Soybean Extracts (Glycine Max) with Curcuma, Boswellia, Pinus and Urtica Are Able to Improve Quality of Life in Patients Affected by CP/CPPS: Is the Pro-Inflammatory Cytokine IL-8 Level Decreasing the Physiopathological Link?
Abstract
1. Introduction
2. Materials and Methods
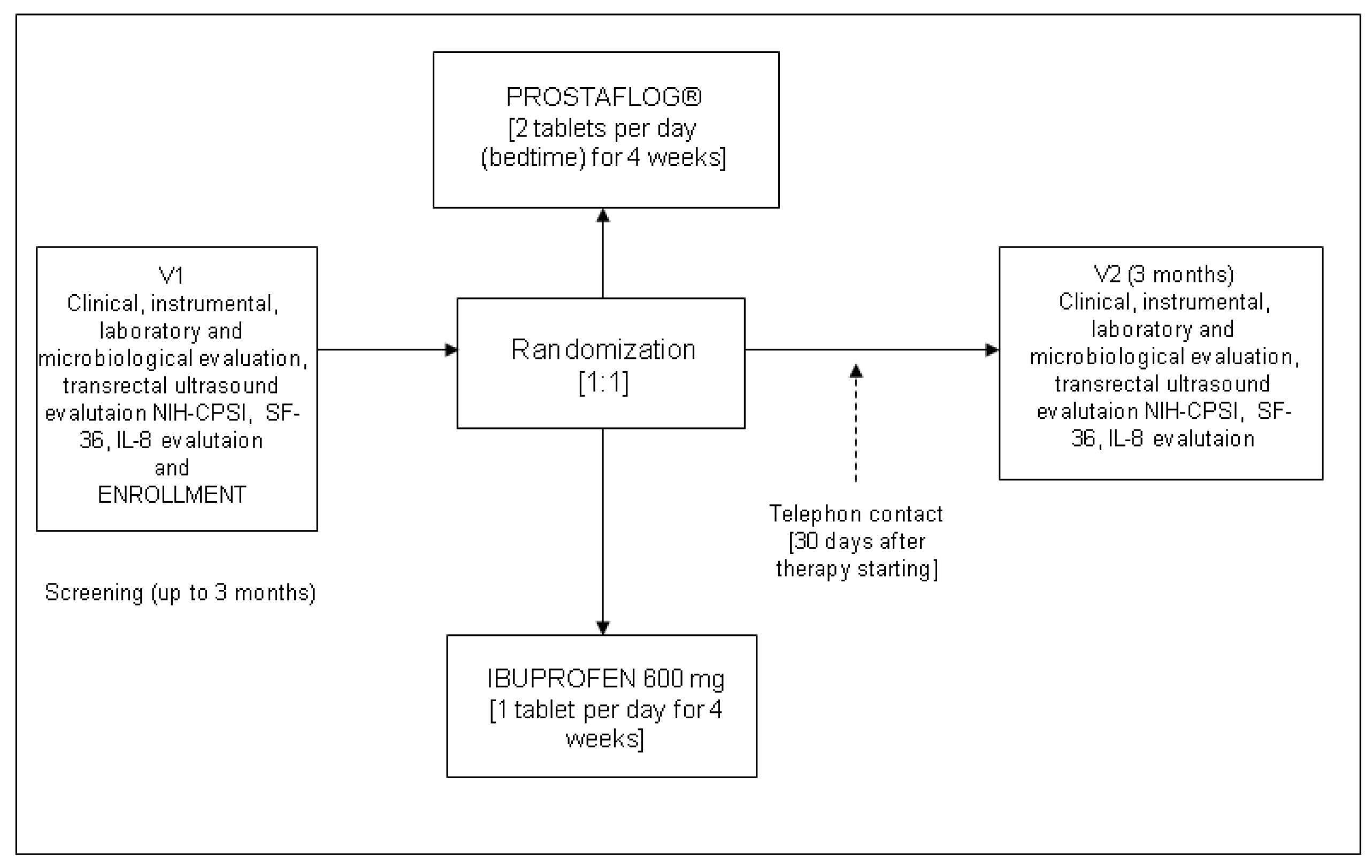
2.1. Study Design and Schedule
2.2. Outcome Measures
2.3. Inclusion and Exclusion Criteria
2.4. Composition and Characterization of the Extracts Used
2.5. Questionnaires and Urological Examinations
2.6. Microbiological Considerations, Sample Collection and Laboratory Procedures
2.7. Transrectal Seminal Vesicle Volume Measurement
2.8. Ethical and Statistical Considerations
3. Results
3.1. Clinical and Instrumental Results at the Follow-Up Evaluation
3.2. Laboratory Results at the Follow-Up Evaluation
3.3. Adverse Effects
4. Discussion
4.1. Major Finding
4.2. Results in Comparison with Other Studies
4.3. Strengths and Limitations of the Present Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Collins, M.M.; Stafford, R.S.; O’Leary, M.P.; Barry, M.J. How common is prostatitis? A national survey of physician visits. J. Urol. 1998, 159, 1224–1228. [Google Scholar] [CrossRef]
- Barbalias, G.A. Clinical and therapeutical guidelines for chronic prostatitis. From bacteriological importance to neuromus-cular considerations. Eur. Urol. 2000, 37, 116–117. [Google Scholar] [CrossRef]
- Nickel, J.C. Role of α1-blockers in chronic prostatitis syndromes. Br. J. Urol. 2008, 101, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Workshop Committee of the National Institute of Diabetes and Digestive and Kidney Disease (NIDDK). In Proceedings of the Chronic Prostatitis Workshop, Bethesda, MD, USA, 7–8 December 1995.
- Schaeffer, A.J. Classification (traditional and national institutes of health) and demographics of prostatitis. Urology 2002, 60, 5–6. [Google Scholar] [CrossRef]
- Tuğcu, V.; Taşçı, A.I.; Fazlıoğlu, A.; Gürbüz, G.; Özbek, E.; Şahin, S.; Kurtuluş, F.; Çek, M. A Placebo-Controlled Comparison of the Efficiency of Triple- and Monotherapy in Category III B Chronic Pelvic Pain Syndrome (CPPS). Eur. Urol. 2007, 51, 1113–1118. [Google Scholar] [CrossRef]
- Nickel, J.; Downey, J.; Clark, J.; Casey, R.W.; Pommerville, P.J.; Barkin, J.; Steinhoff, G.; Brock, G.; Patrick, A.B.; Flax, S.; et al. Levofloxacin for chronic prostatitis/chronic pelvic pain syndrome in men: A randomized placebo-controlled multicenter trial. Urology 2003, 62, 614–617. [Google Scholar] [CrossRef]
- Herati, A.S.; Moldwin, R.M. Alternative therapies in the management of chronic prostatitis/chronic pelvic pain syndrome. World J. Urol. 2013, 31, 761–766. [Google Scholar] [CrossRef]
- Shoskes, D.A.; Zeitlin, S.I.; Shahed, A.; Rajfer, J. Quercetin in men with category III chronic prostatitis: A preliminary prospective, double-blind, placebo-controlled trial. Urology 1999, 54, 960–963. [Google Scholar] [CrossRef]
- Cai, T.; Verze, P.; La Rocca, R.; Anceschi, U.; De Nunzio, C.; Mirone, V. The role of flower pollen extract in managing patients af-fected by chronic prostatitis/chronic pelvic pain syndrome: A comprehensive analysis of all published clinical trials. BMC Urol. 2017, 17, 32. [Google Scholar] [CrossRef]
- Kim, I.-S. Current Perspectives on the Beneficial Effects of Soybean Isoflavones and Their Metabolites for Humans. Antioxidants 2021, 10, 1064. [Google Scholar] [CrossRef]
- Cai, T.; Cocci, A.; Tiscione, D.; Puglisi, M.; Di Maida, F.; Malossini, G.; Verze, P.; Palmieri, A.; Mirone, V.; Bjerklund Johansen, T.E. L-Methionine associated with Hibiscus sabdariffa and Boswellia serrata extracts are not inferior to antibiotic treatment for symptoms relief in patients affected by recurrent uncomplicated urinary tract infections: Focus on antibiotic-sparing ap-proach. Arch Ital. Urol. Androl. 2018, 90, 97–100. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.; Mazzoli, S.; Bechi, A.; Addonisio, P.; Mondaini, N.; Pagliai, R.C.; Bartoletti, R. Serenoa repens associated with Urtica dioica (ProstaMEV®) and curcumin and quercitin (FlogMEV®) extracts are able to improve the efficacy of prulifloxacin in bacterial prostatitis patients: Results from a prospective randomised study. Int. J. Antimicrob. Agents 2009, 33, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-P.; Liang, S.-B.; Liang, N.; Bu, F.-L.; Lai, B.-Y.; Zhang, Y.-P.; Cao, H.-J.; Fei, Y.-T.; Robinson, N. The potential effects and use of Chinese herbal medicine pine pollen (Pinus pollen): A bibliometric analysis of pharmacological and clinical studies. World J. Tradit. Chin. Med. 2020, 6, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Penna, G.; Mondaini, N.; Amuchastegui, S.; Degli Innocenti, S.; Carini, M.; Giubilei, G.; Fibbi, B.; Colli, E.; Maggi, M.; Adorini, L. Sem-inal plasma cytokines and chemokines in prostate inflammation: Interleukin 8 as a predictive biomarker in chronic prosta-titis/chronic pelvic pain syndrome and benign prostatic hyperplasia. Eur. Urol. 2007, 51, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Khadra, A.; Fletcher, P.; Luzzi, G.; Shattock, R.; Hay, P. Interleukin-8 levels in seminal plasma in chronic prostatitis/chronic pel-vic pain syndrome and nonspecific urethritis. BJU Int. 2006, 97, 1043–1046. [Google Scholar] [CrossRef]
- Cai, T.; Verze, P.; La Rocca, R.; Palmieri, A.; Tiscione, D.; Luciani, L.G.; Mazzoli, S.; Mirone, V.; Malossini, G. The Clinical Efficacy of Pollen Extract and Vitamins on Chronic Prostatitis/Chronic Pelvic Pain Syndrome Is Linked to a Decrease in the Pro-Inflammatory Cytokine Interleukin. World J. Men’s Health 2017, 35, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Propert, K.J.; Alexander, R.B.; Nickel, J.; Kusek, J.W.; Litwin, M.S.; Landis, J.; Nyberg, L.M.; Schaeffer, A.J. Design of a multicenter randomized clinical trial for chronic prostatitis/chronic pelvic pain syndrome. Urology 2002, 59, 870–876. [Google Scholar] [CrossRef]
- European Association of Urology Guidelines. Available online: http://uroweb.org/wp-content/uploads/19-Urological-infections_LR2.pdf (accessed on 18 August 2021).
- Cai, T.; Wagenlehner, F.M.; Luciani, L.G.; Tiscione, D.; Malossini, G.; Verze, P.; Mirone, V.; Bartoletti, R. Pollen extract in association with vitamins provides early pain relief in patients affected by chronic prostatitis/chronic pelvic pain syndrome. Exp. Ther. Med. 2014, 8, 1032–1038. [Google Scholar] [CrossRef][Green Version]
- Giubilei, G.; Mondaini, N.; Crisci, A.; Raugei, A.; Lombardi, G.; Travaglini, F.; Del Popolo, G.; Bartoletti, R. The Italian Version of the National Institutes of Health Chronic Prostatitis Symptom Index. Eur. Urol. 2005, 47, 805–811. [Google Scholar] [CrossRef]
- Kaplan, R.M.; Bush, J.W.; Berry, C.C. Health status: Types of validity and the index of well-being. Health Serv. Res. 1976, 11, 478–507. [Google Scholar]
- Nickel, J.C.; Downey, J.; Hunter, D.; Clark, J. Prevalence of prostatitis-like symptoms population based study using the Na-tional Institutes of Health chronic prostates symptoms index. J. Urol. 2001, 165, 843–845. [Google Scholar] [CrossRef]
- Mazzoli, S.; Cai, T.; Rupealta, V.; Gavazzi, A.; Pagliai, R.C.; Mondaini, N.; Bartoletti, R. Interleukin 8 and Anti-Chlamydia trachomatis Mucosal IgA as Urogenital Immunologic Markers in Patients with C. trachomatis Prostatic Infection. Eur. Urol. 2007, 51, 1385–1393. [Google Scholar] [CrossRef] [PubMed]
- Sibona, M.; Destefanis, P.; Agnello, M.; Lillaz, B.; Giuliano, M.; Cai, T.; Gontero, P. The association of Boswellia resin extract and propolis derived polyphenols can improve quality of life in patients affected by prostatitis-like symptoms. Arch. Ital. Urol. Androl. 2020, 91, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Bagherniya, M.; Askari, G.; Alikiaii, B.; Abbasi, S.; Soleimani, D.; Sathyapalan, T.; Jamialahmadi, T.; Sahebkar, A. Curcumin for the Treatment of Prostate Diseases: A Systematic Review of Controlled Clinical Trials. Adv. Exp. Med. Biol. 2021, 1291, 345–362. [Google Scholar] [CrossRef] [PubMed]
- Razavi, B.M.; Rahbardar, M.G.; Hosseinzadeh, H. A review of therapeutic potentials of turmeric (Curcuma longa) and its active constituent, curcumin, on inflammatory disorders, pain, and their related patents. Phytother. Res. 2021, 35, 6489–6513. [Google Scholar] [CrossRef]
- Ferguson, J.J.A.; Abbott, K.A.; Garg, M.L. Anti-inflammatory effects of oral supplementation with curcumin: A systematic review and meta-analysis of randomized controlled trials. Nutr. Rev. 2021, 79, 1043–1066. [Google Scholar] [CrossRef] [PubMed]
- van Die, M.D.; Bone, K.M.; Emery, J.; Williams, S.G.; Pirotta, M.V.; Paller, C.J. Phytotherapeutic interventions in the management of biochemically recurrent prostate cancer: A systematic review of randomised trials. BJU Int. 2016, 117, 17–34. [Google Scholar] [CrossRef]
- Vicari, E.; Malaguarnera, G.; Vicari, B.O.; Salmeri, M.; Salemi, M.; Castiglione, R. Differentially Enhancing Effects of Long-term Treatment with Serrazyme, Boswellia and Pine on Seminal Bacterial Detection in Patients with Chronic Bacterial or In-flammatory Prostatitis, Probably Related to Several Degrees of Bacterial Adherence. Curr. Clin. Pharmacol. 2018, 13, 183–189. [Google Scholar] [CrossRef] [PubMed]
| PROSTAFLOG® Group | Ibuprofen Group | ||
|---|---|---|---|
| Mean (SD * or %) | Mean (SD * or %) | ||
| Patients (n°) | 39 | 38 | |
| Age | 34.1 ± 5.2 | 34.4 ± 4.7 | 0.82 |
| Educational qualification | |||
| Primary School | 3 (7.6) | 2 (5.2) | 0.65 |
| High School | 26 (66.6) | 22 (57.8) | |
| University | 10 (25.8) | 14 (37.0) | |
| Sexual behaviour | |||
| 1 partner | 31 (79.4) | 32 (84.2) | 0.43 |
| >1 partners | 8 (20.6) | 6 (15.8) | |
| Contraceptive use | |||
| Condom | 28 (71.7) | 26 (68.4) | 0.67 |
| Coitus interruptus | 11 (28.3) | 12 (31.6) | |
| Start of CP/CPPS # history (months) | 19.8 ± 4.9 | 19.5 ± 5.1 | 0.61 |
| Symptoms Score at baseline (mean) (range) | |||
| NIH-CPSI § | 25.8 ± 1.9 | 25.9 ± 1.7 | 0.43 |
| SF-36 ‡ | 93.6 ± 1.0 | 93.8 ± 1.1 | 0.49 |
| Clinical presentation | |||
| Dysuria | 20 (51.2) | 18 (47.3) | 0.75 |
| Urgency | 1 (2.4) | 2 (5.4) | |
| Dysuria + Frequency | 8 (20.6) | 7 (18.4) | |
| Burning | 10 (25.8) | 11 (28.9) | |
| Pain | |||
| Perineal | 15 (38.4) | 16 (42.1) | 0.83 |
| Scrotal | 5 (12.8) | 4 (10.5) | |
| Suprapubic | 10 (25.8) | 10 (26.3) | |
| Lower Abdominal | 9 (23.0) | 8 (21.1) | |
| Pain frequency | |||
| Daily | 29 (74.2) | 30 (78.9) | 0.86 |
| Weekly | 10 (25.8) | 8 (21.1) | |
| IL-8 mean level (pg/mL) | |||
| 917 (332–>12,000) | 912 (418–>12,000) | 0.89 |
| PROSTAFLOG® Group | Ibuprofen Group | ||
| Mean (SD *) | Mean (SD *) | ||
| NIH-CPSI ° | |||
| pre-treatment | 25.8 ± 1.9 | 25.9 ± 1.7 | 0.43 |
| post-treatment | 10.8 ± 2.8 | 21.7 ± 4.1 | <0.001 |
| p | <0.001 | 0.0003 | |
| (NIH-CPSI pain domain) | |||
| pre-treatment | 11.5 ± 1.9 | 11.1 ± 2.6 | 0.31 |
| post-treatment | 6.6 ± 1.4 | 8.8 ± 2.2 | 0.003 |
| p | <0.001 | <0.001 | |
| Reduction of NIH-CPSI pain domain | −5.4 ± 0.2 | −3.0 ± 0.5 | <0.001 |
| SF-36 ‡ | |||
| pre-treatment | 93.6 ± 1.0 | 93.8 ± 1.1 | 0.91 |
| post-treatment | 97.9 ± 1.8 | 94.6 ± 3.9 | <0.001 |
| p | <0.001 | 0.13 | |
| IL-8 mean level (pg/mL) | |||
| pre-treatment | 917 (332–>12,000) | 912 (418–>12,000) | 0.89 |
| post-treatment | 301 (<100–3460) | 527 (346–>12,000) | <0.001 |
| p | <0.001 | 0.003 | |
| Seminal vesicles total volume (mL) | |||
| pre-treatment | 18.3 ± 7.1 | 19.1 ± 6.2 | 0.77 |
| post-treatment | 11.2 ± 2.4 | 15.9 ± 9.1 | <0.001 |
| p | <0.001 | 0.09 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, T.; Anceschi, U.; Tamanini, I.; Verze, P.; Palmieri, A. Soybean Extracts (Glycine Max) with Curcuma, Boswellia, Pinus and Urtica Are Able to Improve Quality of Life in Patients Affected by CP/CPPS: Is the Pro-Inflammatory Cytokine IL-8 Level Decreasing the Physiopathological Link? Uro 2022, 2, 40-48. https://doi.org/10.3390/uro2010006
Cai T, Anceschi U, Tamanini I, Verze P, Palmieri A. Soybean Extracts (Glycine Max) with Curcuma, Boswellia, Pinus and Urtica Are Able to Improve Quality of Life in Patients Affected by CP/CPPS: Is the Pro-Inflammatory Cytokine IL-8 Level Decreasing the Physiopathological Link? Uro. 2022; 2(1):40-48. https://doi.org/10.3390/uro2010006
Chicago/Turabian StyleCai, Tommaso, Umberto Anceschi, Irene Tamanini, Paolo Verze, and Alessandro Palmieri. 2022. "Soybean Extracts (Glycine Max) with Curcuma, Boswellia, Pinus and Urtica Are Able to Improve Quality of Life in Patients Affected by CP/CPPS: Is the Pro-Inflammatory Cytokine IL-8 Level Decreasing the Physiopathological Link?" Uro 2, no. 1: 40-48. https://doi.org/10.3390/uro2010006
APA StyleCai, T., Anceschi, U., Tamanini, I., Verze, P., & Palmieri, A. (2022). Soybean Extracts (Glycine Max) with Curcuma, Boswellia, Pinus and Urtica Are Able to Improve Quality of Life in Patients Affected by CP/CPPS: Is the Pro-Inflammatory Cytokine IL-8 Level Decreasing the Physiopathological Link? Uro, 2(1), 40-48. https://doi.org/10.3390/uro2010006








