Aggressive Surgical Management of Bilateral Metachronous Lung Metastases in Fibrolamellar Hepatocellular Carcinoma, a Case Report
Abstract
:1. Background
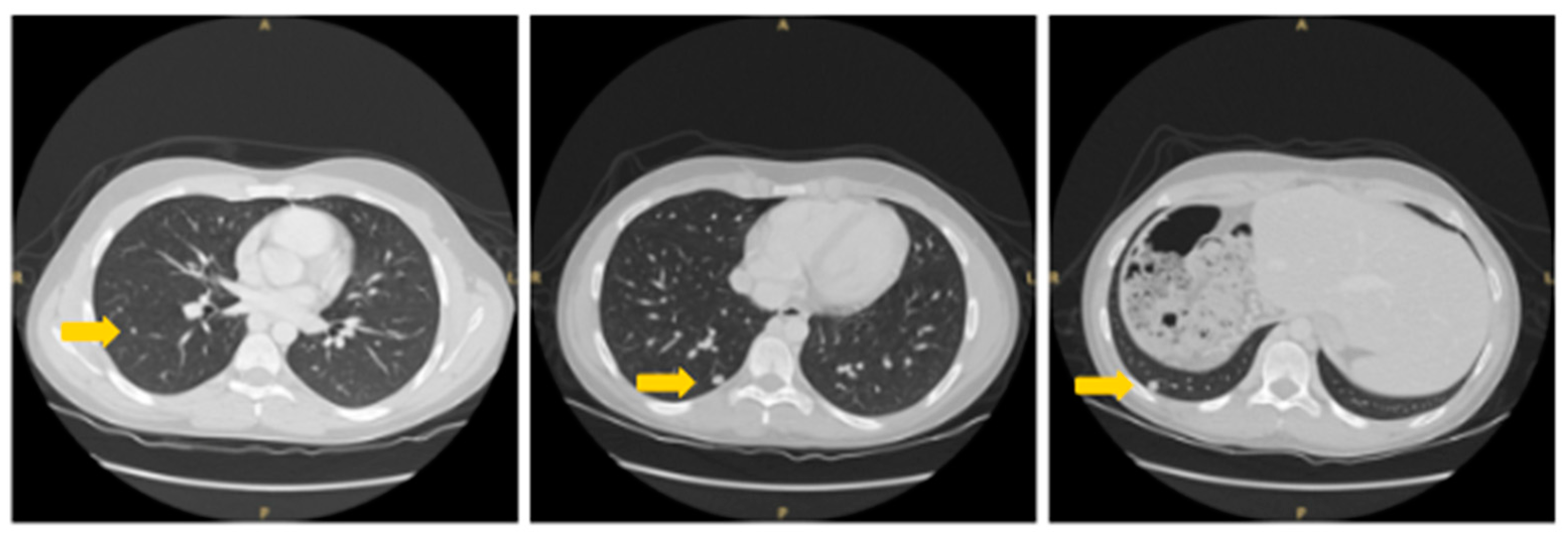
2. Patient Information, Clinical Findings and Diagnostic Assessment
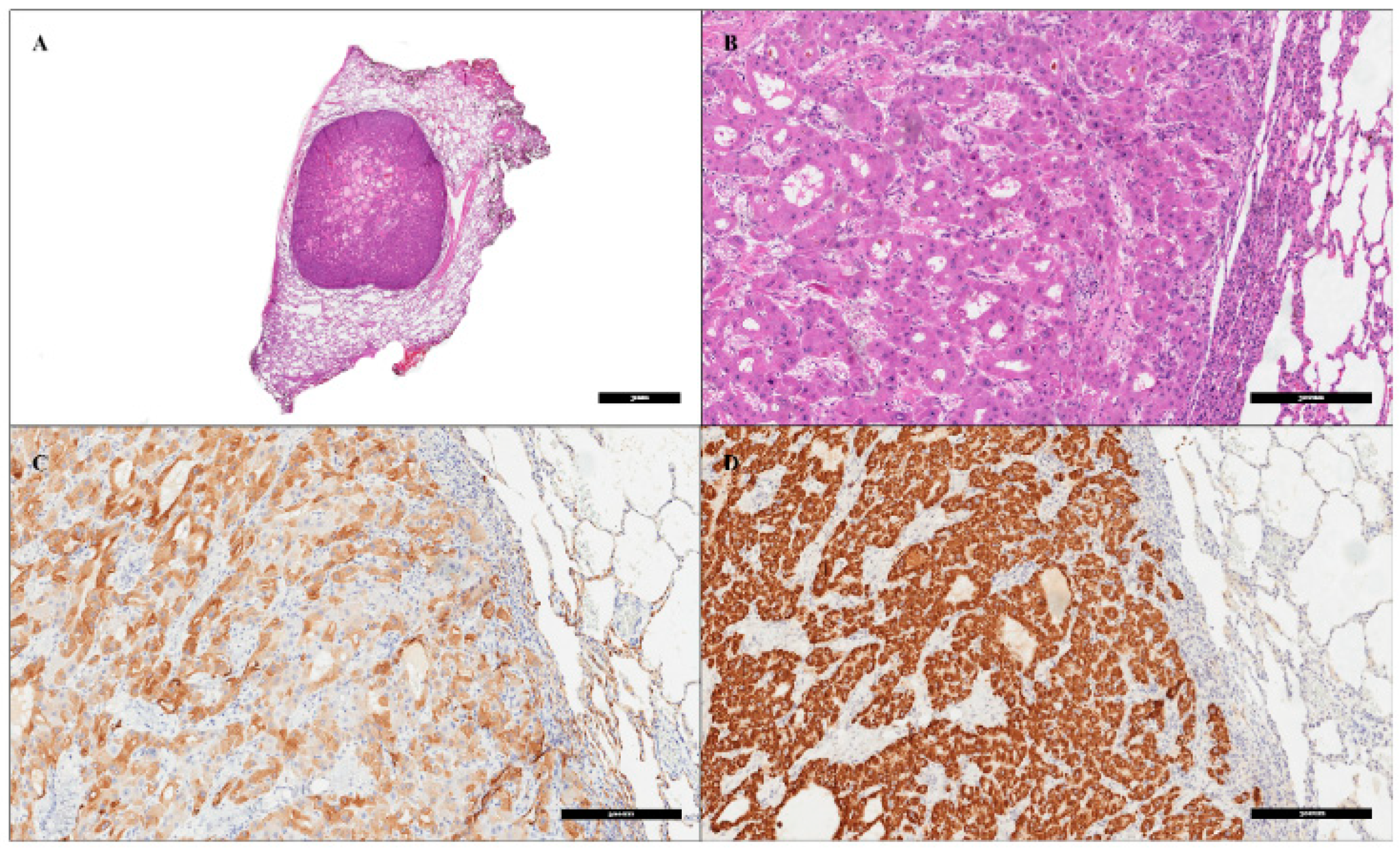
3. Therapeutic Intervention and Outcome
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lin, C.-C.; Yang, H.-M. Fibrolamellar Carcinoma: A Concise Review. Arch. Pathol. Lab. Med. 2018, 142, 1141–1145. [Google Scholar] [CrossRef] [PubMed]
- El-Serag, H.B.; Davila, J.A. Is Fibrolamellar Carcinoma Different from Hepatocellular Carcinoma? A US Population-Based Study. Hepatology 2004, 39, 798–803. [Google Scholar] [CrossRef] [PubMed]
- Cornella, H.; Alsinet, C.; Sayols, S.; Zhang, Z.; Hao, K.; Cabellos, L.; Hoshida, Y.; Villanueva, A.; Thung, S.; Ward, S.C.; et al. Unique Genomic Profile of Fibrolamellar Hepatocellular Carcinoma. Gastroenterology 2015, 148, 806–818.e10. [Google Scholar] [CrossRef] [PubMed]
- Kastenhuber, E.R.; Lalazar, G.; Houlihan, S.L.; Tschaharganeh, D.F.; Baslan, T.; Chen, C.-C.; Requena, D.; Tian, S.; Bosbach, B.; Wilkinson, J.E.; et al. DNAJB1-PRKACA Fusion Kinase Interacts with β-Catenin and the Liver Regenerative Response to Drive Fibrolamellar Hepatocellular Carcinoma. Proc. Natl. Acad. Sci. USA 2017, 114, 13076–13084. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, V.A.; Khobragade, K.; Bhandare, M.; Shrikhande, S.V. Management of Fibrolamellar Hepatocellular Carcinoma. Chin. Clin. Oncol. 2018, 7, 51. [Google Scholar] [CrossRef] [PubMed]
- Epstein, B.E.; Pajak, T.F.; Haulk, T.L.; Herpst, J.M.; Order, S.E.; Abrams, R.A. Metastatic Nonresectable Fibrolamellar Hepatoma: Prognostic Features and Natural History. Am. J. Clin. Oncol. 1999, 22, 22–28. [Google Scholar] [CrossRef]
- Weeda, V.B.; Murawski, M.; McCabe, A.J.; Maibach, R.; Brugières, L.; Roebuck, D.; Fabre, M.; Zimmermann, A.; Otte, J.B.; Sullivan, M.; et al. Fibrolamellar Variant of Hepatocellular Carcinoma Does Not Have a Better Survival than Conventional Hepatocellular Carcinoma--Results and Treatment Recommendations from the Childhood Liver Tumour Strategy Group (SIOPEL) Experience. Eur. J. Cancer 2013, 49, 2698–2704. [Google Scholar] [CrossRef] [PubMed]
- Kaseb, A.O.; Shama, M.; Sahin, I.H.; Nooka, A.; Hassabo, H.M.; Vauthey, J.-N.; Aloia, T.; Abbruzzese, J.L.; Subbiah, I.M.; Janku, F.; et al. Prognostic Indicators and Treatment Outcome in 94 Cases of Fibrolamellar Hepatocellular Carcinoma. Oncology 2013, 85, 197–203. [Google Scholar] [CrossRef]
- Groeschl, R.T.; Miura, J.T.; Wong, R.K.; Bloomston, M.; Lidsky, M.L.; Clary, B.M.; Martin, R.C.G.; Belli, G.; Buell, J.F.; Gamblin, T.C. Multi-Institutional Analysis of Recurrence and Survival after Hepatectomy for Fibrolamellar Carcinoma. J. Surg. Oncol. 2014, 110, 412–415. [Google Scholar] [CrossRef]
- Mammana, M.; Baldi, M.; Melan, L.; Dell’Amore, A.; Rea, F. Laser-Assisted Lung Metastasectomy: A Systematic Review. Updates Surg. 2023, 75, 1783–1793. [Google Scholar] [CrossRef]
- Edmondson, H.A. Differential Diagnosis of Tumors and Tumor-like Lesions of Liver in Infancy and Childhood. AMA J. Dis. Child. 1956, 91, 168–186. [Google Scholar] [CrossRef]
- Cannella, R.; Zins, M.; Brancatelli, G. ESR Essentials: Diagnosis of Hepatocellular Carcinoma-Practice Recommendations by ESGAR. Eur. Radiol. 2024, 34, 2127–2139. [Google Scholar] [CrossRef] [PubMed]
- Friedman, A.C.; Lichtenstein, J.E.; Goodman, Z.; Fishman, E.K.; Siegelman, S.S.; Dachman, A.H. Fibrolamellar Hepatocellular Carcinoma. Radiology 1985, 157, 583–587. [Google Scholar] [CrossRef]
- Ganeshan, D.; Szklaruk, J.; Kundra, V.; Kaseb, A.; Rashid, A.; Elsayes, K.M. Imaging Features of Fibrolamellar Hepatocellular Carcinoma. AJR Am. J. Roentgenol. 2014, 202, 544–552. [Google Scholar] [CrossRef]
- Alshareefy, Y.; Shen, C.Y.; Prekash, R.J. Exploring the Molecular Pathogenesis, Diagnosis and Treatment of Fibrolamellar Hepatocellular Carcinoma: A State of Art Review of the Current Literature. Pathol. Res. Pract. 2023, 248, 154655. [Google Scholar] [CrossRef]
- Patt, Y.Z.; Hassan, M.M.; Lozano, R.D.; Brown, T.D.; Vauthey, J.N.; Curley, S.A.; Ellis, L.M. Phase II Trial of Systemic Continuous Fluorouracil and Subcutaneous Recombinant Interferon Alfa-2b for Treatment of Hepatocellular Carcinoma. J. Clin. Oncol. 2003, 21, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Shimada, Y.; Furumoto, H.; Makino, Y.; Kudo, Y.; Maehara, S.; Hagiwara, M.; Kakihana, M.; Kajiwara, N.; Ohira, T.; et al. Comparative Analysis of the Results of Video-Assisted Thoracic Surgery Lobectomy Simulation Using the Three-Dimensional-Printed Biotexture Wet-Lung Model and Surgeons’ Experience. Interact. Cardiovasc. Thorac. Surg. 2021, 32, 284–290. [Google Scholar] [CrossRef]
- Kyoden, Y. Fibrolamellar Hepatocellular Carcinoma: A Case Report of 24-Year Postoperative Survival. HPB 2024, 26, S214–S215. [Google Scholar] [CrossRef]
- Ichiki, Y.; Sugio, K.; Baba, T.; Mizukami, M.; Oga, T.; Takenoyama, M.; Hanagiri, T.; Okamoto, K.; Yamaguchi, K.; Katagiri, S.; et al. Mediastinal Metastasis from a Fibrolamellar Hepatocellular Carcinoma: Report of a Case. Surg. Today 2010, 40, 360–364. [Google Scholar] [CrossRef]
- Bauer, J.; Köhler, N.; Maringer, Y.; Bucher, P.; Bilich, T.; Zwick, M.; Dicks, S.; Nelde, A.; Dubbelaar, M.; Scheid, J.; et al. The Oncogenic Fusion Protein DNAJB1-PRKACA Can Be Specifically Targeted by Peptide-Based Immunotherapy in Fibrolamellar Hepatocellular Carcinoma. Nat. Commun. 2022, 13, 6401. [Google Scholar] [CrossRef]
- Neumayer, C.; Ng, D.; Requena, D.; Jiang, C.S.; Qureshi, A.; Vaughan, R.; Prakash, T.P.; Revenko, A.; Simon, S.M. GalNAc-Conjugated siRNA Targeting the DNAJB1-PRKACA Fusion Junction in Fibrolamellar Hepatocellular Carcinoma. Mol. Ther. 2024, 32, 140–151. [Google Scholar] [CrossRef] [PubMed]
- Gummadi, J.; Wang, X.; Xie, C. Current Advances in the Treatment of Fibrolamellar Carcinoma of Liver. J. Hepatocell. Carcinoma 2023, 10, 745–752. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicotra, S.; Melan, L.; Busetto, A.; Bonis, A.; Lione, L.; Verzeletti, V.; Pezzuto, F.; Dell’Amore, A.; Calabrese, F.; Rea, F. Aggressive Surgical Management of Bilateral Metachronous Lung Metastases in Fibrolamellar Hepatocellular Carcinoma, a Case Report. Livers 2024, 4, 398-405. https://doi.org/10.3390/livers4030029
Nicotra S, Melan L, Busetto A, Bonis A, Lione L, Verzeletti V, Pezzuto F, Dell’Amore A, Calabrese F, Rea F. Aggressive Surgical Management of Bilateral Metachronous Lung Metastases in Fibrolamellar Hepatocellular Carcinoma, a Case Report. Livers. 2024; 4(3):398-405. https://doi.org/10.3390/livers4030029
Chicago/Turabian StyleNicotra, Samuele, Luca Melan, Alberto Busetto, Alessandro Bonis, Luigi Lione, Vincenzo Verzeletti, Federica Pezzuto, Andrea Dell’Amore, Fiorella Calabrese, and Federico Rea. 2024. "Aggressive Surgical Management of Bilateral Metachronous Lung Metastases in Fibrolamellar Hepatocellular Carcinoma, a Case Report" Livers 4, no. 3: 398-405. https://doi.org/10.3390/livers4030029
APA StyleNicotra, S., Melan, L., Busetto, A., Bonis, A., Lione, L., Verzeletti, V., Pezzuto, F., Dell’Amore, A., Calabrese, F., & Rea, F. (2024). Aggressive Surgical Management of Bilateral Metachronous Lung Metastases in Fibrolamellar Hepatocellular Carcinoma, a Case Report. Livers, 4(3), 398-405. https://doi.org/10.3390/livers4030029






