Antihypertensive Amaranth Protein Hydrolysates Encapsulation in Alginate/Pectin Beads: Influence on Bioactive Properties upon In Vitro Digestion
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Encapsulation by Ionic Gelation
2.3. Particle Size and Roundness Percentage
2.4. Encapsulation Efficiency
2.5. Thermogravimetric Analysis
2.6. Inhibitory Activity of ACE-I In Vitro
2.7. Simulated Gastrointestinal Digestion of Encapsulated Protein Hydrolysates
2.8. Statistical Analysis
3. Results and Discussion
3.1. Particle Size and Roundness Percentage
3.2. Encapsulation Efficiency (EE)
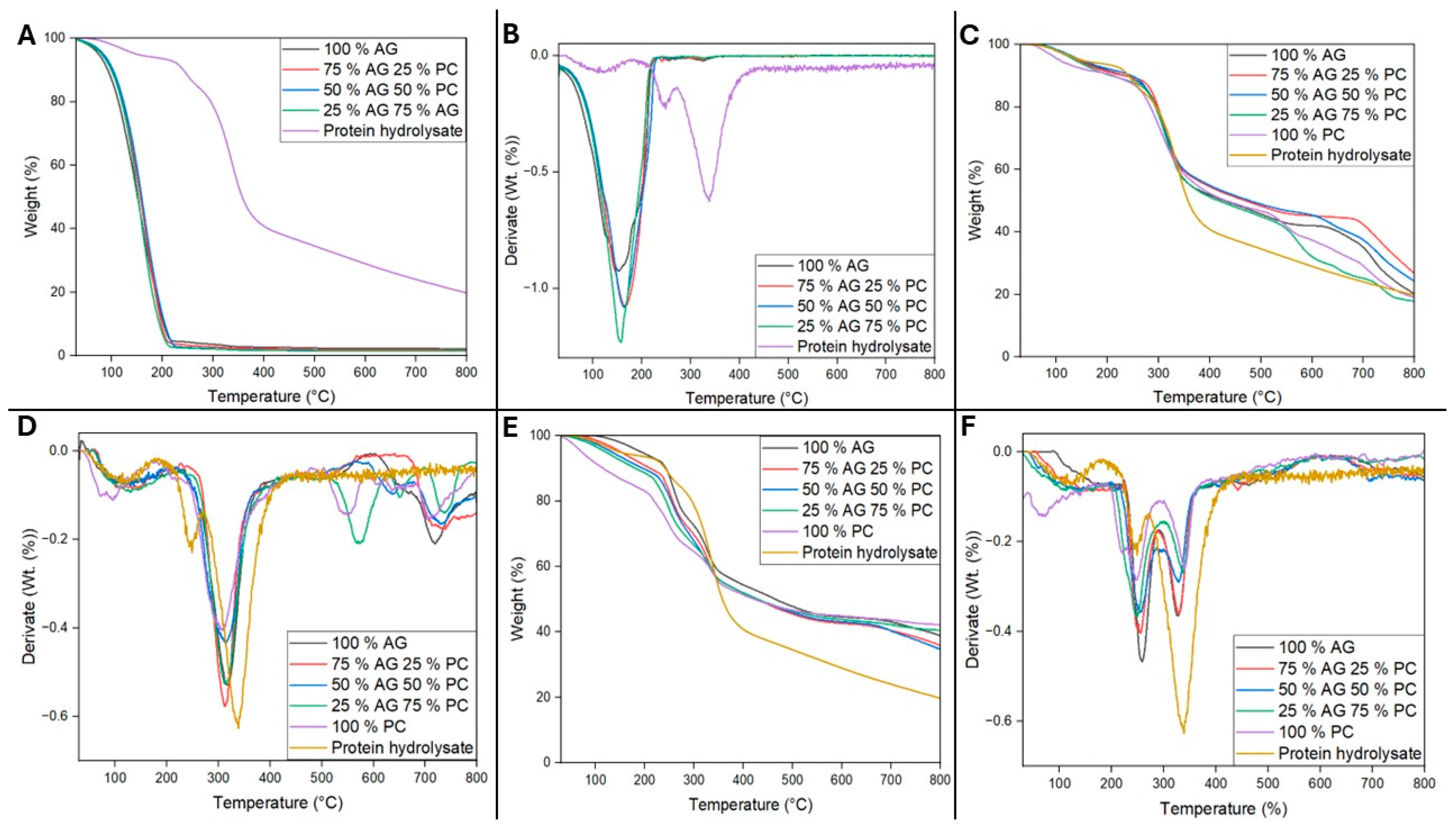
3.3. Thermogravimetric Analysis
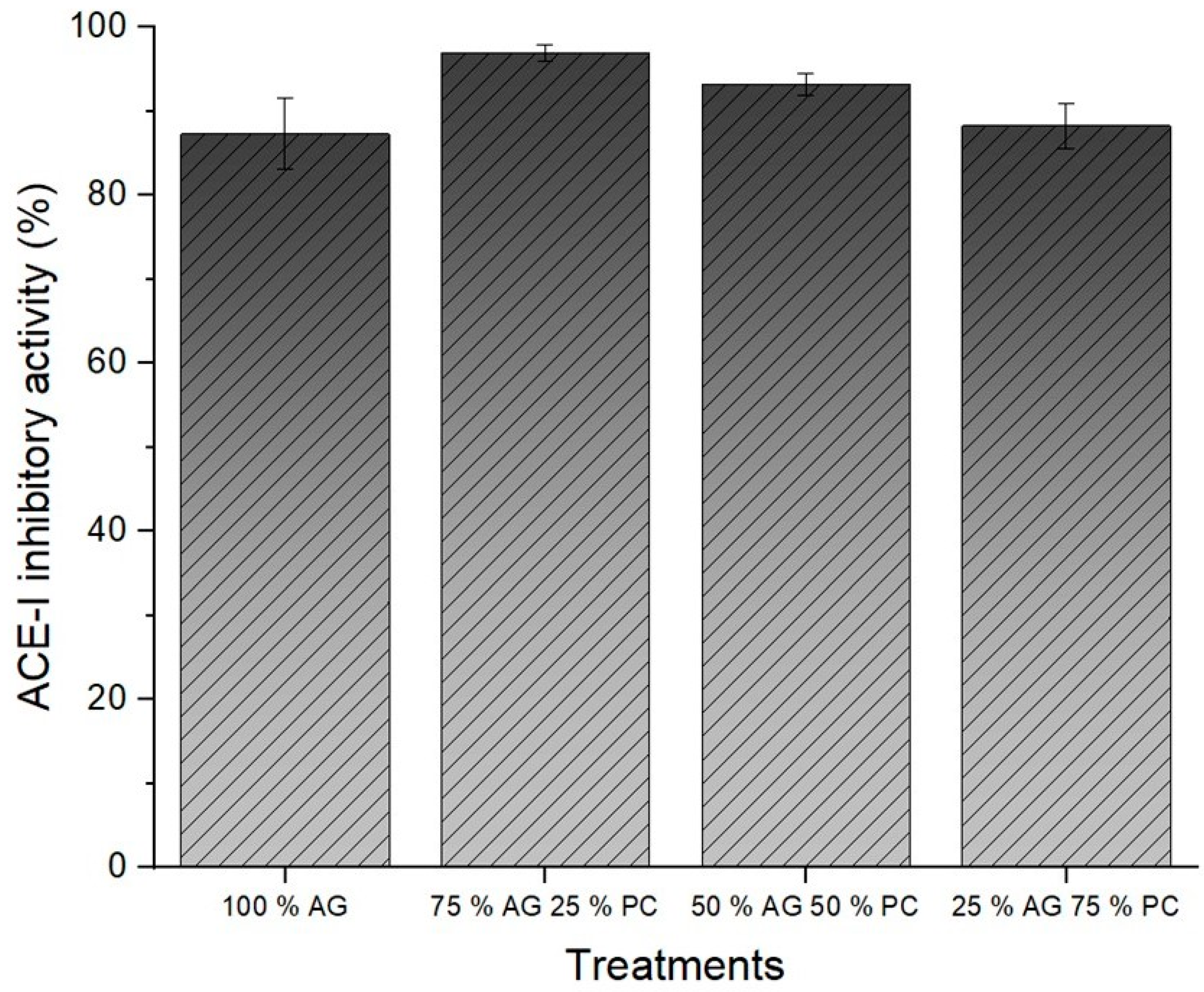
3.4. ACE-I Inhibitory Activity
3.5. Simulated Gastrointestinal Digestion of Encapsulated Protein Hydrolysates
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bekkering, C.S.; Tian, L. Thinking Outside of the Cereal Box: Breeding Underutilized (Pseudo)Cereals for Improved Human Nutrition. Front. Genet. 2019, 10, 1289. [Google Scholar] [CrossRef] [PubMed]
- Taniya, M.S.; Reshma, M.V.; Shanimol, P.S.; Krishnan, G.; Priya, S. Bioactive Peptides from Amaranth Seed Protein Hydrolysates Induced Apoptosis and Antimigratory Effects in Breast Cancer Cells. Food Biosci. 2020, 35, 100588. [Google Scholar] [CrossRef]
- Mekonnen, G.; Woldesenbet, M.; Teshale, T.; Biru, T. Amaranthus Caudatus Production and Nutrition Contents for Food Security and Healthy Living in Menit Shasha, Menit Goldya and Maji Districts of Bench Maji Zone, South Western Ethiopia. Nutr. Food Sci. Int. J. 2018, 7, 1–7. [Google Scholar] [CrossRef]
- Graziano, S.; Marando, S.; Prandi, B.; Boukid, F.; Marmiroli, N.; Francia, E.; Pecchioni, N.; Sforza, S.; Visioli, G.; Gullì, M. Technological Quality and Nutritional Value of Two Durum Wheat Varieties Depend on Both Genetic and Environmental Factors. J. Agric. Food Chem. 2019, 67, 2384–2395. [Google Scholar] [CrossRef] [PubMed]
- Jan, N.; Hussain, S.Z.; Naseer, B.; Bhat, T.A. Amaranth and Quinoa as Potential Nutraceuticals: A Review of Anti-Nutritional Factors, Health Benefits and Their Applications in Food, Medicinal and Cosmetic Sectors. Food Chem. X 2023, 18, 100687. [Google Scholar] [CrossRef]
- Kurek, M.A.; Karp, S.; Wyrwisz, J.; Niu, Y. Physicochemical Properties of Dietary Fibers Extracted from Gluten-Free Sources: Quinoa (Chenopodium quinoa), Amaranth (Amaranthus caudatus) and Millet (Panicum miliaceum). Food Hydrocoll. 2018, 85, 321–330. [Google Scholar] [CrossRef]
- Cruz-Casas, D.E.; Aguilar, C.N.; Ascacio-Valdés, J.A.; Rodríguez-Herrera, R.; Chávez-González, M.L.; Flores-Gallegos, A.C. Bioactive Protein Hydrolysates Obtained from Amaranth by Fermentation with Lactic Acid Bacteria and Bacillus Species. Heliyon 2023, 9, e13491. [Google Scholar] [CrossRef]
- Garbacz, K.; Wawrzykowski, J.; Czelej, M.; Czernecki, T.; Waśko, A. Recent Trends in the Application of Oilseed-Derived Protein Hydrolysates as Functional Foods. Foods 2023, 12, 3861. [Google Scholar] [CrossRef]
- Nikhita, R.; Sachindra, N.M. Optimization of Chemical and Enzymatic Hydrolysis for Production of Chicken Blood Protein Hydrolysate Rich in Angiotensin-I Converting Enzyme Inhibitory and Antioxidant Activity. Poult. Sci. 2021, 100, 101047. [Google Scholar] [CrossRef]
- Samaei, S.P.; Martini, S.; Tagliazucchi, D.; Gianotti, A.; Babini, E. Antioxidant and Angiotensin I-Converting Enzyme (ACE) Inhibitory Peptides Obtained from Alcalase Protein Hydrolysate Fractions of Hemp (Cannabis sativa L.) Bran. J. Agric. Food Chem. 2021, 69, 9220–9228. [Google Scholar] [CrossRef]
- Cruz-Chamorro, I.; Santos-Sánchez, G.; Álvarez-López, A.I.; Pedroche, J.; Lardone, P.J.; Arnoldi, A.; Lammi, C.; Carrillo-Vico, A. Pleiotropic Biological Effects of Lupinus Spp. Protein Hydrolysates. Trends Food Sci. Technol. 2023, 133, 244–266. [Google Scholar] [CrossRef]
- Heffernan, S.; Giblin, L.; O’Brien, N. Assessment of the Biological Activity of Fish Muscle Protein Hydrolysates Using In Vitro Model Systems. Food Chem. 2021, 359, 129852. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Pan, X.; Chi, C.F.; Sun, K.L.; Wang, B. Ten New Pentapeptides from Protein Hydrolysate of Miiuy Croaker (Miichthys miiuy) Muscle: Preparation, Identification, and Antioxidant Activity Evaluation. LWT-Food Sci. Technol. 2019, 105, 1–8. [Google Scholar] [CrossRef]
- Sánchez-López, F.; Robles-Olvera, V.J.; Hidalgo-Morales, M.; Tsopmo, A. Angiotensin-I Converting Enzyme Inhibitory Activity of Amaranthus hypochondriacus Seed Protein Hydrolysates Produced with Lactic Bacteria and Their Peptidomic Profiles. Food Chem. 2021, 363, 130320. [Google Scholar] [CrossRef]
- Tamang, J.P.; Cotter, P.D.; Endo, A.; Han, N.S.; Kort, R.; Liu, S.Q.; Mayo, B.; Westerik, N.; Hutkins, R. Fermented Foods in a Global Age: East Meets West. Compr. Rev. Food Sci. Food Saf. 2020, 19, 184–217. [Google Scholar] [CrossRef]
- Ozyurt, G.; Boga, M.; Uçar, Y.; Boga, E.K.; Polat, A. Chemical, Bioactive Properties and in Vitro Digestibility of Spray-Dried Fish Silages: Comparison of Two Discard Fish (Equulites klunzingeri and Carassius gibelio) Silages. Aquac. Nutr. 2018, 24, 998–1005. [Google Scholar] [CrossRef]
- Bao, C.; Jiang, P.; Chai, J.; Jiang, Y.; Li, D.; Bao, W.; Liu, B.; Liu, B.; Norde, W.; Li, Y. The Delivery of Sensitive Food Bioactive Ingredients: Absorption Mechanisms, Influencing Factors, Encapsulation Techniques and Evaluation Models. Food Res. Int. 2019, 120, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Gao, Z.; Chen, L.; Kang, L.; Huang, W.; Jin, M.; Wang, Q.; Bae, Y.H. Multifunctional Oral Delivery Systems for Enhanced Bioavailability of Therapeutic Peptides/Proteins. Acta Pharm. Sin. B 2019, 9, 902–922. [Google Scholar] [CrossRef]
- Devaraju, R.; Pushpadass, H.A.; Emerald, F.M.E.; Padaki, N.V.; Nath, B.S. Nanoencapsulation of Casein-Derived Peptides within Electrospun Nanofibres. J. Sci. Food Agric. 2021, 102, 1684–1698. [Google Scholar] [CrossRef]
- Yun, P.; Devahastin, S.; Chiewchan, N. Microstructures of Encapsulates and Their Relations with Encapsulation Efficiency and Controlled Release of Bioactive Constituents: A Review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 1768–1799. [Google Scholar] [CrossRef]
- Bodade, R.G.; Bodade, A.G. Microencapsulation of Bioactive Compounds and Enzymes for Therapeutic Applications; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128168981. [Google Scholar]
- Gheorghita, R.; Anchidin-Norocel, L.; Filip, R.; Dimian, M.; Covasa, M. Applications of Biopolymers for Drugs and Probiotics Delivery. Polymers 2021, 13, 2729. [Google Scholar] [CrossRef]
- Das, S. Pectin Based Multi-Particulate Carriers for Colon-Specific Delivery of Therapeutic Agents. Int. J. Pharm. 2021, 605, 120814. [Google Scholar] [CrossRef] [PubMed]
- Riseh, R.S.; Skorik, Y.A.; Thakur, V.K.; Pour, M.M.; Tamanadar, E.; Noghabi, S.S. Encapsulation of Plant Biocontrol Bacteria with Alginate as a Main Polymer Material. Int. J. Mol. Sci. 2021, 22, 11165. [Google Scholar] [CrossRef] [PubMed]
- Ayala-Niño, A.; Rodríguez-Serrano, G.M.; Jiménez-Alvarado, R.; Bautista-Avila, M.; Sánchez-Franco, J.A.; González-Olivares, L.G.; Cepeda-Saez, A. Bioactivity of Peptides Released during Lactic Fermentation of Amaranth Proteins with Potential Cardiovascular Protective Effect: An In Vitro Study. J. Med. Food 2019, 22, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Pamunuwa, G.; Anjalee, N.; Kukulewa, D.; Edirisinghe, C.; Shakoor, F.; Karunaratne, D.N. Tailoring of Release Properties of Folic Acid Encapsulated Nanoparticles via Changing Alginate and Pectin Composition in the Matrix. Carbohydr. Polym. Technol. Appl. 2020, 1, 100008. [Google Scholar] [CrossRef]
- Zhao, Q.Q.; Zhang, X.Y.; Tang, X.F.; Qiao, H. A Novel and Oral Colon Targeted Isoliquiritigenin Delivery System: Development, Optimization, Characterization and in Vitro Evaluation. J. Drug Deliv. Sci. Technol. 2021, 66, 102777. [Google Scholar] [CrossRef]
- Garzón, A.G.; Cian, R.E.; Drago, S.R. Effects of Agar-Carrageenan Wall Materials and Core-to-Wall Material Ratio on Physicochemical Properties and in Vitro Bioaccessibility of Microencapsulated Bioactive Peptides. Food Hydrocoll. 2023, 139, 108570. [Google Scholar] [CrossRef]
- Church, F.C.; Swaisgood, H.E.; Porter, D.H.; Catignani, G.L. Spectrophotometric Assay Using O-Phthaldialdehyde for Determination of Proteolysis in Milk and Isolated Milk Proteins. J. Dairy Sci. 1983, 66, 1219–1227. [Google Scholar] [CrossRef]
- Muhialdin, B.J.; Abdul Rani, N.F.; Meor Hussin, A.S. Identification of Antioxidant and Antibacterial Activities for the Bioactive Peptides Generated from Bitter Beans (Parkia speciosa) via Boiling and Fermentation Processes. LWT-Food Sci. Technol. 2020, 131, 109776. [Google Scholar] [CrossRef]
- Díaz-Gómez, J.L.; Neundorf, I.; López-Castillo, L.M.; Castorena-Torres, F.; Serna-Saldívar, S.O.; García-Lara, S. In Silico Analysis and In Vitro Characterization of the Bioactive Profile of Three Novel Peptides Identified from 19 KDa α-Zein Sequences of Maize. Molecules 2020, 25, 5405. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST Static in Vitro Simulation of Gastrointestinal Food Digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef] [PubMed]
- Sampaio, G.L.A.; Pacheco, S.; Ribeiro, A.P.O.; Galdeano, M.C.; Gomes, F.S.; Tonon, R.V. Encapsulation of a Lycopene-Rich Watermelon Concentrate in Alginate and Pectin Beads: Characterization and Stability. LWT 2019, 116, 108589. [Google Scholar] [CrossRef]
- Stachowiak, N.; Kowalonek, J.; Kozlowska, J. Freeze-Dried Matrices Composed of Degradable Polymers with Surfactant-Loaded Microparticles Based on Pectin and Sodium Alginate. Materials 2021, 14, 3044. [Google Scholar] [CrossRef]
- Santagapita, P.R.; Mazzobre, M.F.; Buera, M. del P. Invertase Stability in Alginate Beads. Effect of Trehalose and Chitosan Inclusion and of Drying Methods. Food Res. Int. 2012, 47, 321–330. [Google Scholar] [CrossRef]
- Alvarado, Y.; Muro, C.; Illescas, J.; del Carmen Díaz, M.; Riera, F. Encapsulation of Antihypertensive Peptides from Whey Proteins and Their Releasing in Gastrointestinal Conditions. Biomolecules 2019, 9, 164. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Al-Assaf, S.; Phillips, G.O.; Nishinari, K.; Funami, T.; Williams, P.A. Binding Behavior of Calcium to Polyuronates: Comparison of Pectin with Alginate. Carbohydr. Polym. 2008, 72, 334–341. [Google Scholar] [CrossRef]
- Vivek, K.; Mishra, S.; Pradhan, R.C.; Nagarajan, M.; Kumar, P.K.; Singh, S.S.; Manvi, D.; Gowda, N.N. A Comprehensive Review on Microencapsulation of Probiotics: Technology, Carriers and Current Trends. Appl. Food Res. 2023, 3, 100248. [Google Scholar] [CrossRef]
- López, D.N.; Ingrassia, R.; Busti, P.; Bonino, J.; Delgado, J.F.; Wagner, J.; Boeris, V.; Spelzini, D. Structural Characterization of Protein Isolates Obtained from Chia (Salvia hispanica L.) Seeds. LWT 2018, 90, 396–402. [Google Scholar] [CrossRef]
- Ricci, L.; Umiltà, E.; Righetti, M.C.; Messina, T.; Zurlini, C.; Montanari, A.; Bronco, S.; Bertoldo, M. On the Thermal Behavior of Protein Isolated from Different Legumes Investigated by DSC and TGA. J. Sci. Food Agric. 2018, 98, 5368–5377. [Google Scholar] [CrossRef]
- Paswan, M.; Chandel, A.K.S.; Malek, N.I.; Dholakiya, B.Z. Preparation of Sodium Alginate/Cur-PLA Hydrogel Beads for Curcumin Encapsulation. Int. J. Biol. Macromol. 2024, 254, 128005. [Google Scholar] [CrossRef]
- Ayouch, I.; Barrak, I.; Kassab, Z.; El Achaby, M.; Barhoun, A.; Draoui, K. Impact of the Drying Process on the Efficiency of Alginate Beads for Cadmium Removal from Water: Kinetic, Isotherm and Thermodynamic Study. Environ. Technol. Innov. 2020, 20, 101157. [Google Scholar] [CrossRef]
- Wang, W.; Ma, X.; Jiang, P.; Hu, L.; Zhi, Z.; Chen, J.; Ding, T.; Ye, X.; Liu, D. Characterization of Pectin from Grapefruit Peel: A Comparison of Ultrasound-Assisted and Conventional Heating Extractions. Food Hydrocoll. 2016, 61, 730–739. [Google Scholar] [CrossRef]
- Fasolin, L.H.; Santana, R.C.; Cunha, R.L. Influence of Organic Acids on Surfactant Self-Assemblies in Surfactant/Oil/Water Systems. Colloids Surf. A Physicochem. Eng. Asp. 2014, 459, 290–297. [Google Scholar] [CrossRef]
- Freitas, C.M.P.; Coimbra, J.S.R.; Souza, V.G.L.; Sousa, R.C.S. Structure and Applications of Pectin in Food, Biomedical, and Pharmaceutical Industry: A Review. Coatings 2021, 11, 922. [Google Scholar] [CrossRef]
- Sen, O.; Manna, S.; Nandi, G.; Jana, S.; Jana, S. Recent Advances in Alginate Based Gastroretentive Technologies for Drug Delivery Applications. Med. Nov. Technol. Devices 2023, 18, 100236. [Google Scholar] [CrossRef]
- Alvarado-Pérez, Y.; Muro-Urista, C.; Illescas-Martínez, J.; Díaz-Nava, M.D.C.; Riera-Rodríguez, F.A. Functionalized Polymers for Enhance Oral Bioavailability of Sensitive Molecules. Polymers 2016, 8, 214. [Google Scholar] [CrossRef]
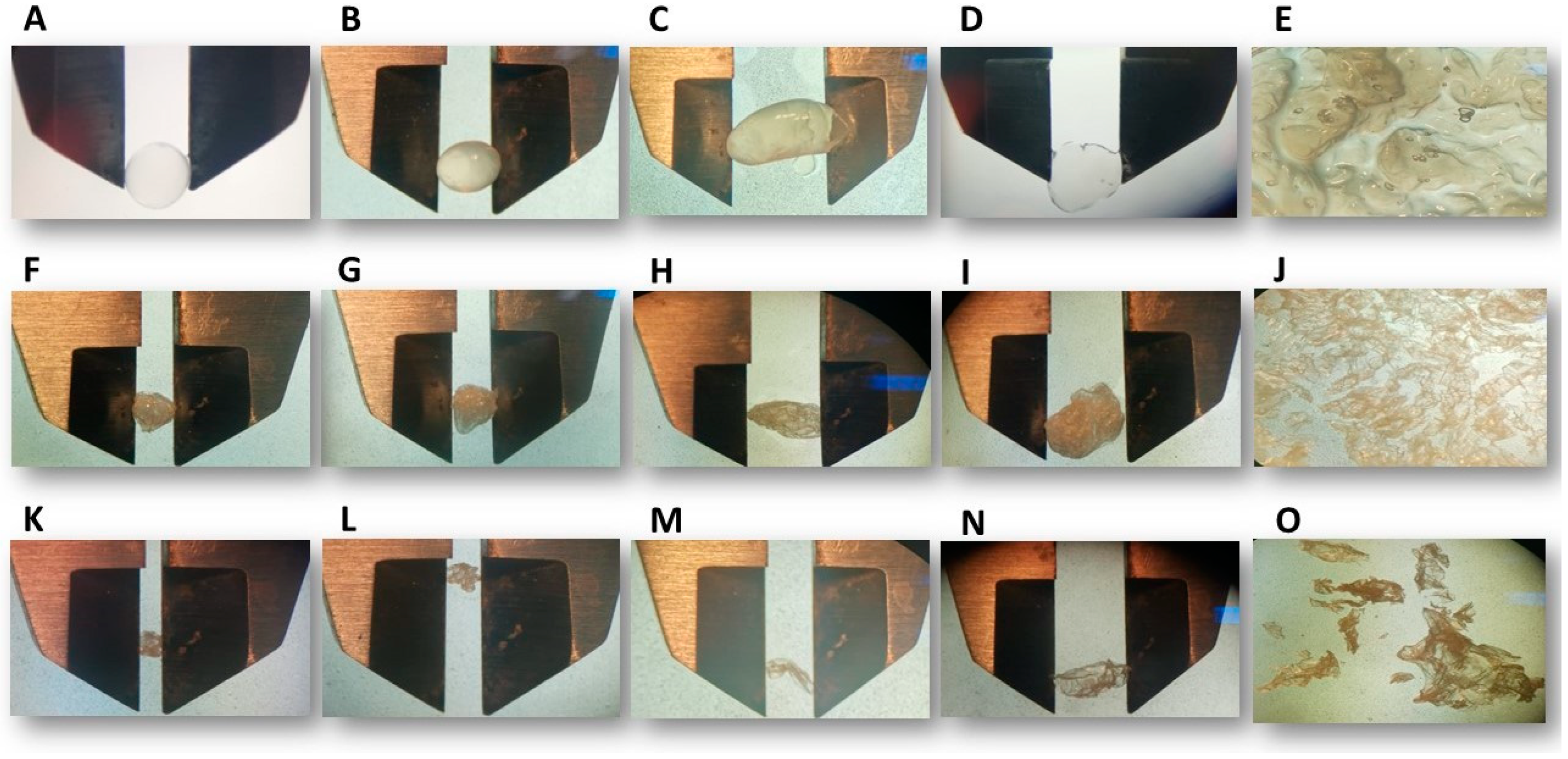

| Sample | Encapsulating Biopolymer Treatment (%) | Dmax (mm) | Dmin (mm) | Mean (mm) | RD (%) |
|---|---|---|---|---|---|
| Wet | 100 AG | 3.7 ± 0.2 | 3.5 ± 0.2 | 3.6 ± 0.2 a | 94.0 ± 4.3 a |
| 75 AG 25 PC | 4.2 ± 0.4 | 3.5 ± 0.3 | 3.9 ± 0.3 ab | 85.0 ± 8.4 ab | |
| 50 AG 50 PC | 4.4 ± 0.7 | 3.6 ± 0.5 | 4.0 ± 0.5 b | 82.1 ± 12.8 bc | |
| 25 AG 75 PC | 6.7 ± 1.1 | 4.9 ± 1.0 | 5.84 ± 0.90 c | 73.9 ± 15.0 cd | |
| 100 PC | - | - | - | - | |
| Freeze-dried | 100 AG | 3.0 ± 0.6 | 2.5 ± 0.9 | 2.7 ± 0.5 a | 83.8 ± 10.6 a |
| 75 AG 25 PC | 3.6 ± 0.8 | 2.6 ± 0.6 | 3.1 ± 0.6 a | 72.5 ± 14.5 bc | |
| 50 AG 50 PC | 4.7 ± 1.1 | 3.1 ± 0.7 | 3.9 ± 0.8 bc | 68.0 ± 18.2 c | |
| 25 AG 75 PC | 5.2 ± 1.3 | 3.7 ± 1.0 | 4.4 ± 0.9 c | 73.1 ± 18.4 bc | |
| 100 PC | - | - | - | - | |
| Air-drying | 100 AG | 2.0 ± 0.3 | 1.5 ± 0.3 | 1.8 ± 0.2 a | 71.7 ± 15.2 a |
| 75 AG 25 PC | 1.9 ± 0.3 | 1.3 ± 0.2 | 1.6 ± 0.2 a | 68.7 ± 15.1 a | |
| 50 AG 50 PC | 3.1 ± 0.8 | 1.9 ± 0.6 | 2.5 ± 0.5 b | 65.0 ± 21.5 a | |
| 25 AG 75 PC | 3.1 ± 1.1 | 2.4 ± 0.8 | 3.0 ± 0.9 c | 65.6 ± 16.0 a | |
| 100 PC | - | - | - | - |
| Digestion Phase | Peptide Release (mg/mL) | ACE-I Inhibition (%) | ||||
|---|---|---|---|---|---|---|
| 100% AG | 75% AG 25% PC | Non-Encapsulated | 100% AG | 75% AG 25% PC | Non-Encapsulated | |
| Oral | 0.005 ± 0.001 c | 0.007 ± 0.003 b | 0.050 ± 0.003 c | 9.97 ± 11.33 | 23.68 ± 10.05 | 0 ± 0 |
| Gastric | 0.142 ± 0.005 b | 0.108 ± 0.006 b | 0.126 ± 0.007 b | 0 ± 0 | 17.46 ± 5.03 | 0 ± 0 |
| Intestinal | 0.532 ± 0.091 a | 0.548 ± 0.015 a | 0.515 ± 0.059 a | 19.93 ± 6.30 | 16.10 ± 8.77 | 0 ± 0 |
| Total | 0.679 ± 0.097 | 0.663 ± 0.024 | 0.691 ± 0.069 | 29.9 ± 17.63 | 57.24 ± 23.85 | 0 ± 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz-Casas, D.E.; Ramos-González, R.; Prado-Barragán, L.A.; Aguilar, C.N.; Rodríguez-Herrera, R.; Iliná, A.; Esparza-González, S.C.; Flores-Gallegos, A.C. Antihypertensive Amaranth Protein Hydrolysates Encapsulation in Alginate/Pectin Beads: Influence on Bioactive Properties upon In Vitro Digestion. Polysaccharides 2024, 5, 450-462. https://doi.org/10.3390/polysaccharides5030028
Cruz-Casas DE, Ramos-González R, Prado-Barragán LA, Aguilar CN, Rodríguez-Herrera R, Iliná A, Esparza-González SC, Flores-Gallegos AC. Antihypertensive Amaranth Protein Hydrolysates Encapsulation in Alginate/Pectin Beads: Influence on Bioactive Properties upon In Vitro Digestion. Polysaccharides. 2024; 5(3):450-462. https://doi.org/10.3390/polysaccharides5030028
Chicago/Turabian StyleCruz-Casas, Dora Elisa, Rodolfo Ramos-González, Lilia Arely Prado-Barragán, Cristóbal N. Aguilar, Raúl Rodríguez-Herrera, Anna Iliná, Sandra Cecilia Esparza-González, and Adriana Carolina Flores-Gallegos. 2024. "Antihypertensive Amaranth Protein Hydrolysates Encapsulation in Alginate/Pectin Beads: Influence on Bioactive Properties upon In Vitro Digestion" Polysaccharides 5, no. 3: 450-462. https://doi.org/10.3390/polysaccharides5030028
APA StyleCruz-Casas, D. E., Ramos-González, R., Prado-Barragán, L. A., Aguilar, C. N., Rodríguez-Herrera, R., Iliná, A., Esparza-González, S. C., & Flores-Gallegos, A. C. (2024). Antihypertensive Amaranth Protein Hydrolysates Encapsulation in Alginate/Pectin Beads: Influence on Bioactive Properties upon In Vitro Digestion. Polysaccharides, 5(3), 450-462. https://doi.org/10.3390/polysaccharides5030028










