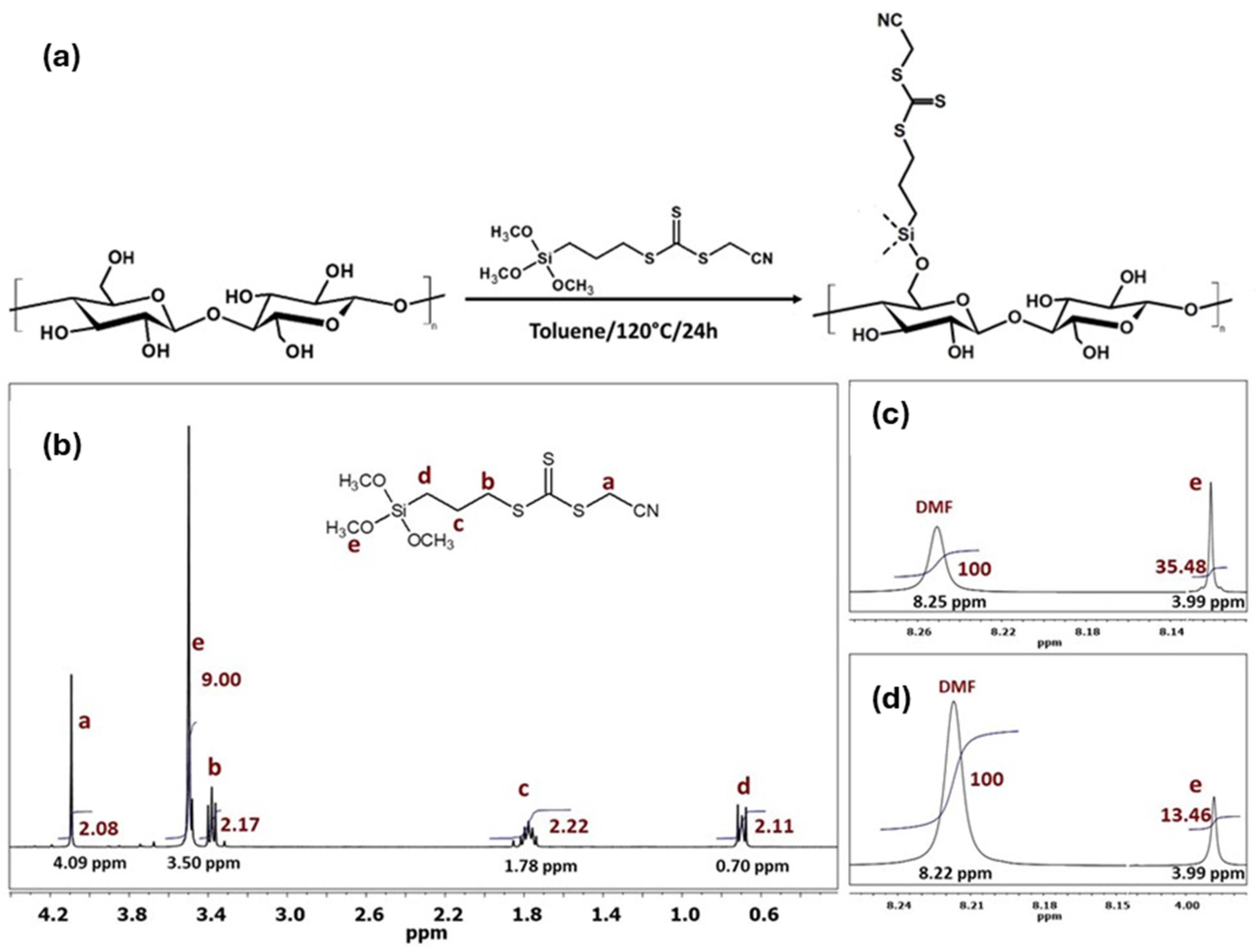
2.3. Modification of CNC Film with RAFT Agent (CNC-g-CTA)
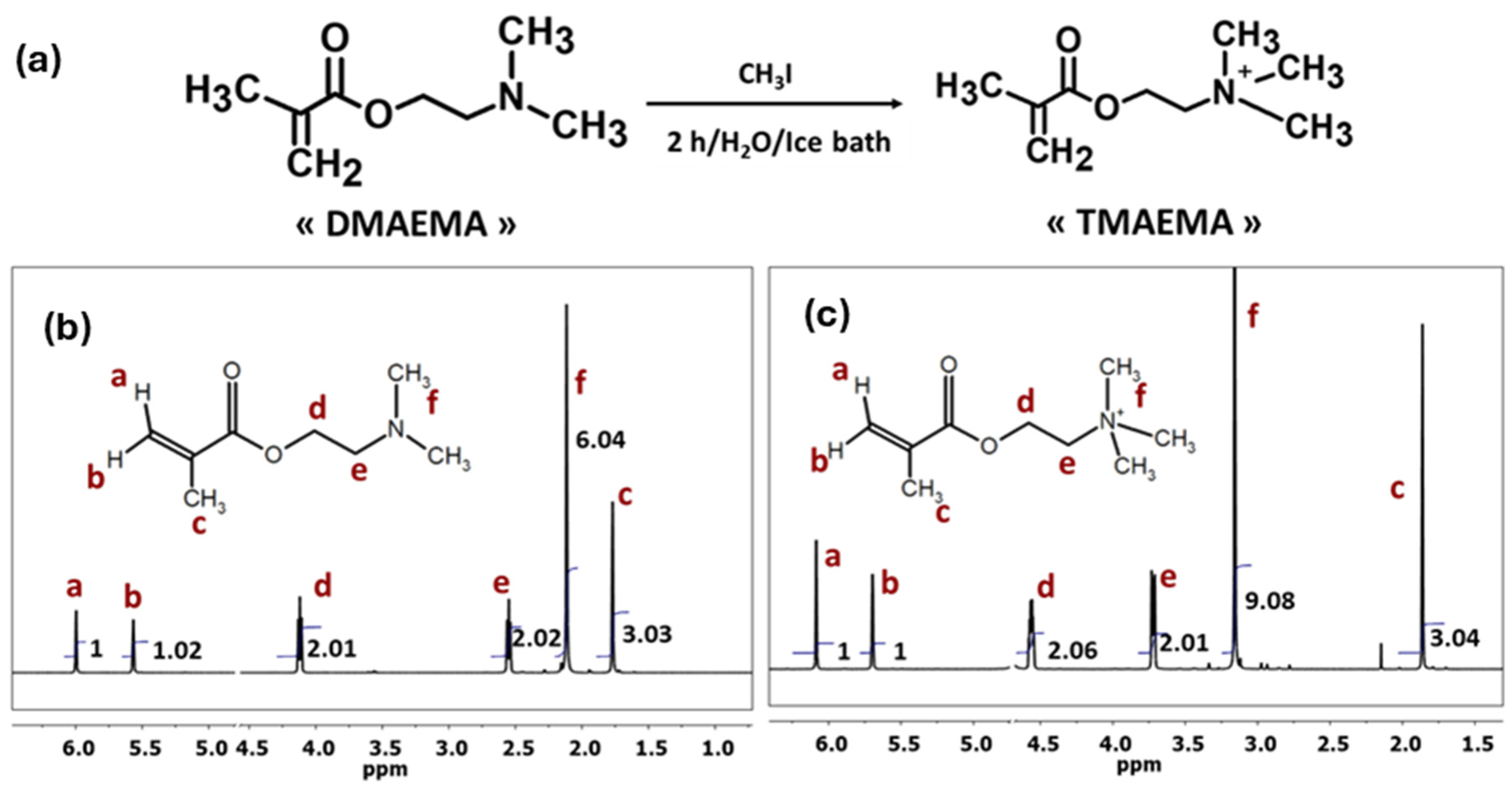
2.4. Quaternization of DMAEMA
2.5. Polymerization of TMAEMA from CNC-g-CTA
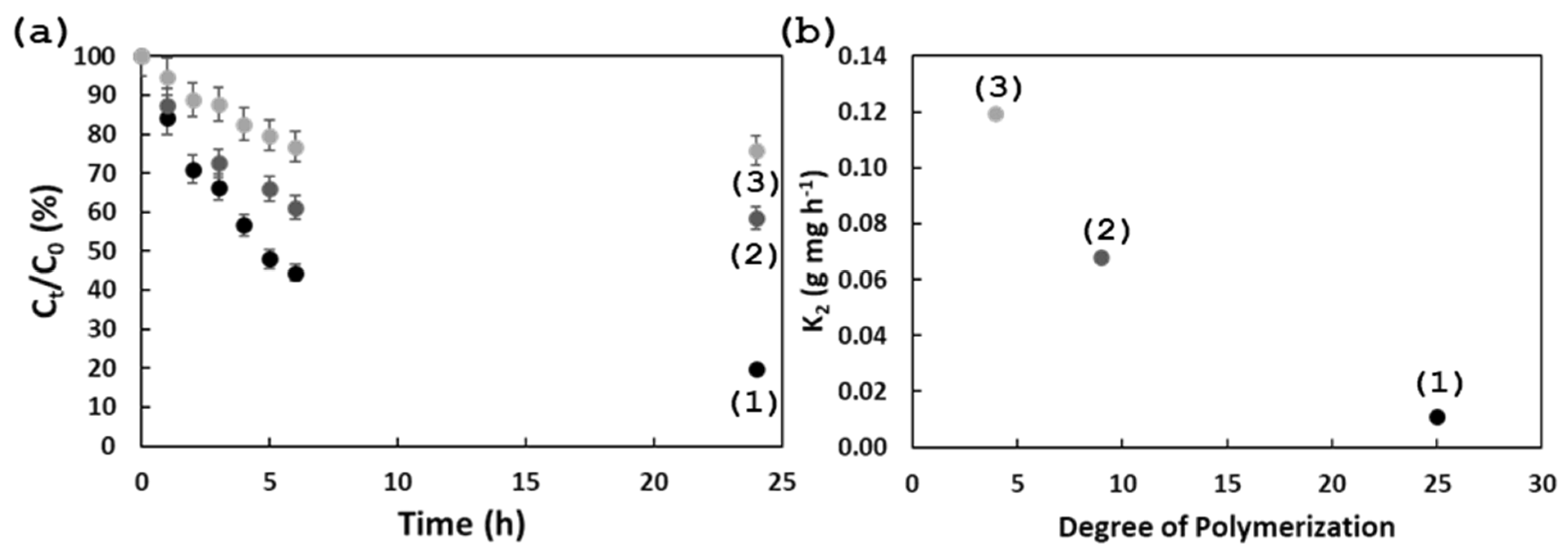
2.6. Adsorption Experiments
2.7. Characterization
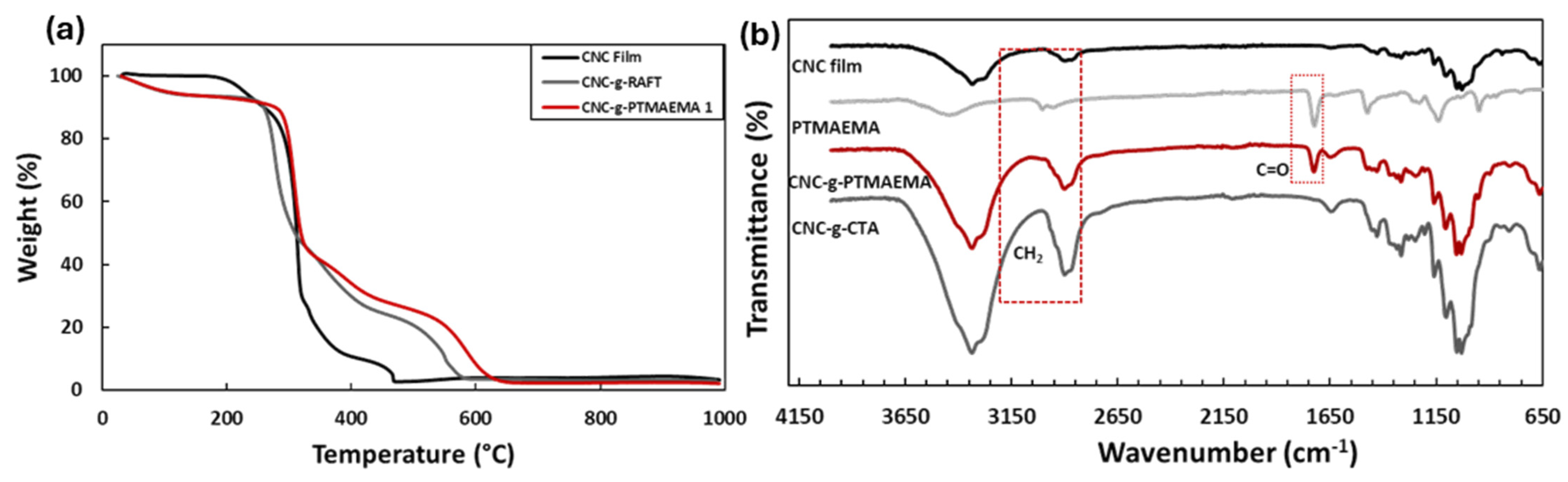
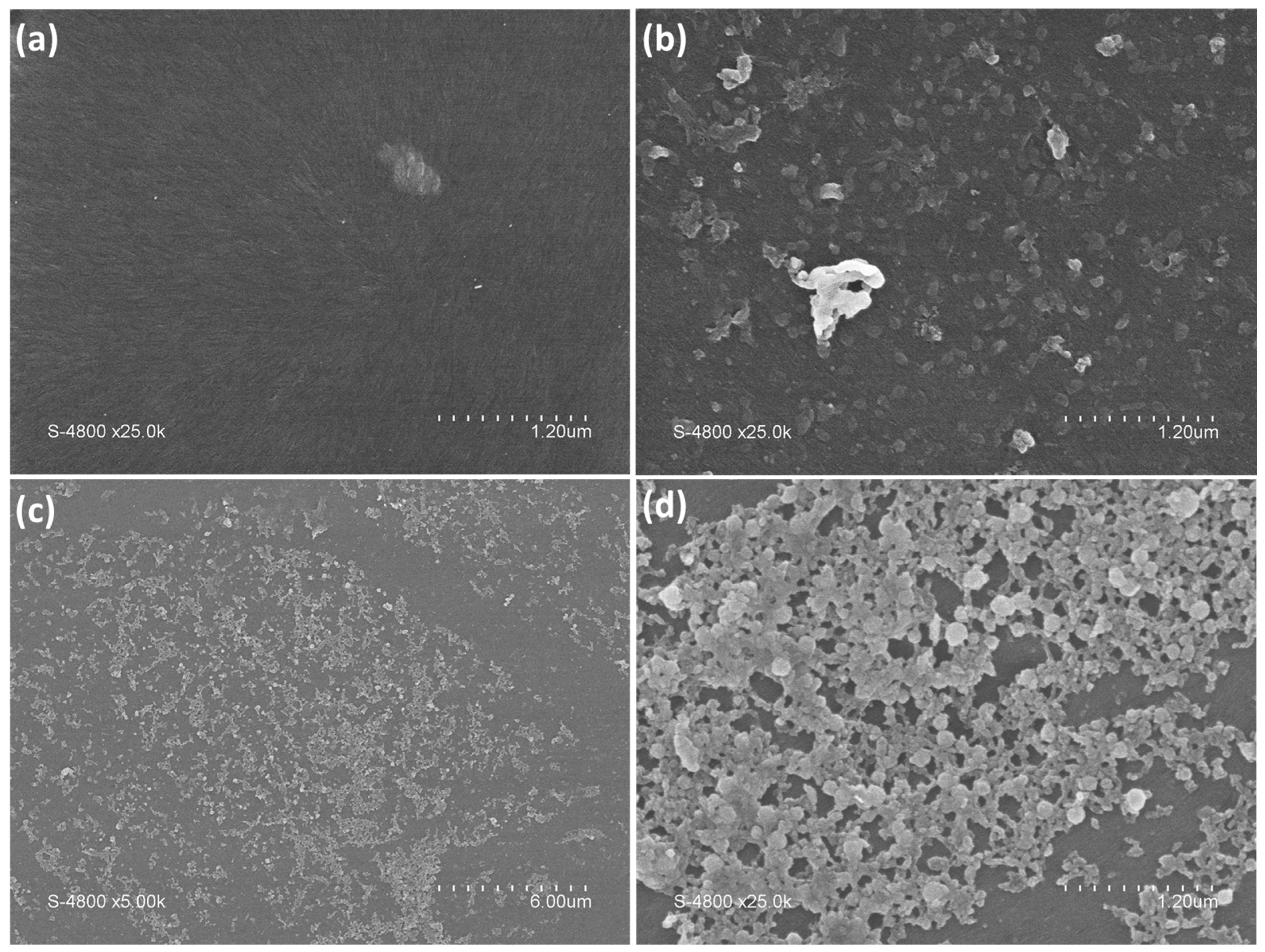
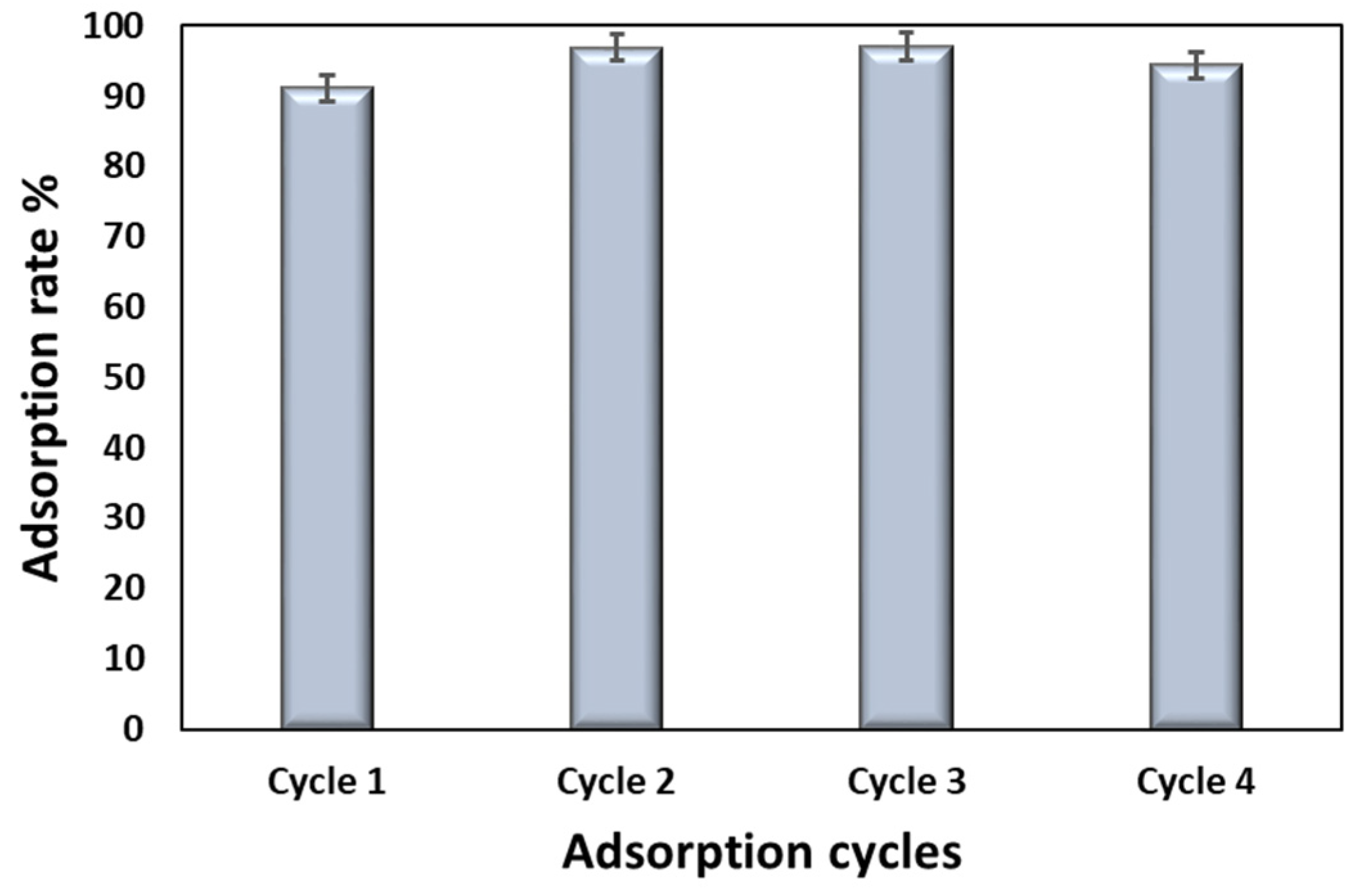
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Garcia-Valdez, O.; Champagne, P.; Cunningham, M.F. Graft modification of natural polysaccharides via reversible deactivation radical polymerization. Prog. Polym. Sci. 2018, 76, 151–173. [Google Scholar] [CrossRef]
- Lagerwall, J.P.F.; Schütz, C.; Salajkova, M.; Noh, J.; Park, J.H.; Scalia, G.; Bergström, L. Cellulose nanocrystal-based materials: From liquid crystal self-assembly and glass formation to multifunctional thin films. NPG Asia Mater. 2014, 6, e80. [Google Scholar] [CrossRef]
- George, J.; Sabapathi, S.N. Cellulose nanocrystals: Synthesis, functional properties, and applications. Nanotechnol. Sci. Appl. 2015, 8, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Roy, D.; Semsarilar, M.; Guthrie, J.T.; Perrier, S. Cellulose modification by polymer grafting: A review. Chem. Soc. Rev. 2009, 38, 2046–2064. [Google Scholar] [CrossRef] [PubMed]
- Semsarilar, M.; Perrier, S. “Green” reversible addition-fragmentation chain-transfer (RAFT) polymerization. Nat. Chem. 2010, 2, 811–820. [Google Scholar] [CrossRef]
- Oberlintner, A.; Likozar, B.; Novak, U. Hydrophobic functionalization reactions of structured cellulose nanomaterials: Mechanisms, kinetics and in silico multi-scale models. Carbohydr. Polym. 2021, 259, 117742. [Google Scholar] [CrossRef]
- Gomri, C.; Cretin, M.; Semsarilar, M. Recent progress on chemical modification of cellulose nanocrystal (CNC) and its application in nanocomposite films and membranes-A comprehensive review. Carbohydr. Polym. 2022, 294, 119790. [Google Scholar] [CrossRef] [PubMed]
- Arredondo, J.; Jessop, P.G.; Champagne, P.; Bouchard, J.; Cunningham, M.F. Synthesis of CO2-responsive cellulose nanocrystals by surface-initiated Cu(0)-mediated polymerisation. Green Chem. 2017, 19, 4141–4152. [Google Scholar] [CrossRef]
- Anžlovar, A.; Huskić, M.; Žagar, E. Modification of nanocrystalline cellulose for application as a reinforcing nanofiller in PMMA composites. Cellulose 2016, 23, 505–518. [Google Scholar] [CrossRef]
- Arredondo, J.; Woodcock, N.M.; Garcia-Valdez, O.; Jessop, P.G.; Champagne, P.; Cunningham, M.F. Surface modification of cellulose nanocrystals via RAFT polymerization of CO2-responsive monomer-tuning hydrophobicity. Langmuir 2020, 36, 13989–13997. [Google Scholar] [CrossRef]
- Eskandari, P.; Roghani-Mamaqani, H.; Salami-Kalajahi, M.; Abousalman-Rezvani, Z. Modification of cellulose nanocrystal with dual temperature- and CO2-responsive block copolymers for ion adsorption applications. J. Mol. Liq. 2020, 310, 113234. [Google Scholar] [CrossRef]
- Du, X.; Zhang, Z.; Liu, W.; Deng, Y. Nanocellulose-based conductive materials and their emerging applications in energy devices—A review. Nano Energy 2017, 35, 299–320. [Google Scholar] [CrossRef]
- Xu, C.; Chen, W.; Gao, H.; Xie, X.; Chen, Y. Cellulose nanocrystal/silver (CNC/Ag) thin-film nanocomposite nanofiltration membranes with multifunctional properties. Environ. Sci. Nano 2020, 7, 803–816. [Google Scholar] [CrossRef]
- Sucinda, E.F.; Abdul Majid, M.S.; Ridzuan, M.J.M.; Cheng, E.M.; Alshahrani, H.A.; Mamat, N. Development and characterisation of packaging film from Napier cellulose nanowhisker reinforced polylactic acid (PLA) bionanocomposites. Int. J. Biol. Macromol. 2021, 187, 43–53. [Google Scholar] [CrossRef]
- Qin, L.; Gao, H.; Xiong, S.; Jia, Y.; Ren, L. Preparation of collagen/cellulose nanocrystals composite films and their potential applications in corneal repair. J. Mater. Sci. Mater. Med. 2020, 31, 55. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Gao, P.; Deng, Y. Destruction of Per- A nd Polyfluoroalkyl Substances (PFAS) with Advanced Reduction Processes (ARPs): A Critical Review. Environ. Sci. Technol. 2020, 54, 3752–3766. [Google Scholar] [CrossRef]
- Domingo, J.L.; Nadal, M. Human exposure to per- and polyfluoroalkyl substances (PFAS) through drinking water: A review of the recent scientific literature. Environ. Res. 2019, 177, 108648. [Google Scholar] [CrossRef]
- Gomri, C.; Tarek, B.; Chaix, A.; Dorandeu, C.; Chopineau, J.; Petit, E.; Aissou, K.; Cot, D.; Cretin, M.; Semsarilar, M. A facile approach to modify cellulose nanocrystal for the adsorption of perfluorooctanoic acid. Carbohydr. Polym. 2023, 319, 121189. [Google Scholar] [CrossRef] [PubMed]
- Semsarilar, M.; Ladmiral, V.; Blanazs, A.; Armes, S.P. Cationic polyelectrolyte-stabilized nanoparticles via RAFT aqueous dispersion polymerization. Langmuir 2013, 29, 7416–7424. [Google Scholar] [CrossRef]
- Zhu, W.; Yao, Y.; Zhang, Y.; Jiang, H.; Wang, Z.; Chen, W.; Xue, Y. Preparation of an Amine-Modified Cellulose Nanocrystal Aerogel by Chemical Vapor Deposition and Its Application in CO2 Capture. Ind. Eng. Chem. Res. 2020, 59, 16660–16668. [Google Scholar] [CrossRef]
- Singh, S.; Dhakar, G.L.; Kapgate, B.P.; Maji, P.K.; Verma, C.; Chhajed, M.; Rajkumar, K.; Das, C. Synthesis and chemical modification of crystalline nanocellulose to reinforce natural rubber composites. Polym. Adv. Technol. 2020, 31, 3059–3069. [Google Scholar] [CrossRef]
- Anpilova, A.Y.; Mastalygina, E.E.; Khrameeva, N.P.; Popov, A.A. Methods for Cellulose Modification in the Development of Polymeric Composite Materials (Review). Russ. J. Phys. Chem. B 2020, 14, 176–182. [Google Scholar] [CrossRef]
- Zhang, D.; Luo, Q.; Gao, B.; Chiang, S.Y.D.; Woodward, D.; Huang, Q. Sorption of perfluorooctanoic acid, perfluorooctane sulfonate and perfluoroheptanoic acid on granular activated carbon. Chemosphere 2016, 144, 2336–2342. [Google Scholar] [CrossRef] [PubMed]


| Samples | m (g) | n (mmol) | Target Dp |
|---|---|---|---|
| CNC-g-PTMAEMA 1 | 0.2 | 0.66 | 40 |
| CNC-g-PTMAEMA 2 | 0.1 | 0.33 | 20 |
| CNC-g-PTMAEMA 3 | 0.05 | 0.16 | 10 |
| Samples | qe Experimental (mg g−1) | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|---|
| qe (mg g−1) | K1 (h−1) | R2 | qe (mg g−1) | K2 (g mg−1 h−1) | R2 | ||
| CNC-g-PTMAEMA 1 | 17.25 | 13.50 | 0.09140 | 0.96 | 19.01 | 0.01065 | 0.99 |
| CNC-g-PTMAEMA 2 | 7.95 | 3.87 | 0.08870 | 0.65 | 8.18 | 0.06766 | 0.99 |
| CNC-g-PTMAEMA 3 | 4.09 | 5.68 | 0.49040 | 0.87 | 4.21 | 0.11918 | 0.99 |
| Sample | Langmuir Model | Freundlich Model | ||||
|---|---|---|---|---|---|---|
| CNC-g-PTMAEMA 1 | KL (L mg−1) | qm (mg g−1) | R2 | Kf ((mg g−1) (L mg−1)1/n | 1/n | R2 |
| 0.0375 | 227 | 0.91 | 8.45 | 0.81 | 0.96 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomri, C.; Benkhaled, B.T.; Chaix, A.; Petit, E.; Cretin, M.; Semsarilar, M. Surface Modification of Cellulose Nanocrystal Films via RAFT Polymerization for Adsorption of PFAS. Polysaccharides 2024, 5, 85-95. https://doi.org/10.3390/polysaccharides5020006
Gomri C, Benkhaled BT, Chaix A, Petit E, Cretin M, Semsarilar M. Surface Modification of Cellulose Nanocrystal Films via RAFT Polymerization for Adsorption of PFAS. Polysaccharides. 2024; 5(2):85-95. https://doi.org/10.3390/polysaccharides5020006
Chicago/Turabian StyleGomri, Chaimaa, Belkacem Tarek Benkhaled, Arnaud Chaix, Eddy Petit, Marc Cretin, and Mona Semsarilar. 2024. "Surface Modification of Cellulose Nanocrystal Films via RAFT Polymerization for Adsorption of PFAS" Polysaccharides 5, no. 2: 85-95. https://doi.org/10.3390/polysaccharides5020006
APA StyleGomri, C., Benkhaled, B. T., Chaix, A., Petit, E., Cretin, M., & Semsarilar, M. (2024). Surface Modification of Cellulose Nanocrystal Films via RAFT Polymerization for Adsorption of PFAS. Polysaccharides, 5(2), 85-95. https://doi.org/10.3390/polysaccharides5020006