Enhancing the Properties of Sodium Alginate with a Glycerol–Silicate Plasticizer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Glycerol–Silicate (GS) Precursor Synthesis
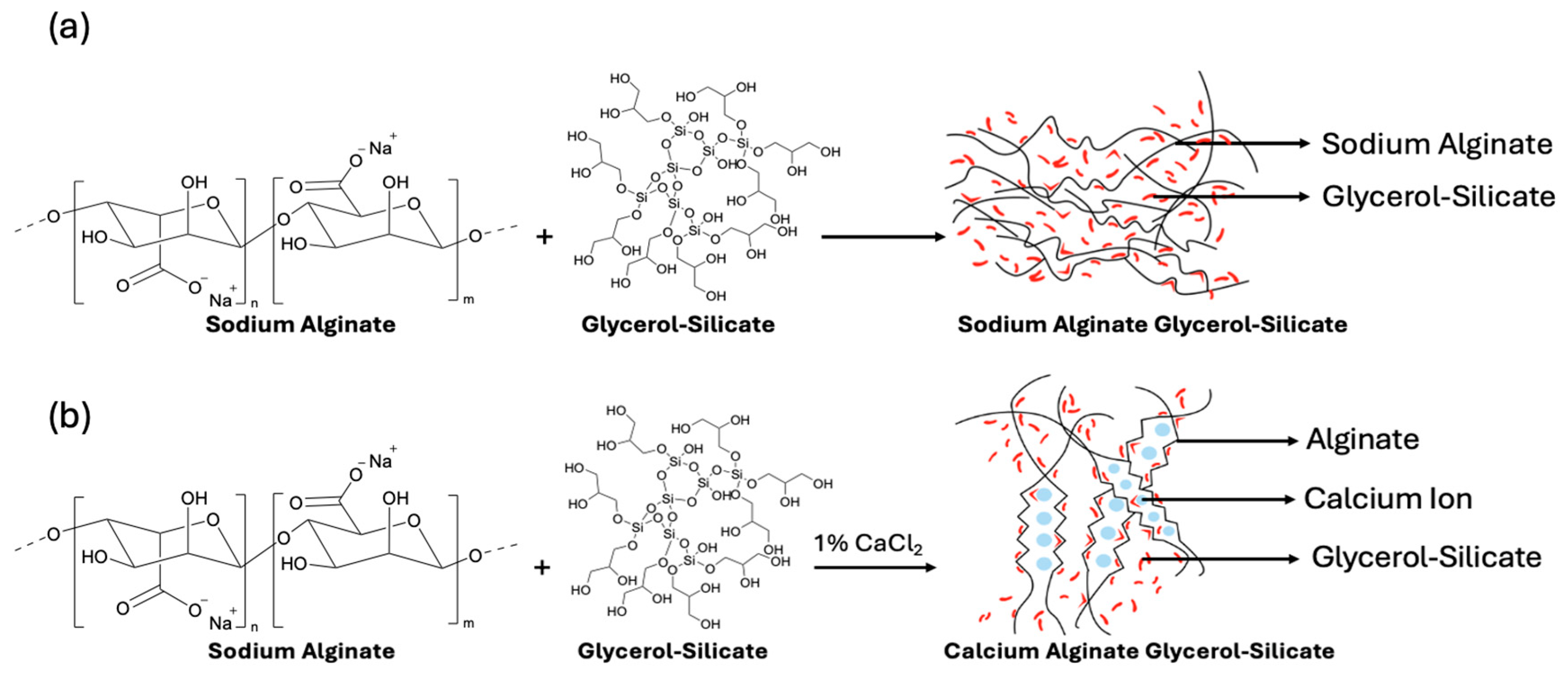
2.3. Alginate Bioplastic Composite Preparation
2.4. Fourier Transmission Infrared Spectroscopy (FTIR)
2.5. Mechanical Properties
2.6. Thermogravimetric Analysis (TGA)
2.7. Advancing Contact Angle
3. Results
3.1. Film Preparation and Morphology
3.2. Spectrometric Analysis
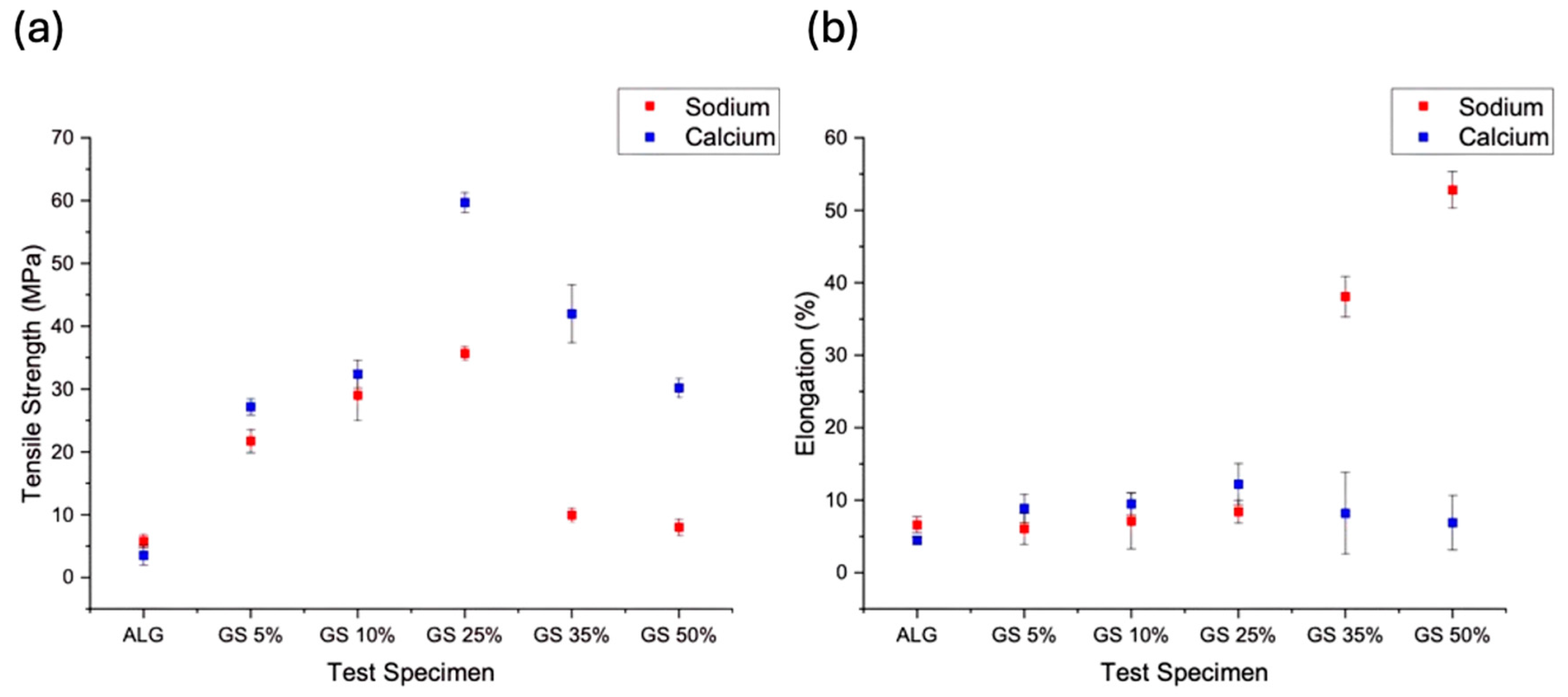
3.3. Mechanical Properties
3.4. Thermal Properties
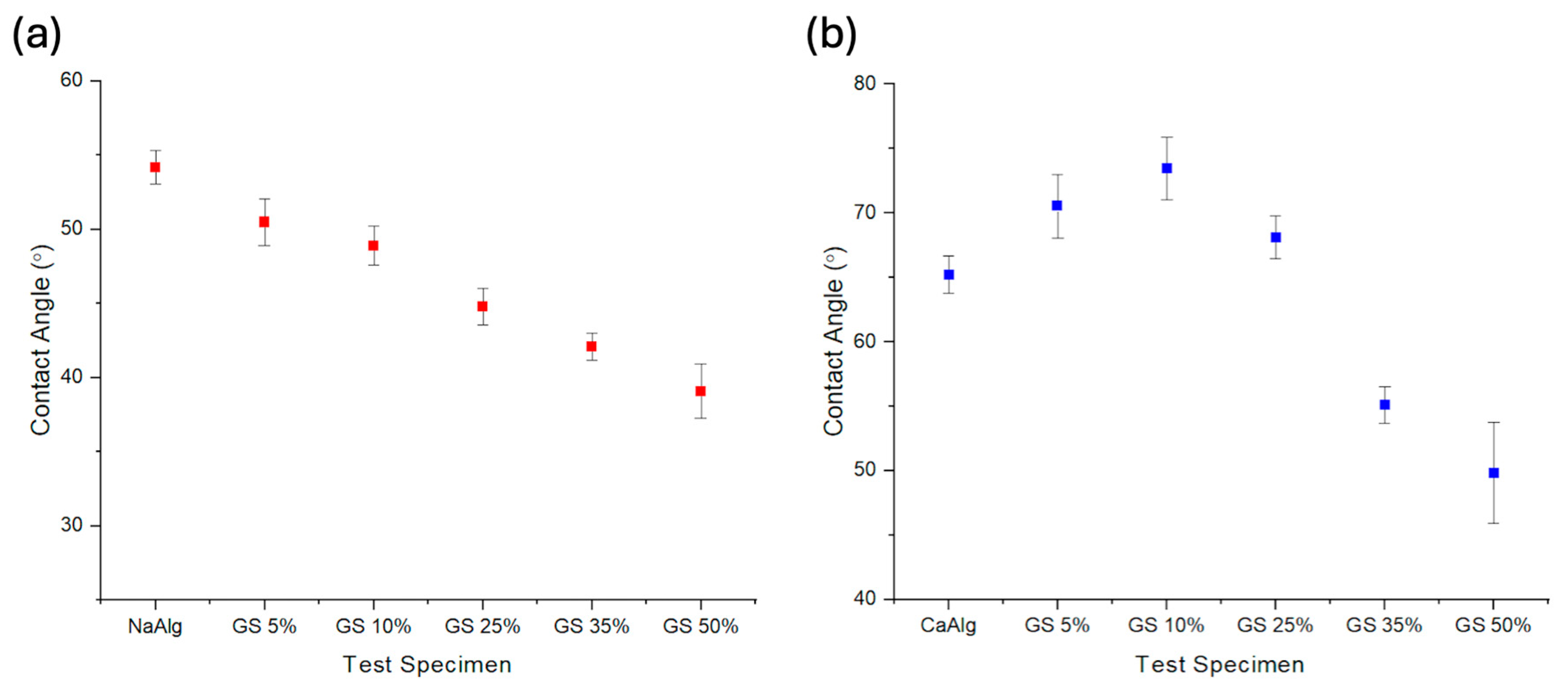
3.5. Advancing Contact Angle
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ye, S.; Xie, C.; Agar, O.T.; Barrow, C.; Dunshea, F.; Suleria, H. Alginates from Brown Seaweeds as a Promising Natural Source: A Review of Its Properties and Health Benefits. Food Rev. Int. 2023, 40, 2682–2710. [Google Scholar] [CrossRef]
- Bouzenad, N.; Ammouchi, N.; Chaib, N.; Belhocine, Y.; Belhaoues, A.; Hamrouche, N.; El Islam Boudagha, S.; Boudagha, I.; Abdennouri, A.; Zahnit, W. Development of Bioplastic Films from Sargassum Muticum Alginate: Properties and Applications in Food Packaging. Iran. J. Chem. Chem. Eng. 2024, 43, 3794–3811. [Google Scholar] [CrossRef]
- Abka-khajouei, R.; Tounsi, L.; Shahabi, N.; Patel, A.K.; Abdelkafi, S.; Michaud, P. Structures, Properties and Applications of Alginates. Mar. Drugs 2022, 20, 364. [Google Scholar] [CrossRef]
- Ahmad Raus, R.; Wan Nawawi, W.M.F.; Nasaruddin, R.R. Alginate and Alginate Composites for Biomedical Applications. Asian J. Pharm. Sci. 2021, 16, 280–306. [Google Scholar] [CrossRef]
- Gao, C.; Pollet, E.; Avérous, L. Properties of Glycerol-Plasticized Alginate Films Obtained by Thermo-Mechanical Mixing. Food Hydrocoll. 2017, 63, 414–420. [Google Scholar] [CrossRef]
- Eslami, Z.; Elkoun, S.; Robert, M.; Adjallé, K. A Review of the Effect of Plasticizers on the Physical and Mechanical Properties of Alginate-Based Films. Molecules 2023, 28, 6637. [Google Scholar] [CrossRef]
- Akshaya, S.; Nathanael, A.J. A Review on Hydrophobically Associated Alginates: Approaches and Applications. ACS Omega 2024, 9, 4246–4262. [Google Scholar] [CrossRef]
- Hu, C.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Ions-Induced Gelation of Alginate: Mechanisms and Applications. Int. J. Biol. Macromol. 2021, 177, 578–588. [Google Scholar] [CrossRef]
- Carafa, R.N.; Foucher, D.A.; Sacripante, G.G. Biobased Polymers from Lignocellulosic Sources. Green Chem. Lett. Rev. 2023, 16, 2153087. [Google Scholar] [CrossRef]
- Jost, V.; Kobsik, K.; Schmid, M.; Noller, K. Influence of Plasticiser on the Barrier, Mechanical and Grease Resistance Properties of Alginate Cast Films. Carbohydr. Polym. 2014, 110, 309–319. [Google Scholar] [CrossRef]
- Giz, A.S.; Berberoglu, M.; Bener, S.; Aydelik-Ayazoglu, S.; Bayraktar, H.; Alaca, B.E.; Catalgil-Giz, H. A Detailed Investigation of the Effect of Calcium Crosslinking and Glycerol Plasticizing on the Physical Properties of Alginate Films. Int. J. Biol. Macromol. 2020, 148, 49–55. [Google Scholar] [CrossRef]
- Foroughi-Dahr, M.; Mostoufi, N.; Sotudeh-Gharebagh, R.; Chaouki, J. Reference Module in Chemistry, Molecular Sciences and Chemical Engineering: Chapter Title: Particle Coating in Fluidized Beds; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar] [CrossRef]
- Mohammed, M.I.; El-Sayed, F. PEG’s Impact as a Plasticizer on the PVA Polymer’s Structural, Thermal, Mechanical, Optical, and Dielectric Characteristics. Opt. Quantum Electron. 2023, 55, 1141. [Google Scholar] [CrossRef]
- Pradhan, D.K.; Choudhary, R.N.P.; Samantaray, B.K.; Karan, N.K.; Katiyar, R.S. Effect of Plasticizer on Structural and Electrical Properties of Polymer Nanocompsoite Electrolytes. Int. J. Electrochem. Sci. 2007, 2, 861–871. [Google Scholar] [CrossRef]
- Aharoni, S.M. Increased Glass Transition Temperature in Motionally Constrained Semicrystalline Polymers. Polym. Adv. Technol. 1998, 9, 169–201. [Google Scholar] [CrossRef]
- Lim, H.; Hoag, S.W. Plasticizer Effects on Physical-Mechanical Properties of Solvent Cast Soluplus® Films. AAPS PharmSciTech 2013, 14, 903–910. [Google Scholar] [CrossRef]
- Dalal, S.R.; El-Naggar, N.E.A.; El Naeem, G.A. Biosynthesis of Sustainable Biodegradable Bioplastics Using Alginate Extracted from Padina Pavonica, Optimization and Characterization. Algal Res. 2023, 76, 103325. [Google Scholar] [CrossRef]
- Silva, R.R.A.; Marques, C.S.; Arruda, T.R.; Teixeira, S.C.; de Oliveira, T.V.; Stringheta, P.C.; dos Santos Pires, A.C.; de Fátima Ferreira Soares, N. Ionic Strength of Methylcellulose-Based Films: An Alternative for Modulating Mechanical Performance and Hydrophobicity for Potential Food Packaging Application. Polysaccharides 2022, 3, 426–440. [Google Scholar] [CrossRef]
- Silva, R.R.A.; de Freitas, P.A.V.; Teixeira, S.C.; de Oliveira, T.V.; Marques, C.S.; Stringheta, P.C.; Pires, A.C.D.S.; de Fátima Ferreira Soares, N. Plasticizer Effect and Ionic Cross-Linking: The Impact of Incorporating Divalent Salts in Methylcellulose Films for Colorimetric Detection of Volatile Ammonia. Food Biophys. 2022, 17, 59–74. [Google Scholar] [CrossRef]
- Yu Larchenko, E.; Shadrina, E.V.; Khonina, T.G.; Chupakhin, O.N. New Hybrid Chitosan–Silicone-Containing Glycerohydrogels. Mendeleev Commun. 2014, 24, 201–202. [Google Scholar] [CrossRef]
- Baccile, N.; Babonneau, F.; Thomas, B.; Coradin, T. Introducing Ecodesign in Silica Sol-Gel Materials. J. Mater. Chem. 2009, 19, 8537–8559. [Google Scholar] [CrossRef]
- Shetranjiwalla, S.; Fasulo, A.; Rhoden, S. Eco-Design and Tunable Structure-Properties of Chitosan-Epoxy-Glycerol-Silicate Biohybrids Using Integrated Crosslinking. Carbohydr. Polym. 2023, 299, 120187. [Google Scholar] [CrossRef] [PubMed]
- ASTM D882-18; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 2017; pp. 1–11.
- Bekin, S.; Sarmad, S.; Gürkan, K.; Keçeli, G.; Gürdaǧ, G. Synthesis, Characterization and Bending Behavior of Electroresponsive Sodium Alginate/Poly(Acrylic Acid) Interpenetrating Network Films under an Electric Field Stimulus. Sens. Actuators B Chem. 2014, 202, 878–892. [Google Scholar] [CrossRef]
- Capron, I.; Robert, P.; Colonna, P.; Brogly, M.; Planchot, V. Starch in Rubbery and Glassy States by FTIR Spectroscopy. Carbohydr. Polym. 2007, 68, 249–259. [Google Scholar] [CrossRef]
- Dharmayanti, N.; Supriatna, J.; Abinawanto; Yasman. Isolation and Partial Characterization of Alginate Extracted from Sargassum Polycystum Collected from Three Habitats in Banten, Indonesia. Biodiversitas 2019, 20, 1776–1785. [Google Scholar] [CrossRef]
- Li, P.; Dai, Y.-N.; Zhang, J.-P.; Wang, A.-Q.; Wei, Q. Chitosan-Alginate Nanoparticles as a Novel Drug Delivery System for Nifedipine. J. Biomed. Sci. 2008, 4, 221–228. [Google Scholar]
- Papageorgiou, S.K.; Kouvelos, E.P.; Favvas, E.P.; Sapalidis, A.A.; Romanos, G.E.; Katsaros, F.K. Metal-Carboxylate Interactions in Metal-Alginate Complexes Studied with FTIR Spectroscopy. Carbohydr. Res. 2010, 345, 469–473. [Google Scholar] [CrossRef]
- Hariyadi, D.M.; Islam, N. Current Status of Alginate in Drug Delivery. Adv. Pharmacol. Pharm. Sci. 2020, 2020, 8886095. [Google Scholar] [CrossRef]
- Pragya, A.; Mutalik, S.; Younas, M.W.; Pang, S.K.; So, P.K.; Wang, F.; Zheng, Z.; Noor, N. Dynamic Cross-Linking of an Alginate-Acrylamide Tough Hydrogel System: Time-Resolvedin Situmapping of Gel Self-Assembly. RSC Adv. 2021, 11, 10710–10726. [Google Scholar] [CrossRef]
- Barbut, S.; Harper, B.A. Dried Ca-Alginate Films: Effects of Glycerol, Relative Humidity, Soy Fibers, and Carrageenan. LWT 2019, 103, 260–265. [Google Scholar] [CrossRef]
- Avella, M.; Pace, E.D.; Immirzi, B.; Impallomeni, G.; Malinconico, M.; Santagata, G. Addition of Glycerol Plasticizer to Seaweeds Derived Alginates: Influence of Microstructure on Chemical-Physical Properties. Carbohydr. Polym. 2007, 69, 503–511. [Google Scholar] [CrossRef]
- Xie, F.; Gao, C.; Avérous, L. Alginate-Based Materials: Enhancing Properties through Multiphase Formulation Design and Processing Innovation. Mater. Sci. Eng. R Rep. 2024, 159, 100799. [Google Scholar] [CrossRef]
- Janik, W.; Nowotarski, M.; Ledniowska, K.; Shyntum, D.Y.; Krukiewicz, K.; Turczyn, R.; Sabura, E.; Furgoł, S.; Kudła, S.; Dudek, G. Modulation of Physicochemical Properties and Antimicrobial Activity of Sodium Alginate Films through the Use of Chestnut Extract and Plasticizers. Sci. Rep. 2023, 13, 11530. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Wu, L.; Wu, L.; Zhang, M. Rheology and Thermal Stability of Polylactide/Clay Nanocomposites. Polym. Degrad. Stab. 2006, 91, 3149–3155. [Google Scholar] [CrossRef]
- Chang, J.H.; An, Y.U.; Sur, G.S. Poly(Lactic Acid) Nanocomposites with Various Organoclays. I. Thermomechanical Properties, Morphology, and Gas Permeability. J. Polym. Sci. B Polym. Phys. 2002, 41, 94–103. [Google Scholar] [CrossRef]
- Davoodi, M.; Kavoosi, G.; Shakeri, R. Preparation and Characterization of Potato Starch-Thymol Dispersion and Film as Potential Antioxidant and Antibacterial Materials. Int. J. Biol. Macromol. 2017, 104, 173–179. [Google Scholar] [CrossRef]
- Sharma, A.; Verma, C.; Mukhopadhyay, S.; Gupta, A.; Gupta, B. Development of Sodium Alginate/Glycerol/Tannic Acid Coated Cotton as Antimicrobial System. Int. J. Biol. Macromol. 2022, 216, 303–311. [Google Scholar] [CrossRef]
- Liang, L.; Bhagia, S.; Li, M.; Huang, C.; Ragauskas, A.J. Cross-Linked Nanocellulosic Materials and Their Applications. ChemSusChem 2020, 13, 78–87. [Google Scholar] [CrossRef]



| Sample | Tonset (°C) | Tdmax (°C) | Sample | Tonset (°C) | Tdmax (°C) |
|---|---|---|---|---|---|
| NaAlg | 216 | 238.3 | CaAlg | 206.4 | 215.6 |
| GS 5% | 206.3 | 218.1 | GS 5% | 209.5 | 218.1 |
| GS 10% | 206.3 | 214.1 | GS 10% | 217.9 | 250.4 |
| GS 25% | 204.3 | 210.6 | GS 25% | 206.9 | 215.7 |
| GS 35% | 203.5 | 208.4 | GS 35% | 206.1 | 213.5 |
| GS 50% | 202.0 | 208.7 | GS 50% | 203.8 | 210.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fasulo, A.; Towie, C.; Mouchiroud, L.; Malik, H.; Foucher, D.; Sacripante, G. Enhancing the Properties of Sodium Alginate with a Glycerol–Silicate Plasticizer. Polysaccharides 2025, 6, 20. https://doi.org/10.3390/polysaccharides6010020
Fasulo A, Towie C, Mouchiroud L, Malik H, Foucher D, Sacripante G. Enhancing the Properties of Sodium Alginate with a Glycerol–Silicate Plasticizer. Polysaccharides. 2025; 6(1):20. https://doi.org/10.3390/polysaccharides6010020
Chicago/Turabian StyleFasulo, Anthony, Corradina Towie, Lucie Mouchiroud, Hamza Malik, Daniel Foucher, and Guerino Sacripante. 2025. "Enhancing the Properties of Sodium Alginate with a Glycerol–Silicate Plasticizer" Polysaccharides 6, no. 1: 20. https://doi.org/10.3390/polysaccharides6010020
APA StyleFasulo, A., Towie, C., Mouchiroud, L., Malik, H., Foucher, D., & Sacripante, G. (2025). Enhancing the Properties of Sodium Alginate with a Glycerol–Silicate Plasticizer. Polysaccharides, 6(1), 20. https://doi.org/10.3390/polysaccharides6010020







