Ultrasound-Assisted Process to Increase the Hydrophobicity of Cellulose from Oat Hulls by Surface Modification with Vegetable Oils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cellulose Extraction
2.3. Cellulose Modification with Vegetable Oils
2.4. Cellulose, Hemicellulose, and Lignin Contents
2.5. Fourier Transform Infrared Spectroscopy (FTIR)
2.6. X-ray Diffraction (XRD)
2.7. Scanning Electron Microscopy (SEM)
2.8. Wettability
2.9. Oil Absorption Capacity (OAC)
2.10. Water Absorption Capacity (WAC)
2.11. Moisture Adsorption Isotherms
2.12. Moisture Adsorption Kinetics
2.13. Statistical Analysis
3. Results and Discussion
3.1. Cellulose Extracted Content
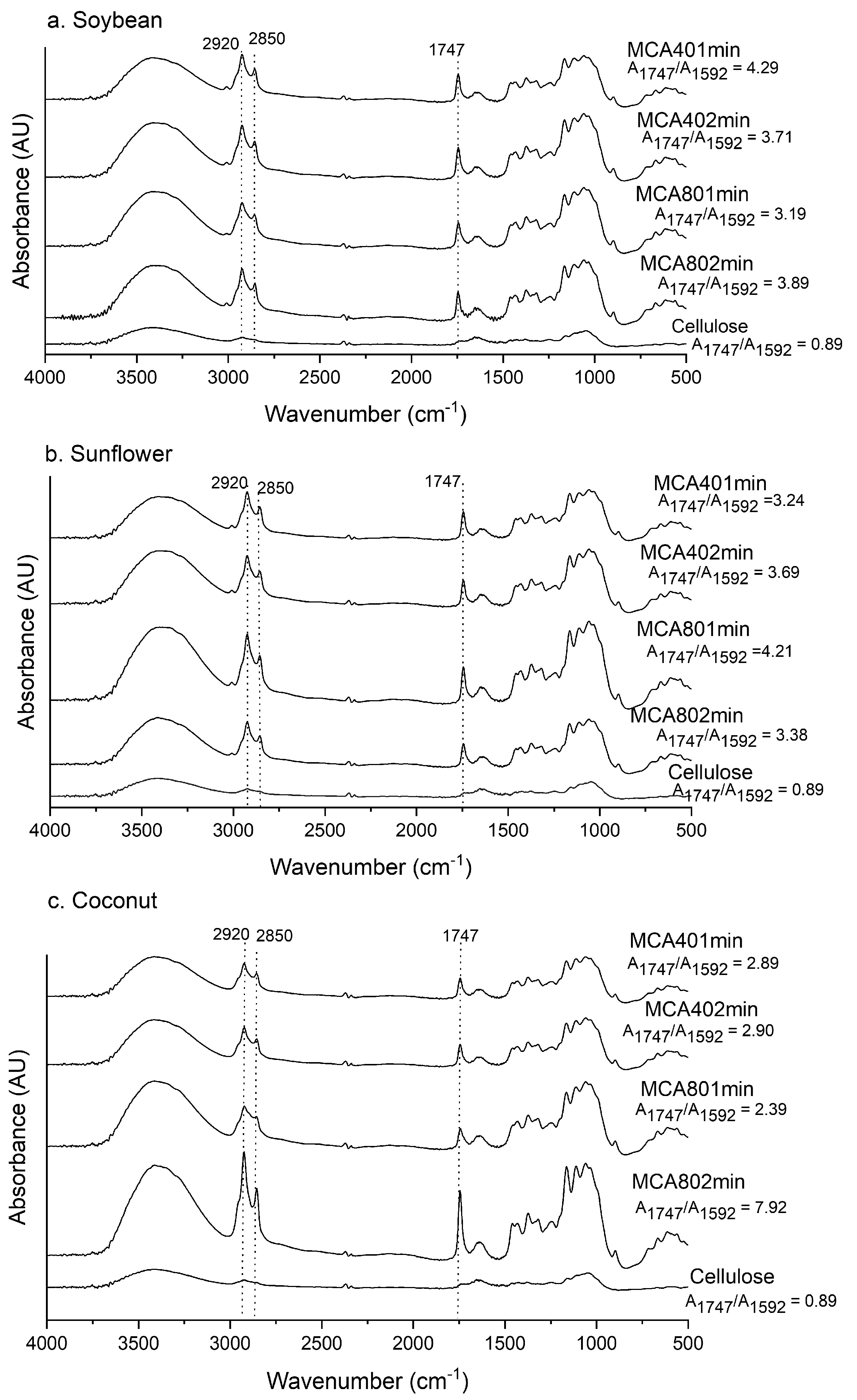
3.2. Fourier-Transform Infrared Spectroscopy (FTIR)
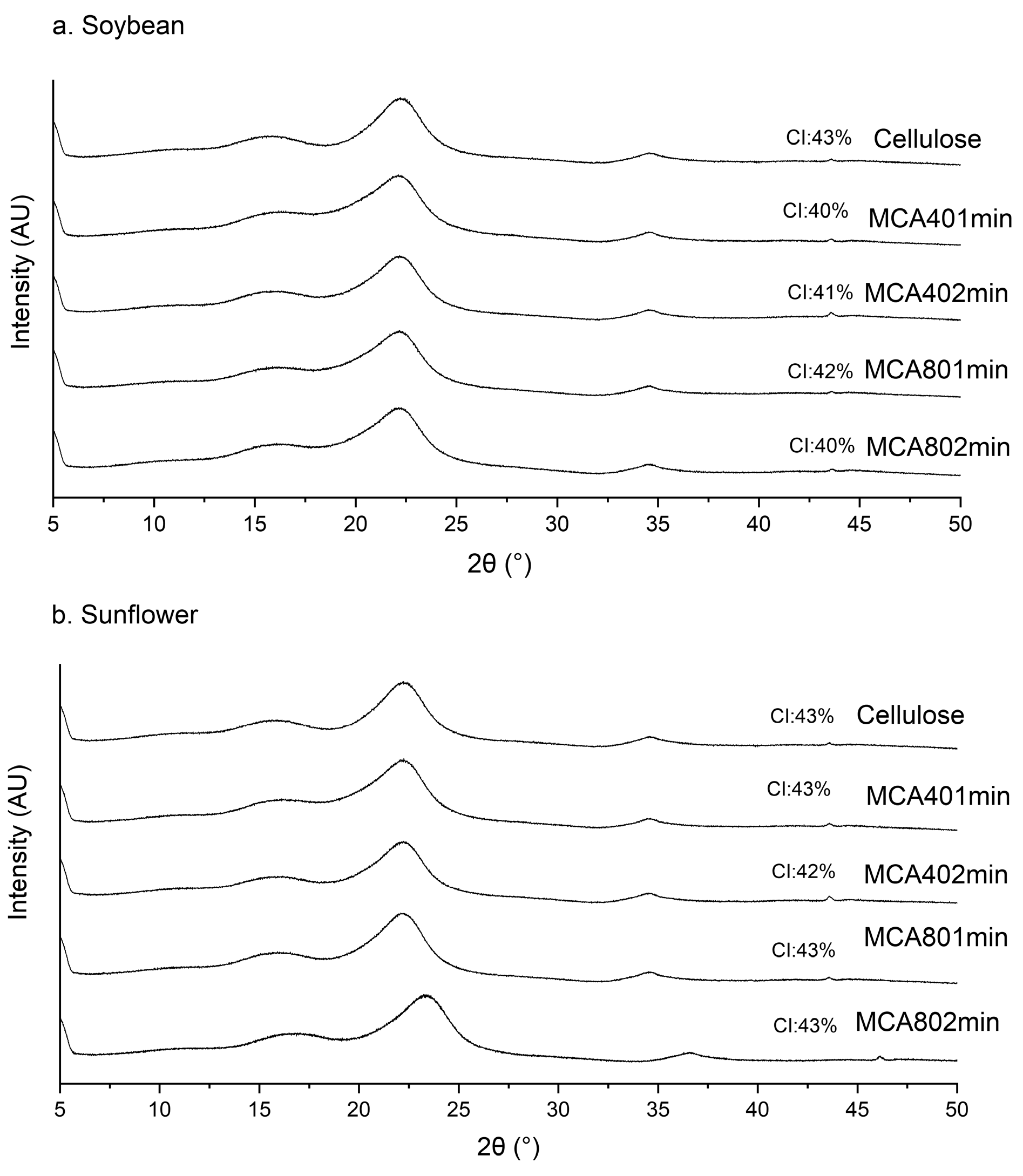
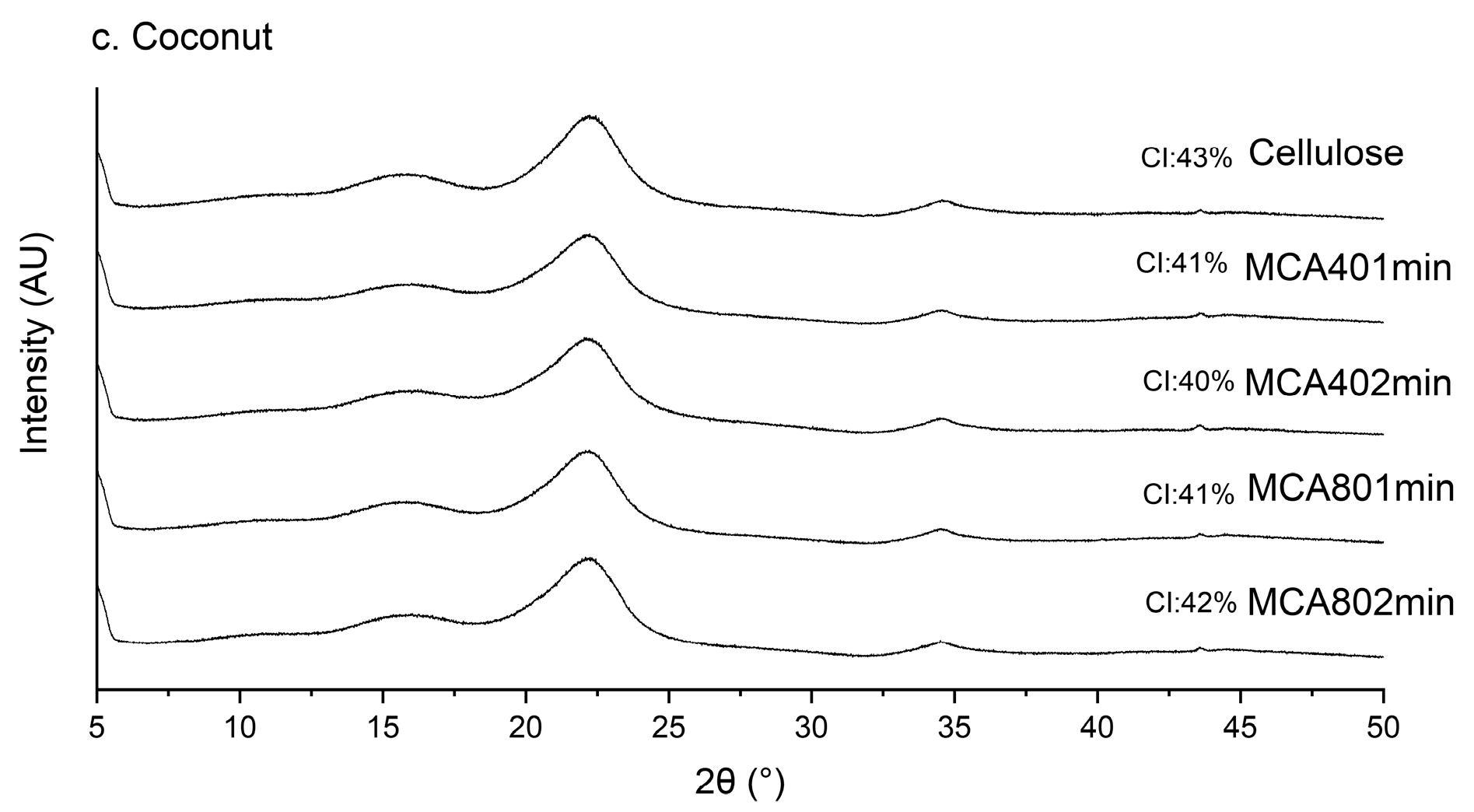
3.3. X-ray Diffraction (XRD)
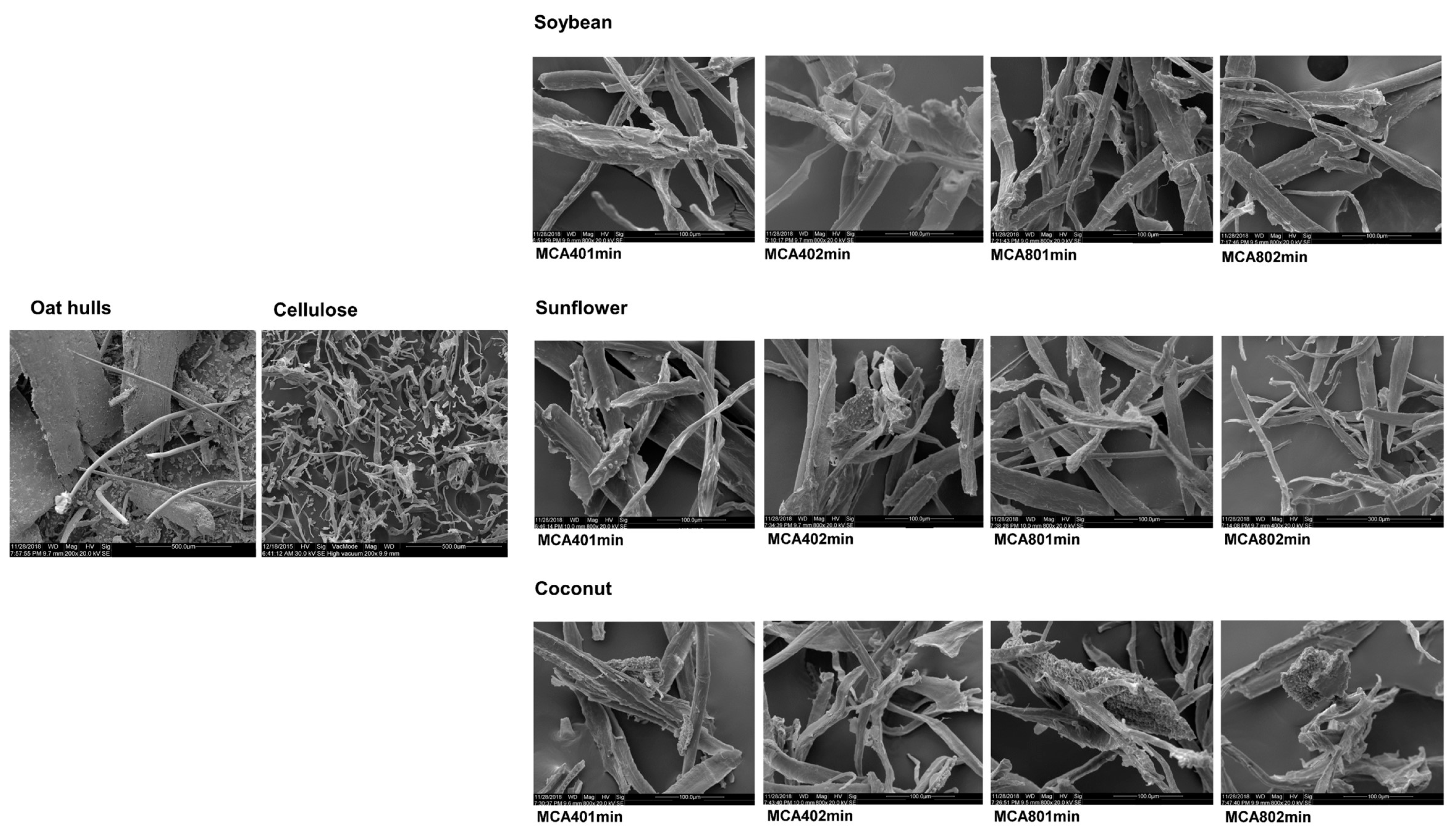
3.4. Scanning Electron Microscopy (SEM)
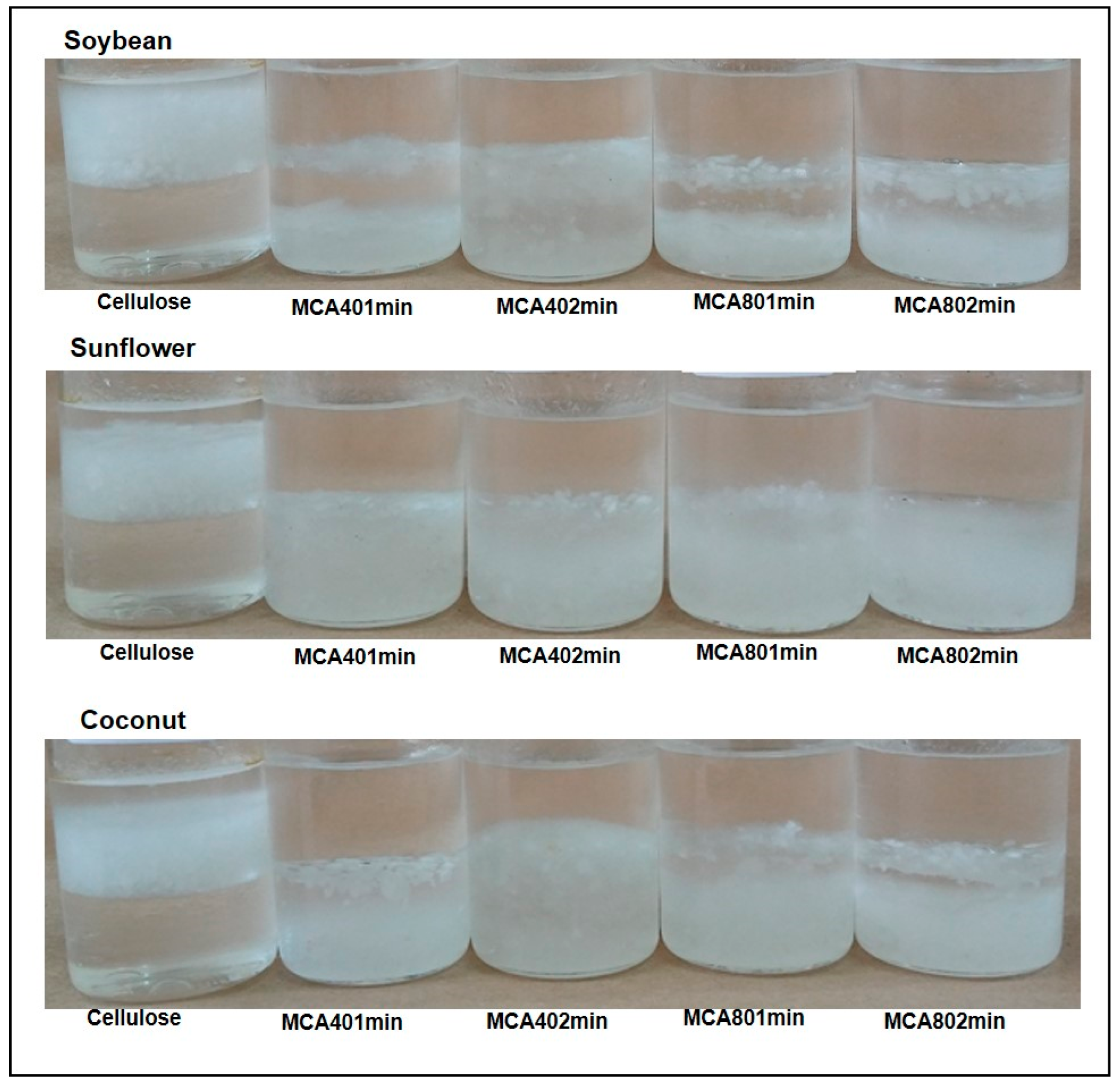
3.5. Wettability
3.6. Water and Oil Absorption Capacities
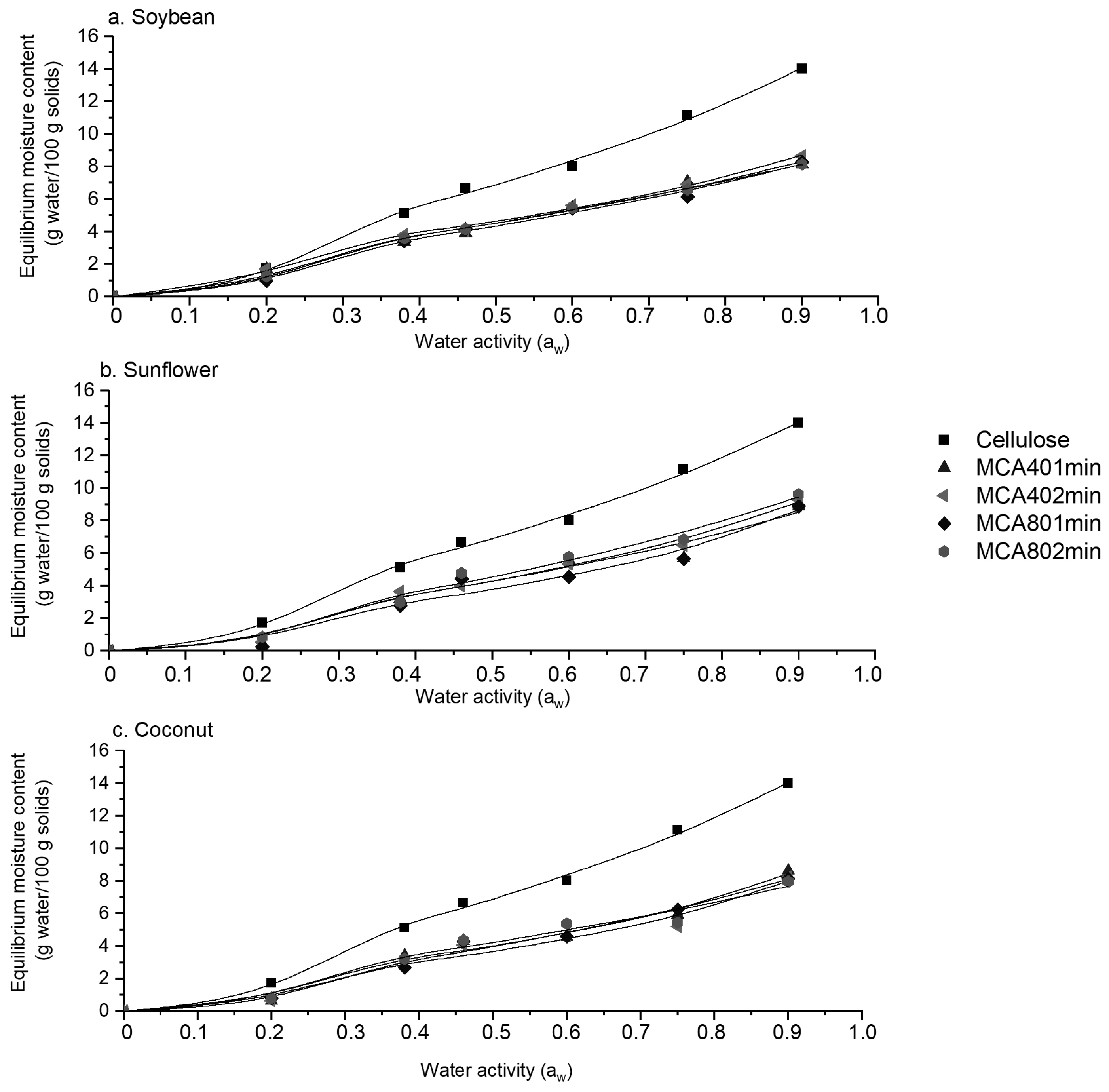
3.7. Moisture Sorption Isotherms
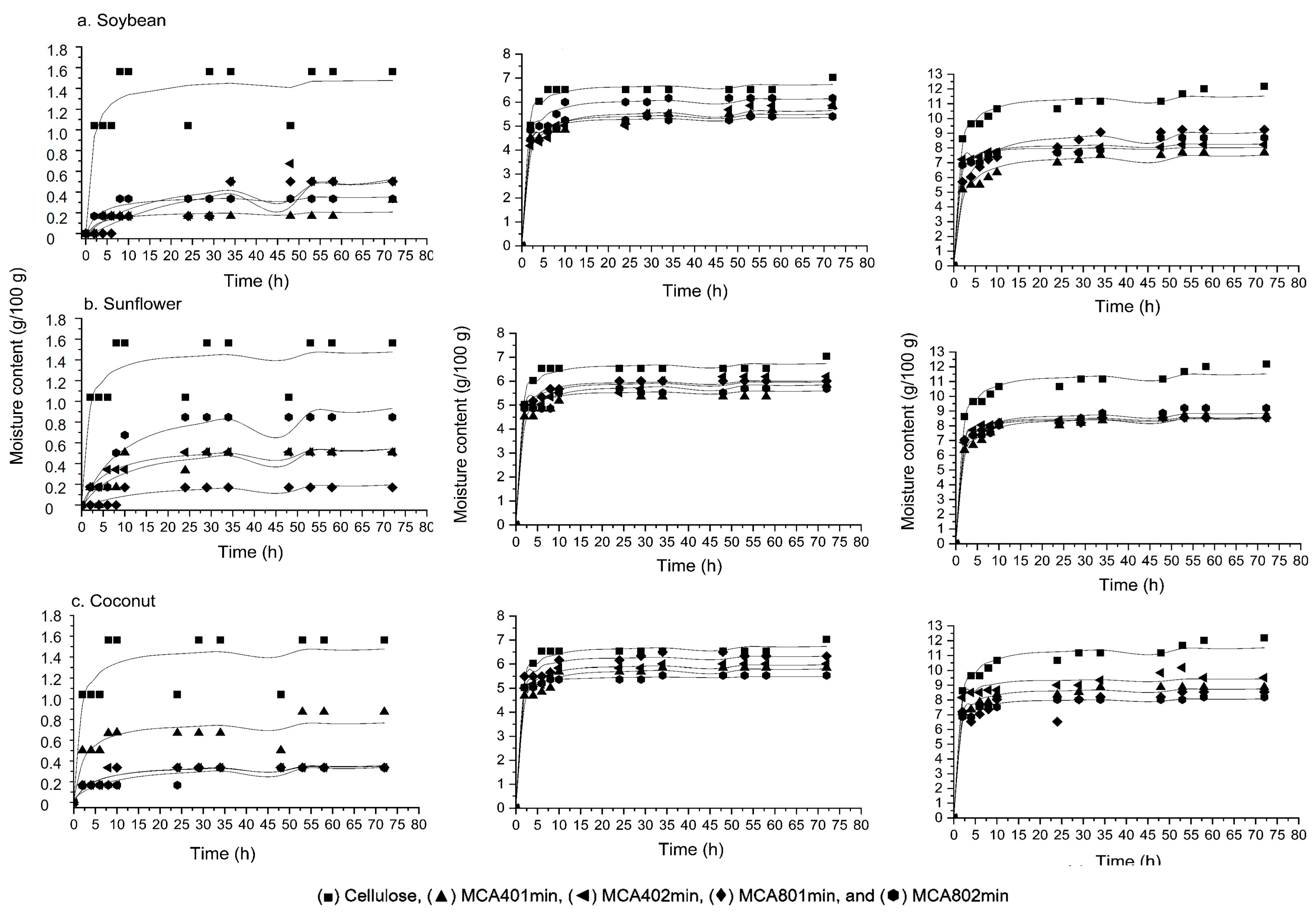
3.8. Moisture Adsorption Kinetics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Wang, X.; Xie, Y.; Zhang, K. Functional nanomaterials through esterification of cellulose: A review of chemistry and application. Cellulose 2018, 25, 3703–3731. [Google Scholar] [CrossRef]
- Debiagi, F.; Faria-Tischer, P.C.S.; Mali, S. Nanofibrillated cellulose obtained from soybean hull using simple and eco-friendly processes based on reactive extrusion. Cellulose 2020, 27, 1975–1988. [Google Scholar] [CrossRef]
- Kumar, S.M.; Rajini, N.; Alavudeen, A.; Siengchin, S.; Rajulu, V.A.; Ayrilmis, N. Development and analysis of completely biodegradable cellulose/banana peel powder composite films. J. Nat. Fibers. 2021, 18, 151–160. [Google Scholar]
- Gil-Giraldo, G.A.; Mantovan, J.; Marim, B.M.; Kishima, J.O.F.; Mali, S. Surface modification of cellulose from oat hull with citric acid using ultrasonication and reactive extrusion assisted processes. Polysaccharides 2021, 2, 218–233. [Google Scholar] [CrossRef]
- Debiagi, F.; Madeira, T.B.; Nixdorf, S.L.; Mali, S. Pretreatment efficiency using autoclave high-pressure steam and ultrasonication in sugar production from liquid hydrolysates and access to the residual solid fractions of wheat bran and oat hulls. Appl. Biochem. Biotechnol. 2019, 190, 166–181. [Google Scholar] [CrossRef]
- Debiagi, F.; Faria-Tischer, P.C.S.; Mali, S. A Green approach based on reactive extrusion to produce nanofibrillated cellulose from oat hull. Waste Biomass. Valor. 2021, 12, 1051–1060. [Google Scholar] [CrossRef]
- Gulvady, A.A.; Brown, R.C.; Bell, J.A. Oat Nutrition and Chemistry: Nutritional Comparison of Oats and Other Commonly Consumed Whole Grains, in Oats Nutrition and Technology; Quaker Oats Center of Excellence, PepsiCo R&D Nutrition: Barrington, IL, USA, 2014. [Google Scholar]
- Paschoal, G.B.; Muller, C.M.O.; Carvalho, G.M.; Tischer, C.A.; Mali, S. Isolation and characterization of nanofibrillated cellulose from oat hulls. Quim. Nova 2015, 38, 478–482. [Google Scholar] [CrossRef]
- Mishra, D.K.; Kumar, S.; Sukla, R.S. Furfuryl alcohol—A promissing plataform chemical. In Biomass, Biofuels, Biochemicals: Recent Advances in Developing of Plataform Chemicals; Saravanamurugan, S., Pandey, A., Li, H., Riisagen, A., Eds.; Elsevier Science Publishers: Oxford, UK, 2020; pp. 323–345. [Google Scholar]
- Zuin, V.G.; Ramin, L.Z. Green and sustainable separation of natural products from agroindustrial waste: Challenges, potentialities, and perspectives on emerging approaches. Top. Curr. Chem. 2018, 3, 229–282. [Google Scholar]
- Agrawal, C.M.; Ong, J.L.; Appleford, M.R.; Mani, G. Natural biomaterials. In Introduction to Biomaterials—Basic Theory with Engineering Application; Saltzman, M.W., Chien, S., Eds.; Cambrigde University Press: Cambridge, UK, 2014; pp. 198–232. [Google Scholar]
- Klemm, D.; Heublein, B.; Fink, H.P.; Bohn, A. Cellulose: Fascinating biopolymer and sustainable raw material. Angew. Chem. Int. Edit. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- Ngwabebhoh, F.A.; Ermem, A.; Yildiz, U.A. A design optimization study on synthesized nanocrystalline cellulose, evaluation and surface modification as a potential biomaterial for prospective biomedical applications. Int. J. Biol. Macromol. 2018, 114, 536–546. [Google Scholar] [CrossRef]
- Singh, P.; Duarte, H.; Alves, L.; Antunes, F.; Moigne, N.L.; Dormanns, J.; Duchemin, B.; Staiger, M.P.; Medronho, B. From cellulose dissolution and regeneration to added value applications—Synergism between molecular understanding and material development. In Cellulose—Fundamental Aspects and Current Trends; Poletto, M., Ornaghi, H.L., Jr., Eds.; IntechOpen: Rijeka, Croatia, 2015; pp. 1–45. [Google Scholar]
- Liyanage, S.; Acharya, S.; Parajuli, P.; Shamshina, J.L.; Abidi, N. Production and surface modification of cellulose bioproducts. Polymers 2021, 13, 3433. [Google Scholar] [CrossRef] [PubMed]
- Asim, N.; Badiei, M.; Mohammad, M. Recent advances in cellulose-based hydrophobic food packaging. Emergent Mater. 2022, 5, 703–718. [Google Scholar] [CrossRef]
- Bohrer, B.; Izadifar, M.; Barbut, S. Structural and functional properties of modified cellulose ingredients and their application in reduced-fat meat batters. Meat Sci. 2023, 195, 109011. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Zhang, C.; Li, Y.; Wu, Y.; Deng, Q.; Ni, X. Edible oleogels fabricated by dispersing cellulose particles in oil phase: Effects from the water addition. Food Hydrocoll. 2023, 134, 108040. [Google Scholar] [CrossRef]
- Wang, Q.; Espert, M.; Larrea, V.; Quiles, A.; Salvador, A.; Sanz, T. Comparison of different indirect approaches to design edible oleogels based on cellulose ethers. Food Hydrocoll. 2023, 134, 108007. [Google Scholar] [CrossRef]
- Zheng, L.; Zhong, J.; Liu, X.; Wang, Q.; Qin, X. Physicochemical properties and intermolecular interactions of a novel diacylglycerol oil oleogel made with ethyl cellulose as affected by γ-oryzanol. Food Hydrocoll. 2023, 138, 108484. [Google Scholar] [CrossRef]
- Agu, O.S.; Tabil, L.G.; Dumonceaux, T. Microwave-assisted alkali pre-treatment, densification and enzymatic saccharification of canola straw and oat hull. Bioengineering 2017, 4, 25. [Google Scholar] [CrossRef]
- Gorade, V.G.; Kotwal, A.; Chaudhary, B.U.; Kale, R.D. Surface modification of microcrystalline cellulose using rice bran oil: A bio-based approach to achieve water repellency. J. Polym. Res. 2019, 26, 217. [Google Scholar] [CrossRef]
- Dankovich, T.A.; Hsieh, Y.L. Surface modification of cellulose with plant triglycerides for hydrophobicity. Cellulose 2007, 14, 469–480. [Google Scholar] [CrossRef]
- Dong, X.; Dong, Y.; Jiang, M.; Wang, L.; Tong, J.; Zhou, J. Modification of microcrystalline cellulose by using soybean oil for surface hydrophobization. Ind. Crop. Prod. 2013, 46, 301–303. [Google Scholar] [CrossRef]
- Kale, D.; Gorade, V.G.; Madye, M.; Chaudhary, B.; Bangde, P.S.; Dandekar, P.P. Preparation and characterization of biocomposite packaging film from poly(lactic acid) and acylated microcrystalline cellulose using rice bran oil. Int. J. Biol. Macromol. 2018, 118, 1090–1102. [Google Scholar] [CrossRef] [PubMed]
- Mantovan, J.; Gil-Giraldo, G.A.; Marim, B.M.; Kishima, J.O.F.; Mal, S. Valorization of orange bagasse through one-step physical and chemical combined processes to obtain a cellulose-rich material. J. Sci. Food Agric. 2021, 101, 2362–2370. [Google Scholar] [CrossRef]
- Van Soest, P.J. Symposium on factors influencing the voluntary intake of herbage by ruminants: Voluntary intake in relation to chemical composition and digestibility. J. Anim. Sci. 1965, 24, 834–843. [Google Scholar] [CrossRef]
- Tappi Test Method T222 om-88, Acid-Insoluble Lignin in Wood and Pulp, in Tappi Test Methods; Tappi Press: Atlanta, GA, USA, 1999.
- Coma, V.; Sebti, I.; Pardon, P.; Pichavant, F.H.; Deschamps, A. Film properties from crosslinking of cellulosic derivatives with a polyfunctional carboxylic acid. Carbohydr. Polym. 2003, 51, 265–271. [Google Scholar] [CrossRef]
- Segal, L.; Creely, J.J.; Martin, A.E.; Conrad, C.M. An empirical method for estimating the degree of crystallinity of native cellulose using the X-ray diffractometer. Text. Res. J. 1959, 29, 786–794. [Google Scholar] [CrossRef]
- Namazi, H.; Dadkhah, A. Convenient method for preparation of hydrophobically modified starch nanocrystals with using fatty acids. CarbohydR. Polym. 2010, 79, 731–737. [Google Scholar] [CrossRef]
- Lu, H.; Gui, Y.; Zheng, L.; Liu, X. Morphological, crystalline, thermal and physicochemical properties of cellulose nanocrystals obtained from sweet potato residue. Food. Res. Int. 2013, 50, 121–128. [Google Scholar] [CrossRef]
- Rockland, L.B. Saturated salt solutions for static control of relative humidity between 5° and 40 °C. Anal. Chem. 1960, 32, 1375–1376. [Google Scholar] [CrossRef]
- Bizot, H. Using the GAB model to construct sorption isotherms. In Physical Properties of Foods; Jowitt, R., Escher, F., Hallistrom, B., Meffert, H.F.T., Spiess, W.W.L., Vos, G., Eds.; Applied Science Publishers: London, UK, 1984; pp. 27–41. [Google Scholar]
- Peleg, M. An empirical model for the description of moisture sorption curves. J. Food. Sci. 1988, 53, 1216–1219. [Google Scholar] [CrossRef]
- Cardoso, M.A.P.; Carvalho, G.M.; Yamashita, F.; Mali, S.; Eiras, D.; Demiate, I.M.; Grossmann, M.V.E. Oat hull fibers bleached by reactive extrusion with alkaline hydrogen peroxide in thermoplastic starch/poly (butylene adipate-co-terephthalate) composites. Polym. Compos. 2018, 39, 950–1958. [Google Scholar] [CrossRef]
- Marim, B.M.; Mantovan, J.; Giraldo, G.A.; Mali, S. Environmentally friendly process based on a combination of ultrasound and peracetic acid treatment to obtain cellulose from orange bagasse. J. Chem. Technol. Biotechnol. 2021, 96, 630–638. [Google Scholar] [CrossRef]
- Shang, W.; Huang, J.; Luo, H.; Chang, P.R.; Feng, J.; Xie, G. Hydrophobic modification of cellulose nanocrystal via covalently grafting of castor oil. Cellulose 2013, 20, 179–190. [Google Scholar] [CrossRef]
- Adewuyi, A.; Pereira, F.V. Surface modification of cellulose isolated from Sesamun indicum underutilized seed: A means of enhancing cellulose hydrophobicity. J. Sci. Adv. Mater. Devices 2017, 2, 326–332. [Google Scholar] [CrossRef]
- Demitri, C.; Del Sole, R.; Scalera, F.; Sannino, A.; Vasapollo, G.; Maffezzoli, A.; Nicolais, L. Novel superabsorbent cellulose-based hydrogels crosslinked with citric acid. J. Appl. Polym. Sci. 2008, 110, 2453–2460. [Google Scholar] [CrossRef]
- De Oliveira, J.P.; Bruni, G.P.; El-Halal, M.S.L.; Bertoldi, F.C.; Guerra-Dias, A.R.; Da Rosa-Zavareze, E. Cellulose nanocrystals from rice and oat husks and their application in aerogels for food packaging. Int. J. Biol. Macromol. 2019, 124, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhang, J.; Xiao, P.; Tian, W.; Zhang, J. Novel thermoplastic cellulose esters containing bulky moieties and soft segments. ACS Sustain. Chem. Eng. 2018, 6, 4931–4939. [Google Scholar] [CrossRef]
- David, G.; Gontard, N.; Guerin, D.; Heux, L.; Lecomte, J.; Molina-Boisseau, S.; Angellier-Coussy, H. Exploring the potential of gas-phase esterification to hydrophobize the surface of micrometric cellulose particles. Eur. Polym. J. 2019, 115, 138–146. [Google Scholar] [CrossRef]
- Sai, H.; Fu, R.; Xin, L.; Xiang, J.; Li, Z.; Li, F.; Zhang, T. Surface modification of bacterial cellulose aerogels’ web-like skeleton for oil/water separation. ACS Appl. Mater. Inter. 2015, 7, 7373–7381. [Google Scholar] [CrossRef]
- Mali, S.; Sakanaka, L.S.; Yamashita, F.; Grossmann, M.V.E. Water sorption and mechanical properties of cassava starch films and their relation to plasticizing effect. Carbohydr. Polym. 2005, 60, 283–289. [Google Scholar] [CrossRef]
| Sample | WAC (g/g) | OAC (g/g) |
|---|---|---|
| Cellulose | 10.20 ± 0.00 a | 1.92 ± 0.22 b |
| MCA401mimS | 1.87 ± 0.05 b | 6.06 ± 1.14 a |
| MCA402minS | 1.78 ± 0.03 b | 4.99 ± 0.42 a |
| MCA801minS | 1.33 ± 0.34 b | 5.24 ± 0.59 a |
| MCA802minS | 1.89 ± 0.13 b | 5.62 ± 0.49 a |
| MCA401minSF | 1.57 ± 0.24 b | 5.03 ± 0.45 a |
| MCA402minSF | 1.15 ± 0.63 b | 5.38 ± 0.39 a |
| MCA801minSF | 1.71 ± 0.14 b | 5.22 ± 0.30 a |
| MCA802minSF | 1.44 ± 0.54 b | 5.19 ± 0.37 a |
| MCA401minC | 1.70 ± 0.29 b | 5.02 ± 0.22 a |
| MCA402minC | 1.60 ± 0.25 b | 5.36 ± 0.68 a |
| MCA801minC | 1.57 ± 0.12 b | 5.17 ± 0.60 a |
| MCA802minC | 1.74 ± 0.32 b | 5.53 ± 0.52 a |
| Sample | m0 | C | K | r2 |
|---|---|---|---|---|
| Cellulose | 8.72 | 3.19 | 0.57 | 0.99 |
| MCA401minS | 4.86 | 5.16 | 0.56 | 0.99 |
| MCA402minS | 4.05 | 7.68 | 0.64 | 0.99 |
| MCA801minS | 5.44 | 4.01 | 0.53 | 0.99 |
| MCA802minS | 5.88 | 4.35 | 0.49 | 0.99 |
| MCA401minSF | 5.47 | 3.31 | 0.56 | 0.98 |
| MCA402minSF | 4.88 | 3.29 | 0.63 | 0.99 |
| MCA801minSF | 3.69 | 3.64 | 0.70 | 0.99 |
| MCA802minSF | 6.62 | 2.61 | 0.55 | 0.99 |
| MCA401minC | 3.69 | 4.96 | 0.68 | 0.99 |
| MCA402minC | 3.36 | 4.72 | 0.69 | 0.99 |
| MCA801minC | 5.57 | 2.82 | 0.55 | 0.98 |
| MCA802minC | 5.30 | 4.28 | 0.51 | 0.98 |
| Sample | 20% RH | 60% RH | 90% RH | ||||||
|---|---|---|---|---|---|---|---|---|---|
| k1 | k2 | r2 | k1 | k2 | r2 | k1 | k2 | r2 | |
| Cellulose | 0.81 | 0.67 | 0.80 | 0.08 | 0.15 | 0.99 | 0.07 | 0.09 | 0.99 |
| MCA401minS | 14.16 | 4.35 | 0.70 | 0.13 | 0.18 | 0.99 | 0.20 | 0.13 | 0.98 |
| MCA402minS | 24.46 | 1.60 | 0.70 | 0.19 | 0.18 | 0.99 | 0.04 | 0.12 | 0.99 |
| MCA801minS | 37.37 | 1.24 | 0.80 | 0.12 | 0.16 | 0.99 | 0.20 | 0.11 | 0.98 |
| MCA802minS | 7.93 | 2.71 | 0.90 | 0.11 | 0.19 | 0.99 | 0.07 | 0.12 | 0.98 |
| MCA401minSF | 14.91 | 1.60 | 0.80 | 0.15 | 0.17 | 0.99 | 0.11 | 0.12 | 0.99 |
| MCA402minSF | 9.01 | 1.70 | 0.95 | 0.10 | 0.17 | 0.98 | 0.06 | 0.12 | 0.99 |
| MCA801minSF | 45.86 | 3.73 | 0.80 | 0.09 | 0.17 | 0.99 | 0.06 | 0.12 | 0.99 |
| MCA802minSF | 7.51 | 0.90 | 0.90 | 0.09 | 0.18 | 0.99 | 0.09 | 0.11 | 0.98 |
| MCA401minC | 2.94 | 1.30 | 0.85 | 0.13 | 0.17 | 0.99 | 0.07 | 0.11 | 0.99 |
| MCA402minC | 11.60 | 2.79 | 0.85 | 0.09 | 0.17 | 0.99 | 0.05 | 0.11 | 0.99 |
| MCA801minC | 12.32 | 2.72 | 0.90 | 0.07 | 0.16 | 0.99 | 0.06 | 0.12 | 0.97 |
| MCA802minC | 27.02 | 2.95 | 0.80 | 0.04 | 0.18 | 0.99 | 0.06 | 0.12 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil-Giraldo, G.A.; Mantovan, J.; Marim, B.M.; Kishima, J.O.F.; Beluci, N.C.L.; Mali, S. Ultrasound-Assisted Process to Increase the Hydrophobicity of Cellulose from Oat Hulls by Surface Modification with Vegetable Oils. Polysaccharides 2024, 5, 463-477. https://doi.org/10.3390/polysaccharides5030029
Gil-Giraldo GA, Mantovan J, Marim BM, Kishima JOF, Beluci NCL, Mali S. Ultrasound-Assisted Process to Increase the Hydrophobicity of Cellulose from Oat Hulls by Surface Modification with Vegetable Oils. Polysaccharides. 2024; 5(3):463-477. https://doi.org/10.3390/polysaccharides5030029
Chicago/Turabian StyleGil-Giraldo, Gina A., Janaina Mantovan, Beatriz M. Marim, João O. F. Kishima, Natália C. L. Beluci, and Suzana Mali. 2024. "Ultrasound-Assisted Process to Increase the Hydrophobicity of Cellulose from Oat Hulls by Surface Modification with Vegetable Oils" Polysaccharides 5, no. 3: 463-477. https://doi.org/10.3390/polysaccharides5030029
APA StyleGil-Giraldo, G. A., Mantovan, J., Marim, B. M., Kishima, J. O. F., Beluci, N. C. L., & Mali, S. (2024). Ultrasound-Assisted Process to Increase the Hydrophobicity of Cellulose from Oat Hulls by Surface Modification with Vegetable Oils. Polysaccharides, 5(3), 463-477. https://doi.org/10.3390/polysaccharides5030029











