Chondroitin Sulfate/Cyanocobalamin–Chitosan Polyelectrolyte Complexes for Improved Oral Delivery of Colistin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
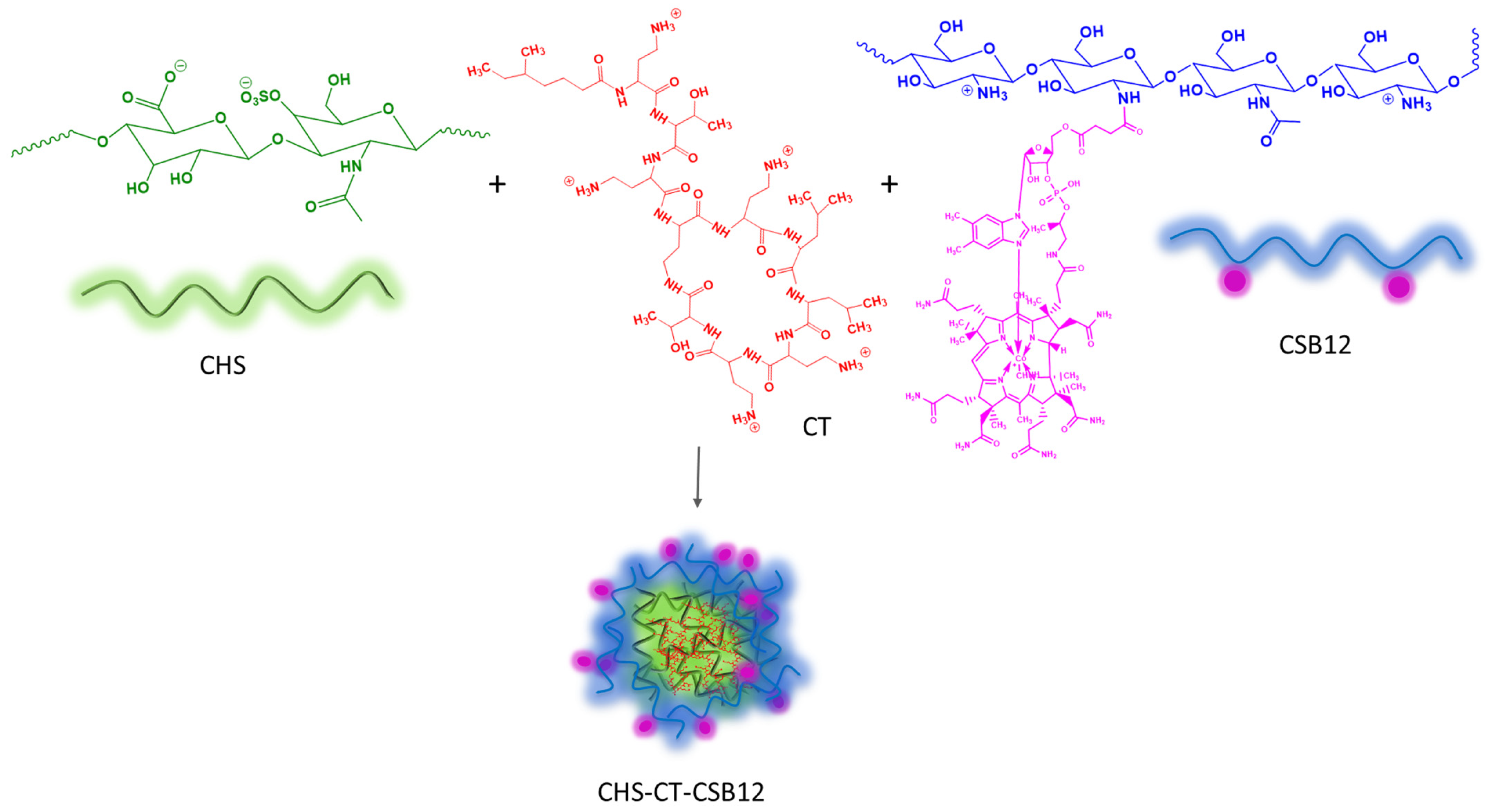
2.2. Preparation of PECs
2.3. Characterization of PECs
2.4. Encapsulation Efficiency and CT Content
2.5. In Vitro CT Release Kinetics
2.6. Mucoadhesion Properties
2.7. Caco-2 Cell Permeability
2.8. Antimicrobial Activity of PECs Against P. aeruginosa
3. Results
3.1. Formation and Characterization of Polyelectrolyte Complexes Based on CT, CHS, and CSB12
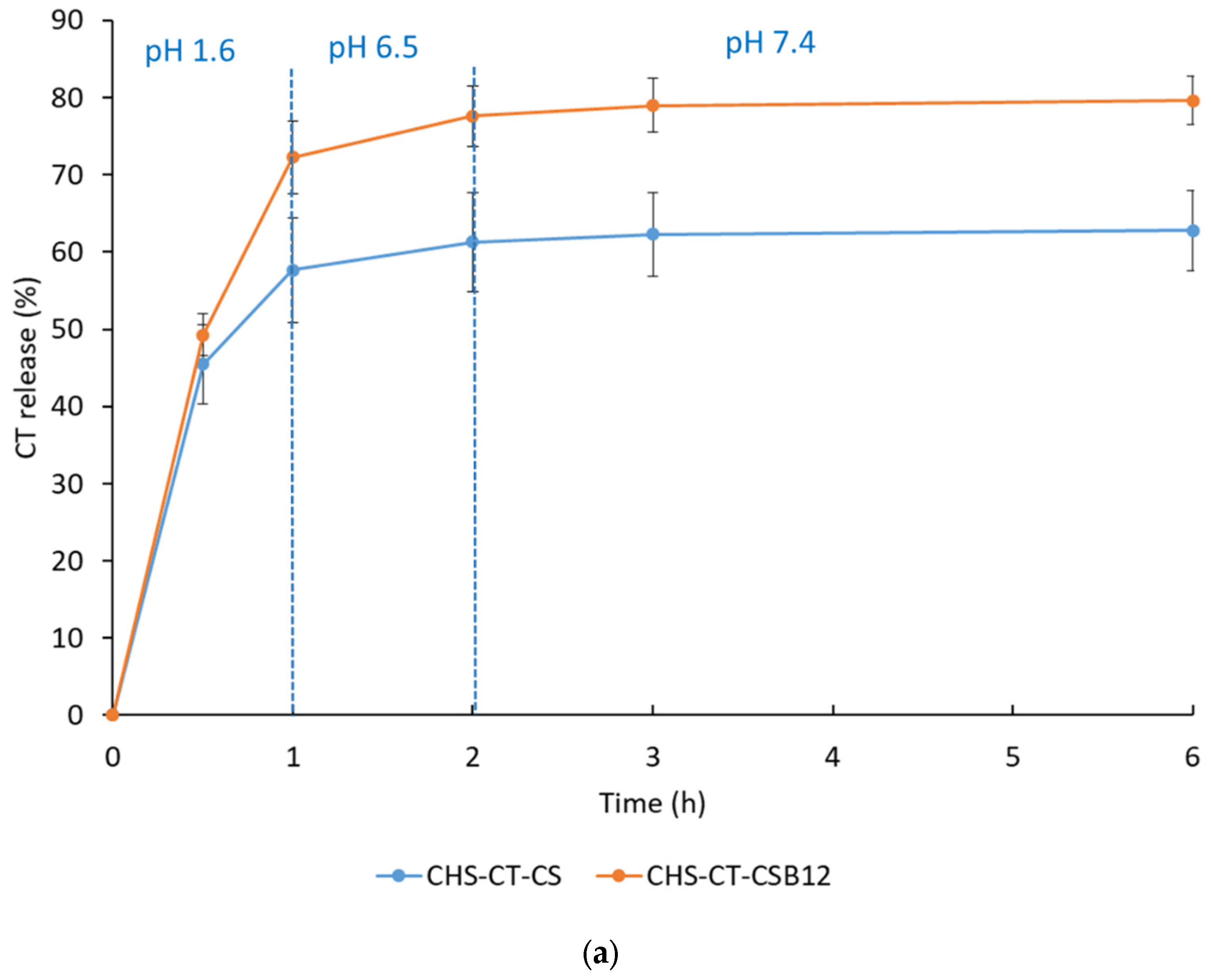
3.2. In Vitro CT Release Profile
3.3. Antimicrobial Activity
3.4. Mucoadhesive Properties
3.5. Caco-2 Cell Permeability Assay
4. Discussion and Outlook
5. Conclusions
- (i)
- The optimal conditions and component mass ratios for the formation of colloidally stable PECs based on CHS and CSB12 were identified. Polymer particles with suitable hydrodynamic sizes (330 and 384 nm) and ζ-potentials (25–27 mV) were formed at pH 3.5 and CSB12/CHS mass ratios of 2 and 3.
- (ii)
- Two-component intrapolymer complexes based on CHS and CT were prepared, demonstrating extremely effective encapsulation efficiencies (EE) of 100% over various CS:CT mass ratios up to 1:1. Subsequently, stable tri-component systems based on CHS, CT, and CSB12 were obtained at a component mass ratio of 1:1:3, with a hydrodynamic diameter of 446 nm and a ζ-potential of 28.2 mV.
- (iii)
- The developed CHS-CT-CSB12 PECs retained antimicrobial activity against P. aeruginosa comparable to that of pure CT. In addition, they exhibited an apparent permeability coefficient similar to that of vitamin B12. Combined with high mucoadhesion, these properties make the resulting formulations promising candidates for improved oral delivery of CT.
- (iv)
- Another important aspect of this study is the potential use of sulfated polysaccharides to create polymer complexes for enhanced delivery of polymyxins.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Hernando-Amado, S.; Coque, T.M.; Baquero, F.; Martínez, J.L. Defining and combating antibiotic resistance from one health and global health perspectives. Nat. Microbiol. 2019, 4, 1432–1442. [Google Scholar] [CrossRef]
- Roope, L.S.; Smith, R.D.; Pouwels, K.B.; Buchanan, J.; Abel, L.; Eibich, P.; Butler, C.C.; Tan, P.S.; Walker, A.S.; Robotham, J.V. The challenge of antimicrobial resistance: What economics can contribute. Science 2019, 364, eaau4679. [Google Scholar] [CrossRef]
- Coque, T.M.; Cantón, R.; Pérez-Cobas, A.E.; Fernández-de-Bobadilla, M.D.; Baquero, F. Antimicrobial resistance in the global health network: Known unknowns and challenges for efficient responses in the 21st century. Microorganisms 2023, 11, 1050. [Google Scholar] [CrossRef] [PubMed]
- Larsson, D.; Flach, C.-F. Antibiotic resistance in the environment. Nat. Rev. Microbiol. 2022, 20, 257–269. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Ma, Y.X.; Wang, C.Y.; Li, Y.Y.; Li, J.; Wan, Q.Q.; Chen, J.H.; Tay, F.R.; Niu, L.N. Considerations and caveats in combating eskape pathogens against nosocomial infections. Adv. Sci. 2020, 7, 1901872. [Google Scholar] [CrossRef]
- Aljeldah, M.M. Antimicrobial resistance and its spread is a global threat. Antibiotics 2022, 11, 1082. [Google Scholar] [CrossRef]
- Miller, W.R.; Arias, C.A. Eskape pathogens: Antimicrobial resistance, epidemiology, clinical impact and therapeutics. Nature Rev. Microbiol. 2024, 22, 598–616. [Google Scholar] [CrossRef] [PubMed]
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging strategies to combat eskape pathogens in the era of antimicrobial resistance: A review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef]
- Singh, A.; Tanwar, M.; Singh, T.; Sharma, S.; Sharma, P. An escape from eskape pathogens: A comprehensive review on current and emerging therapeutics against antibiotic resistance. Int. J. Biol. Macromol. 2024, 279, 135253. [Google Scholar] [CrossRef]
- Shah, S.N.; Bhat, M.A.; Bhat, M.A.; Jan, A.T. Antimicrobial resistance: An overview. In Nanotechnology Based Strategies for Combating Antimicrobial Resistance; Wani, M.Y., Wani, I.A., Rai, A., Eds.; Springer: Singapore, 2024; pp. 1–44. [Google Scholar] [CrossRef]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial antibiotic resistance: The most critical pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Su, T.-Y.; Ye, J.-J.; Hsu, P.-C.; Kuo, A.-J.; Chia, J.-H.; Lee, M.-H. Risk factors and clinical significance of bacteremia caused by pseudomonas aeruginosa resistant only to carbapenems. J. Microbiol. Immunol. Infect. 2017, 50, 677–683. [Google Scholar] [CrossRef]
- De Oliveira, D.M.; Forde, B.M.; Kidd, T.J.; Harris, P.N.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial resistance in eskape pathogens. Clin. Microbiol. Rev. 2020, 33, e00181-19. [Google Scholar] [CrossRef] [PubMed]
- El-Sayed Ahmed, M.A.E.-G.; Zhong, L.-L.; Shen, C.; Yang, Y.; Doi, Y.; Tian, G.-B. Colistin and its role in the era of antibiotic resistance: An extended review (2000–2019). Emerg. Microbes Infect. 2020, 9, 868–885. [Google Scholar] [CrossRef]
- Band, V.I.; Weiss, D.S. Mechanisms of antimicrobial peptide resistance in gram-negative bacteria. Antibiotics 2014, 4, 18–41. [Google Scholar] [CrossRef]
- Grégoire, N.; Aranzana-Climent, V.; Magréault, S.; Marchand, S.; Couet, W. Clinical pharmacokinetics and pharmacodynamics of colistin. Clin. Pharmacokinet. 2017, 56, 1441–1460. [Google Scholar] [CrossRef]
- Vaara, M. Polymyxins and their potential next generation as therapeutic antibiotics. Front. Microbiol. 2019, 10, 1689. [Google Scholar]
- Slingerland, C.J.; Martin, N.I. Recent advances in the development of polymyxin antibiotics: 2010–2023. ACS Infect. Dis. 2024, 10, 1056–1079. [Google Scholar] [CrossRef]
- Nation, R.L.; Rigatto, M.H.P.; Falci, D.R.; Zavascki, A.P. Polymyxin acute kidney injury: Dosing and other strategies to reduce toxicity. Antibiotics 2019, 8, 24. [Google Scholar] [CrossRef]
- Doymaz, M.Z.; Karaaslan, E. Comparison of antibacterial activities of polymyxin b and colistin against multidrug resistant gram negative bacteria. Infect. Dis. 2019, 51, 676–682. [Google Scholar] [CrossRef]
- Aboumanei, M.H.; Mahmoud, A.F.; Motaleb, M. Formulation of chitosan coated nanoliposomes for the oral delivery of colistin sulfate: In vitro characterization, 99mtc-radiolabeling and in vivo biodistribution studies. Drug Dev. Ind. Pharm. 2021, 47, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Ardebili, A.; Izanloo, A.; Rastegar, M. Polymyxin combination therapy for multidrug-resistant, extensively-drug resistant, and difficult-to-treat drug-resistant gram-negative infections: Is it superior to polymyxin monotherapy? Expert Rev. Anti-Infect. Ther. 2023, 21, 387–429. [Google Scholar] [CrossRef]
- Nation, R.L. Polymyxins. In Kucers’ the Use of Antibiotics; CRC Press: Cambridge, UK, 2017; pp. 1420–1449. [Google Scholar]
- Wang, R.; van Dorp, L.; Shaw, L.P.; Bradley, P.; Wang, Q.; Wang, X.; Jin, L.; Zhang, Q.; Liu, Y.; Rieux, A.; et al. The global distribution and spread of the mobilized colistin resistance gene mcr-1. Nat. Commun. 2018, 9, 1179. [Google Scholar] [CrossRef]
- Olaitan, A.O.; Chabou, S.; Okdah, L.; Morand, S.; Rolain, J.-M. Dissemination of the mcr-1 colistin resistance gene. Lancet Infect. Dis. 2016, 16, 147. [Google Scholar] [CrossRef]
- Hamel, M.; Rolain, J.-M.; Baron, S.A. The history of colistin resistance mechanisms in bacteria: Progress and challenges. Microorganisms 2021, 9, 442. [Google Scholar] [CrossRef] [PubMed]
- Anyanwu, M.U.; Jaja, I.F.; Nwobi, O.C. Occurrence and characteristics of mobile colistin resistance (mcr) gene-containing isolates from the environment: A review. Int. J. Environ. Res. Public Health 2020, 17, 1028. [Google Scholar] [CrossRef]
- Azad, M.A.; Nation, R.L.; Velkov, T.; Li, J. Mechanisms of polymyxin-induced nephrotoxicity. In Polymyxin Antibiotics: From Laboratory Bench to Bedside. Advances in Experimental Medicine and Biology, Vol 1145; Li, J., Nation, R., Kaye, K., Eds.; Springer: Cham, Switzerland, 2019; pp. 305–319. [Google Scholar] [CrossRef]
- Alqahtani, M.S.; Kazi, M.; Alsenaidy, M.A.; Ahmad, M.Z. Advances in oral drug delivery. Front. Pharmacol. 2021, 12, 618411. [Google Scholar] [CrossRef]
- Hung, Y.-P.; Chen, Y.-F.; Tsai, P.-J.; Huang, I.-H.; Ko, W.-C.; Jan, J.-S. Advances in the application of nanomaterials as treatments for bacterial infectious diseases. Pharmaceutics 2021, 13, 1913. [Google Scholar] [CrossRef] [PubMed]
- Jampilek, J.; Kralova, K. Advances in nanostructures for antimicrobial therapy. Materials 2022, 15, 2388. [Google Scholar] [CrossRef]
- Brown, P.; Dawson, M.J. Development of new polymyxin derivatives for multi-drug resistant gram-negative infections. J. Antibiot. 2017, 70, 386–394. [Google Scholar] [CrossRef]
- Dubashynskaya, N.V.; Skorik, Y.A. Polymyxin delivery systems: Recent advances and challenges. Pharmaceuticals 2020, 13, 83. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Nie, X.; Zou, M.; Shi, Y.; Cheng, G. Recent advances in materials for extended-release antibiotic delivery system. J. Antibiot. 2011, 64, 625–634. [Google Scholar] [CrossRef]
- Dubashynskaya, N.V.; Bokatyi, A.N.; Dobrodumov, A.V.; Kudryavtsev, I.V.; Trulioff, A.S.; Rubinstein, A.A.; Aquino, A.D.; Dubrovskii, Y.A.; Knyazeva, E.S.; Demyanova, E.V. Succinyl chitosan-colistin conjugates as promising drug delivery systems. Int. J. Mol. Sci. 2022, 24, 166. [Google Scholar] [CrossRef]
- Le, H.; Karakasyan, C.; Jouenne, T.; Le Cerf, D.; Dé, E. Application of polymeric nanocarriers for enhancing the bioavailability of antibiotics at the target site and overcoming antimicrobial resistance. Appl. Sci. 2021, 11, 10695. [Google Scholar] [CrossRef]
- Skwarczynski, M.; Bashiri, S.; Yuan, Y.; Ziora, Z.M.; Nabil, O.; Masuda, K.; Khongkow, M.; Rimsueb, N.; Cabral, H.; Ruktanonchai, U. Antimicrobial activity enhancers: Towards smart delivery of antimicrobial agents. Antibiotics 2022, 11, 412. [Google Scholar] [CrossRef]
- Sharma, S.; Bhende, M. An overview: Non-toxic and eco-friendly polysaccharides—Its classification, properties, and diverse applications. Polym. Bull. 2024, 81, 12383–12429. [Google Scholar] [CrossRef]
- Pal, D.; Saha, S. Chondroitin: A natural biomarker with immense biomedical applications. RSC Adv. 2019, 9, 28061–28077. [Google Scholar] [CrossRef]
- Dubashynskaya, N.V.; Raik, S.V.; Dubrovskii, Y.A.; Shcherbakova, E.S.; Demyanova, E.V.; Shasherina, A.Y.; Anufrikov, Y.A.; Poshina, D.N.; Dobrodumov, A.V.; Skorik, Y.A. Hyaluronan/colistin polyelectrolyte complexes: Promising antiinfective drug delivery systems. Int. J. Biol. Macromol. 2021, 187, 157–165. [Google Scholar] [CrossRef]
- Dubashynskaya, N.V.; Raik, S.V.; Dubrovskii, Y.A.; Demyanova, E.V.; Shcherbakova, E.S.; Poshina, D.N.; Shasherina, A.Y.; Anufrikov, Y.A.; Skorik, Y.A. Hyaluronan/diethylaminoethyl chitosan polyelectrolyte complexes as carriers for improved colistin delivery. Int. J. Mol. Sci. 2021, 22, 8381. [Google Scholar] [CrossRef]
- Dubashynskaya, N.V.; Bokatyi, A.N.; Sall, T.S.; Egorova, T.S.; Demyanova, E.V.; Dubrovskii, Y.A.; Murashko, E.A.; Anufrikov, Y.A.; Shasherina, A.Y.; Vlasova, E.N. Hyaluronan/b12-chitosan polyelectrolyte complex for oral colistin administration. Int. J. Biol. Macromol. 2024, 263, 130177. [Google Scholar] [CrossRef]
- Fu, J.; Fares, H.M.; Schlenoff, J.B. Ion-pairing strength in polyelectrolyte complexes. Macromolecules 2017, 50, 1066–1074. [Google Scholar] [CrossRef]
- Shchipunov, Y.A.; Postnova, I.V. Water-soluble polyelectrolyte complexes of oppositely charged polysaccharides. Compos. Interfaces 2009, 16, 251–279. [Google Scholar] [CrossRef]
- Petrus, A.K.; Fairchild, T.J.; Doyle, R.P. Traveling the vitamin b12 pathway: Oral delivery of protein and peptide drugs. Angew. Chem. Int. Ed. 2009, 48, 1022–1028. [Google Scholar] [CrossRef]
- Dubashynskaya, N.V.; Bokatyi, A.N.; Sall, T.S.; Egorova, T.S.; Nashchekina, Y.A.; Dubrovskii, Y.A.; Murashko, E.A.; Vlasova, E.N.; Demyanova, E.V.; Skorik, Y.A. Cyanocobalamin-modified colistin–hyaluronan conjugates: Synthesis and bioactivity. Int. J. Mol. Sci. 2023, 24, 11550. [Google Scholar] [CrossRef] [PubMed]
- Stepanova, M.; Levit, M.; Egorova, T.; Nashchekina, Y.; Sall, T.; Demyanova, E.; Guryanov, I.; Korzhikova-Vlakh, E. Poly (2-deoxy-2-methacrylamido-d-glucose)-based complex conjugates of colistin, deferoxamine and vitamin b12: Synthesis and biological evaluation. Pharmaceutics 2024, 16, 1080. [Google Scholar] [CrossRef] [PubMed]
- Fedosov, S.N. Physiological and molecular aspects of cobalamin transport. Water Soluble Vitam. Clin. Res. Future Appl. 2012, 56, 347–367. [Google Scholar]
- Russell-Jones, G.; Westwood, S.; Habberfield, A. Vitamin b12 mediated oral delivery systems for granulocyte-colony stimulating factor and erythropoietin. Bioconjugate Chem. 1995, 6, 459–465. [Google Scholar] [CrossRef]
- Russell-Jones, G.; Westwood, S.; Farnworth, P.; Findlay, J.; Burger, H. Synthesis of lhrh antagonists suitable for oral administration via the vitamin b12 uptake system. Bioconjugate Chem. 1995, 6, 34–42. [Google Scholar] [CrossRef]
- Verma, A.; Sharma, S.; Gupta, P.K.; Singh, A.; Teja, B.V.; Dwivedi, P.; Gupta, G.K.; Trivedi, R.; Mishra, P.R. Vitamin b12 functionalized layer by layer calcium phosphate nanoparticles: A mucoadhesive and ph responsive carrier for improved oral delivery of insulin. Acta Biomater. 2016, 31, 288–300. [Google Scholar] [CrossRef]
- Dubashynskaya, N.V.; Bokatyi, A.N.; Trulioff, A.S.; Rubinstein, A.A.; Novikova, V.P.; Petrova, V.A.; Vlasova, E.N.; Malkov, A.V.; Kudryavtsev, I.V.; Skorik, Y.A. Delivery system for dexamethasone phosphate based on a Zn2+-crosslinked polyelectrolyte complex of diethylaminoethyl chitosan and chondroitin sulfate. Carbohydr. Polym. 2024, 348, 122899. [Google Scholar] [CrossRef]
- Raik, S.V.; Poshina, D.N.; Lyalina, T.A.; Polyakov, D.S.; Vasilyev, V.B.; Kritchenkov, A.S.; Skorik, Y.A. N-[4-(N,N,N-trimethylammonium) benzyl] chitosan chloride: Synthesis, interaction with DNA and evaluation of transfection efficiency. Carbohydr. Polym. 2018, 181, 693–700. [Google Scholar] [CrossRef] [PubMed]
- Iudin, D.; Zashikhina, N.; Demyanova, E.; Korzhikov-Vlakh, V.; Shcherbakova, E.; Boroznjak, R.; Tarasenko, I.; Zakharova, N.; Lavrentieva, A.; Skorik, Y.; et al. Polypeptide self-assembled nanoparticles as delivery systems for polymyxins b and e. Pharmaceutics 2020, 12, 868. [Google Scholar] [CrossRef]
- Wu, T.; Zivanovic, S.; Hayes, D.G.; Weiss, J. Efficient reduction of chitosan molecular weight by high-intensity ultrasound: Underlying mechanism and effect of process parameters. J. Agric. Food Chem. 2008, 56, 5112–5119. [Google Scholar] [CrossRef] [PubMed]
- Dubashynskaya, N.V.; Bokatyi, A.N.; Trulioff, A.S.; Rubinstein, A.A.; Kudryavtsev, I.V.; Skorik, Y.A. Development and bioactivity of zinc sulfate cross-linked polysaccharide delivery system of dexamethasone phosphate. Pharmaceutics 2023, 15, 2396. [Google Scholar] [CrossRef]
- Hejjaji, E.M.; Smith, A.M.; Morris, G.A. Evaluation of the mucoadhesive properties of chitosan nanoparticles prepared using different chitosan to tripolyphosphate (CS:TPP) ratios. Int. J. Biol. Macromol. 2018, 120, 1610–1617. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Lin, Y.-S.; Wu, S.-J.; Mi, F.-L. Mutlifunctional nanoparticles prepared from arginine-modified chitosan and thiolated fucoidan for oral delivery of hydrophobic and hydrophilic drugs. Carbohydr. Polym. 2018, 193, 163–172. [Google Scholar] [CrossRef]
- Elbi, S.; Nimal, T.; Rajan, V.; Baranwal, G.; Biswas, R.; Jayakumar, R.; Sathianarayanan, S. Fucoidan coated ciprofloxacin loaded chitosan nanoparticles for the treatment of intracellular and biofilm infections of salmonella. Colloids Surf. B Biointerfaces 2017, 160, 40–47. [Google Scholar]
- Hosseini-Ashtiani, N.; Tadjarodi, A.; Zare-Dorabei, R. Low molecular weight chitosan-cyanocobalamin nanoparticles for controlled delivery of ciprofloxacin: Preparation and evaluation. Int. J. Biol. Macromol. 2021, 176, 459–467. [Google Scholar] [CrossRef]
- Ashford, M. Gastrointestinal tract–physiology and drug absorption. In Aulton’s Pharmaceutics E-Book: The Design and Manufacture of Medicines, 5th ed.; Taylor, K.M.G., Aulton, M.E., Eds.; Elsevier: Edinburgh, UK, 2017; pp. 300–318. [Google Scholar]
- Kozyraki, R.; Cases, O. Vitamin b12 absorption: Mammalian physiology and acquired and inherited disorders. Biochimie 2013, 95, 1002–1007. [Google Scholar] [CrossRef]
- Pathomthongtaweechai, N.; Muanprasat, C. Potential applications of chitosan-based nanomaterials to surpass the gastrointestinal physiological obstacles and enhance the intestinal drug absorption. Pharmaceutics 2021, 13, 887. [Google Scholar] [CrossRef]
- Sangnim, T.; Dheer, D.; Jangra, N.; Huanbutta, K.; Puri, V.; Sharma, A. Chitosan in oral drug delivery formulations: A review. Pharmaceutics 2023, 15, 2361. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-C.; Mi, F.-L.; Liao, Z.-X.; Hsiao, C.-W.; Sonaje, K.; Chung, M.-F.; Hsu, L.-W.; Sung, H.-W. Recent advances in chitosan-based nanoparticles for oral delivery of macromolecules. Adv. Drug Deliv. Rev. 2013, 65, 865–879. [Google Scholar] [CrossRef] [PubMed]
- Ways, T.M.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yu, M.; Fan, W.; Gan, Y.; Hovgaard, L.; Yang, M. Orally active-targeted drug delivery systems for proteins and peptides. Expert Opin. Drug Deliv. 2014, 11, 1435–1447. [Google Scholar] [CrossRef]
- Yeh, T.-H.; Hsu, L.-W.; Tseng, M.T.; Lee, P.-L.; Sonjae, K.; Ho, Y.-C.; Sung, H.-W. Mechanism and consequence of chitosan-mediated reversible epithelial tight junction opening. Biomaterials 2011, 32, 6164–6173. [Google Scholar] [CrossRef]
- Kumar, R.; Islam, T.; Nurunnabi, M. Mucoadhesive carriers for oral drug delivery. J. Control. Release 2022, 351, 504–559. [Google Scholar] [CrossRef]




| CSB12/CHS Mass Ratio | Volume of CSB12 Solution (mL) | Concentration of CSB12 Solution (mg/mL) | pH | Volume of CHS Solution (mL) | Volume of CT Solution (mL) |
|---|---|---|---|---|---|
| 0.50 | 1.00 | 0.50 | 3.5 | 1.00 | - |
| 1.0 | 1.00 | 1.0 | 3.5 | 1.00 | - |
| 2.0 | 1.00 | 2.0 | 3.5 | 1.00 | - |
| 3.0 | 1.00 | 3.0 | 3.5 | 1.00 | 1.00 |
| 5.0 | 1.00 | 5.0 | 3.5 | 1.00 | - |
| 0.50 | 1.00 | 0.50 | 5.0 | 1.00 | - |
| 1.0 | 1.00 | 1.0 | 5.0 | 1.00 | - |
| 2.0 | 1.00 | 2.0 | 5.0 | 1.00 | - |
| 3.0 | 1.00 | 3.0 | 5.0 | 1.00 | - |
| 5.0 | 1.00 | 5.0 | 5.0 | 1.00 | - |
| Formulation (Component Mass Ratio) | Dh, nm | ζ-Potential, mV | EE, % | CT Content, µg/mg | CT Cumulative Release in 24 h, % |
|---|---|---|---|---|---|
| CHS-CT-CSB12 (1:1:3) | 446 ± 26 | 28.2 ± 0.9 | 100 | 200 | 80% |
| CHS-CSB12 (1:3) | 384 ± 68 | 28.5 ± 1.1 | - | - | - |
| CHS-CT-CS (1:1:3) | 950 ± 188 | 26.6 ± 1.6 | 100 | 200 | 63% |
| CHS-CS (1:3) | 816 ± 134 | 26.9 ± 0.8 | - | - | - |
| Sample | Papp (cm/s) |
|---|---|
| CT | 4.0 × 10−8 |
| CHS-CT-CS | 2.1 × 10−7 |
| CHS-CT-CSB12 | 1.1 × 10−6 |
| B12 | 3.5 × 10−6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dubashynskaya, N.V.; Borovskoy, A.Y.; Bokatyi, A.N.; Sall, T.S.; Egorova, T.S.; Demyanova, E.V.; Murashko, E.A.; Skorik, Y.A. Chondroitin Sulfate/Cyanocobalamin–Chitosan Polyelectrolyte Complexes for Improved Oral Delivery of Colistin. Polysaccharides 2025, 6, 21. https://doi.org/10.3390/polysaccharides6010021
Dubashynskaya NV, Borovskoy AY, Bokatyi AN, Sall TS, Egorova TS, Demyanova EV, Murashko EA, Skorik YA. Chondroitin Sulfate/Cyanocobalamin–Chitosan Polyelectrolyte Complexes for Improved Oral Delivery of Colistin. Polysaccharides. 2025; 6(1):21. https://doi.org/10.3390/polysaccharides6010021
Chicago/Turabian StyleDubashynskaya, Natallia V., Andrey Y. Borovskoy, Anton N. Bokatyi, Tatiana S. Sall, Tatiana S. Egorova, Elena V. Demyanova, Ekaterina A. Murashko, and Yury A. Skorik. 2025. "Chondroitin Sulfate/Cyanocobalamin–Chitosan Polyelectrolyte Complexes for Improved Oral Delivery of Colistin" Polysaccharides 6, no. 1: 21. https://doi.org/10.3390/polysaccharides6010021
APA StyleDubashynskaya, N. V., Borovskoy, A. Y., Bokatyi, A. N., Sall, T. S., Egorova, T. S., Demyanova, E. V., Murashko, E. A., & Skorik, Y. A. (2025). Chondroitin Sulfate/Cyanocobalamin–Chitosan Polyelectrolyte Complexes for Improved Oral Delivery of Colistin. Polysaccharides, 6(1), 21. https://doi.org/10.3390/polysaccharides6010021









