Abstract
The present investigation was performed to evaluate the effects of various synthetic antioxidants (vitamin A, vitamin E, β-carotene, and BHT) on the oxidation of sunflower oil subjected to accelerated thermal storage at 60 °C for three months (12 weeks). The performance of the antioxidants studied was evaluated using several quality parameters: the free fatty acid value (FFA), primary oxidation (via the peroxide value (PV) and K232 value), secondary oxidation products (via the anisidine value (p-AV) and K270 value), and the total oxidation value (TOTOX). The fatty acid composition (FAC), oxidizability value (COX), iodine value (IV), and pigment content (chlorophyll and carotenoid) were also evaluated. The results revealed that the control sample of sunflower oil exhibited higher susceptibility to oxidative deterioration. Antioxidants at 200 ppm were more effective in preserving the oxidative stability of sunflower oil subjected to accelerated storage compared to the control oil. The smallest increases in all stability parameter indexes were recorded for antioxidant-supplemented sunflower oil. However, the IV and chlorophyll and carotenoid contents were reduced. At 200 ppm, vitamin E and β-carotene showed the greatest stability in sunflower oil, while their combination with vitamin A at 100 ppm of each showed the lowest stability. In addition, synthetic antioxidants provided greater protection against the degradation of polyunsaturated fatty acids (PUFAs). The highest level of PUFA degradation was recorded in the control oil, followed by the oil containing vitamin A. In conclusion, adding synthetic antioxidants to sunflower oil improves its stability during storage. However, some authors associated these molecules with a health risk due to carcinogenic effects as these molecules have been listed as “Generally Recognized As Safe” (GRAS).
1. Introduction
Vegetable oils and fats are a valuable part of a healthy human diet [1]. Among these oils is sunflower (Helianthus annuus L.) oil, the most cultivated vegetable oil worldwide [2], which is mainly used in the food, cosmetic, and pharmaceutical industries [3]. It accounts for 12% of the world’s edible oil production, making it the fourth most important oilseed crop [4]. Sunflower oil is a rich source of polyunsaturated fatty acids (PUFAs) and monounsaturated fatty acids (MUFAs), tocopherols, and sterols [5]. Additionally, it has a high content of polyunsaturated fatty acids (85–95%), consisting mostly of linoleic and oleic acids (~90%) [6].
A major problem with oils, fats, and foods incorporating fats is oxidation, which leads to alterations in quality characteristics such as weight, taste, texture, and nutritional value [7,8]. It is a process that depends largely on various parameters such as the unsaturation level, oxygen molecule type, food matrix type, and the availability of metal ions and antioxidants [9]. Indeed, highly unsaturated oils are unambiguously recognized as more susceptible to oxidative deterioration [1,10] during storage or heat treatments [11]. However, due to the relatively higher proportion of unsaturated fatty acids (UFAs) on one hand and beneficial components such as antioxidants, which are also removed during the refining process [12], on the other hand, sunflower oil is more prone to oxidation and/or degradation during processing and storage [2]. This deterioration can occur through several reactions such as the oxidation of fatty acids, the hydrolysis of triacylglycerols, polymerization, and isomerization [13]. Therefore, several products are generated (aldehydes, epoxides, hydroxyketones, dicarboxylic compounds, and carbonyls) that can induce pathological complications such as cardiovascular diseases, diabetes, colon cancer, neurodegenerative diseases, and aging [14].
Stability to oxidation is a vital parameter for assessing the quality of edible oils and fats [15]. This term reflects the ability of oils and fats, during processing and storage, to resist oxidative deterioration (rancidity) [15]. Oxidative stability can be improved by adding suitable antioxidants [16]. The term antioxidant refers to a compound or system that has the ability to retard autoxidation by preventing the formation of free radicals or stopping their spread [17] in foods or biological systems [18]. Antioxidants act as inhibitors of the oxidation process [10,19] through various mechanisms and at various levels [10]. In effect, antioxidants can act as scavengers of peroxidation initiator species, interrupt the auto-oxidation chain reaction, chelate metal ions, and deactivate ●O2− to prevent peroxide formation [17]. For this purpose, synthetic antioxidants are used which are able to scavenge free radicals and chelate transition metals [17]. The antioxidant capacity of antioxidant compounds is dependent on their structural properties, the concentration used, the applied temperature, exposure to light [18], the presence of synergistic and pro-oxidant compounds, and the characteristics of the substrate susceptible to oxidation [20].
Many synthetic antioxidant substances have been developed and applied practically as food additives and supplements [19]. Synthetic antioxidants are used to increase the oxidative stability of oils [21]. The most commonly used antioxidants are Butylhydroxyanisol (BHA), butylhydroxytoluene (BHT), tert-butyl-hydroquinone (TBHQ) [12,22], and propyl gallate (PG) [12]. Traditionally, antioxidants are used to protect vegetable oils from oxidative aging and to extend their shelf life [23]. They are effective and less expensive than antioxidants of natural origin [23]. Antioxidants for use in food must be inexpensive, non-toxic, and effective at low concentrations [7,12].
The use of synthetic and natural antioxidants in food preservation, along with their safety and selection as additives, has been well documented. These compounds are generally classified as “Generally Recognized As Safe” (GRAS) ingredients. However, both the proportions in which antioxidants are added to foods and tolerance thresholds (acceptable quantities) are subject to significant variations. For example, TBHQ can be used alone or in combination with BHA and/or BHT up to a maximum limit of 200 ppm [24].
The aim of this study was to investigate the efficacy of several synthetic antioxidants including vitamin A, vitamin E, β-carotene, and butylated hydroxytoluene (BHT) on the qualitative properties of sunflower vegetable oil and their effectiveness in retarding oxidation during accelerated thermal storage. Conducted at a constant temperature of 60 °C over a three-month period, this study assessed antioxidant activity by determining the extent of the inhibition of the formation of primary and secondary oxidation products by each additive. This research also aimed to explore the behavior of these antioxidants and identify the most effective one for improving the shelf life and maintaining the nutritional quality of sunflower oil under high-temperature storage conditions.
2. Materials and Methods
2.1. Sunflower Oil Sampling
The starting oil was industrially-produced sunflower oil purchased from a local supermarket which contained no oxidation-inhibiting additives. All antioxidants, BHT, β-carotene, vitamin A, and α-tocopherol, were obtained from the company “Huileries du Souss Belhassan (HSB)”. The oil samples and antioxidants were stored at 4 °C before the experiments began. All other chemicals and reagents employed were of analytical grade, supplied by Merck, and used without further purification.
2.2. Mixture Preparation and Storage of Supplemented Sunflower Oil
Synthetic antioxidants (vitamin A, vitamin E, β-carotene, and BHT) were added directly to the sunflower oil. Each antioxidant was added at 200 ppm. A mixture of vitamin E (100 ppm) and β-carotene (100 ppm) was also added. Oil without added antioxidants were considered simple control (SF-C) oils. The prepared samples were individually transferred into glass bottles (60 mL), sealed, and filled. Then, the samples were stored in an oven at 60 ± 2 °C for 90 days. The oxidation state of the control and supplemented oils was examined by determining changes in chemical indicators of oil oxidation. Free fatty acids (FFAs), the peroxide value (PV), the p-anisidine value (p-AV), the K232 value (conjugated dienes), the K270 value (conjugated trienes), the TOTOX value, and the chlorophyll and carotenoid content were measured every fortnight. The fatty acid content (FAC), iodine value (IV), and COX value were determined at the end of each month of storage.
2.3. Analytical Methods
2.3.1. Determination of Free Fatty Acids Value (FFA)
The free fatty acid (FFA) value was determined following the guidelines outlined in [25]. Briefly, 10 g of each oil sample was dissolved in 50 mL of neutral ethanol (95%). The resulting mixture was manually stirred and titrated with potassium hydroxide (0.1 N) using phenolphthalein as an indicator. The FFA value was then expressed as the percentage (g/100 g) of oleic acid present in the oil samples.
2.3.2. Determination of Peroxide Value (PV)
The peroxide value (PV) of the oils was determined using the standard method outlined in [26]. Initially, 5 g of each oil sample was accurately weighed and dissolved in a mixture containing acetic acid and chloroform in a ratio of 3:2 (v/v). Subsequently, 1 mL of saturated iodide solution was added to this mixture. The reaction was allowed to proceed for 5 min at room temperature in the absence of light. Following this, 75 mL of distilled water was added to the solution, and titration was carried out using 0.01 N sodium thiosulfate (Na2O3S2), with starch serving as a color indicator. The titration continued until the blue color disappeared. The results were then expressed in milliequivalents of active oxygen per kilogram of oil (mEq (O2)/Kg).
2.3.3. Determination of K232 (Conjugated Diene) and K270 (Conjugated Triene) Values
The K232 and K270 values were obtained in accordance with the procedures specified in [27]. These values represent the specific extinctions of a mixture solution of 1% (w/v) cyclohexane and sunflower oil, which were determined using a SCILOGEX SP-UV1100 spectrometer fitted with a 1 cm cuvette. The parameters were derived from measuring the absorbance of the oil sample at specific wavelengths, namely 232 nm for K232 (conjugated dienes) and 270 nm for K270 (conjugated trienes) [28].
2.3.4. Determination of p-Anisidine Value (p-AV)
The p-anisidine value (p-AV) was determined following the standardized method outlined in [29]. First, 2 g of the oil sample was accurately weighed and placed into a 25 mL flask. It was then diluted with 25 mL of isooctane to prepare solution A. The absorbance (Ab) of solution A at 350 nm was measured using a SCILOGEX SP-UV1100 spectrometer, with isooctane serving as a blank. Subsequently, 5 mL of solution A was transferred into the first tube, and precisely 1 mL of p-anisidine solution (0.25% w/v in glacial acetic acid) was added. Following a 10 min incubation period, the absorbance (As) at 350 nm was measured using the spectrometer, with the second tube containing isooctane and p-anisidine used as the blank reference. The p-AV was calculated according to the Formula (1) as follows:
p-AV = (25 × (1.2 × (As − Ab)))/m
p-AV is the p-anisidine value. As is the absorbance of the oil solution sample post reaction with p-anisidine. Ab is the absorbance of the non-reacted test solution. m is the mass, in grams, of the test sample. Finally, 25 is the equivalent volume of isooctane dissolving the oil, and 1.2 is a correction factor use to account for the dilution of the test sample by 1 mL of the p-anisidine solution.
2.3.5. Determination of Total Oxidation Value (TOTOX)
The total oxidation value (TOTOX) value, which provides a comprehensive assessment of the early and late stages of oxidation, giving a more complete overview of oxidation stability and overall oil quality, is calculated theoretically by combining the peroxide value (early stage) and the p-anisidine value (late stage) using Formula (2) [30]:
TOTOX = 2 × PV + p-AV
2.3.6. Determination of Fatty Acid Composition (FAC)
In accordance with the established standard in [31], the fatty acid composition was obtained using a gas chromatograph (Agilent-6890, Santa Clara, CA, USA) coupled to a flame ionization detector (GC/FID) after the transformation of the fatty acids into the corresponding fatty acid methyl esters by a transmethylation process. Approximately 0.1 g of each oil sample was carefully vortexed with 2 mL of isooctane and 0.1 mL of a methanolic potassium hydroxide solution (2 N) in a test tube for 1 min. The solution was then left to stand for 2 min. Next, 2 mL of 40% (w/v) sodium chloride solution was added and stirred immediately. The isooctane phase containing the fatty acid methyl esters was mixed with 1 g of sodium hydrogen sulphate, and 1 µL of the phase was injected into the GC in split mode (fractionation ratio: 1:50). The carrier gas was helium (with a flow rate of 1 mL/min), and a DB 23 AG-TRANS capillary column (60 m × 320 μm diameter; 0.25 μm film thickness) was used. The oven, injector, and detector were set at 185, 200, and 230 °C, respectively. A mixture of hydrogen and air was used to generate the flame. The results were expressed as a percentage for each fatty acid detected in the oil [32].
2.3.7. Determination of Iodine Value (IV)
The iodine value (IV) is utilized as an indicator to evaluate the degree of unsaturation in oils, representing the weight of iodine absorbed per 100 g of oil or fat. It is calculated from the percentage of unsaturated fatty acids, which can be determined using Equation (3) [30] as follows:
IV = (% C16:1 × CO1) + (% C18:1 × CO2) + (% C18:2 × CO3) + (% C18:3 × CO3)
The notation C16:1 refers to palmitoleic acid, C18:1 represents oleic acid, C18:2 stands for linoleic acid, and C18:3 denotes linolenic acid. CO1, CO2, CO3, and CO4 correspond, respectively, to iodine values of 1.001, 0.899, 1.814, and 2.737 for each fatty acid.
2.3.8. Determination of Oxidizability Value (COX)
The oxidizability value (COX) is a measurement of an oil’s sensitivity to oxidation and is calculated as a percentage by means of a theoretical formula derived from the fatty acid composition. This computation method allows for a quantitative assessment of the oil’s oxidizability on the basis of its fatty acid composition, thereby assisting in understanding and predicting its stability and shelf life. The equation used to calculate the COX is as follows (4) [28]:
COX = ((% C16:1 + % C18:1) + 10.3 × (% C18:2) + 21.6 × (% C18:3))/100
2.3.9. Determination of Pigment Content
The determination of the oil pigment content followed the protocol outlined by [24]. In a 25 mL volumetric flask, approximately 7.5 g of each oil sample was placed and then diluted with cyclohexane to reach a volume of 25 mL. The content of chlorophylls and carotenoids was quantified by measuring the absorbance at 670 nm and 470 nm, respectively, using Equations (5) and (6) as follows:
Chlorophyll content (mg/Kg) = (A670 × 106)/(613 × 100 × L)
Carotenoid content (mg/Kg) = (A470 × 106)/(2000 × 100 × L)
2.4. Statistical Analysis
The results are presented as mean ± SD (standard deviation, n = 3) values. A statistical analysis to evaluate statistically significant differences in mean values was carried out with the Tukey test at a significance level of 0.05, using R software (version 4.0.5).
3. Results and Discussion
3.1. Effect of Thermal Storage on Free Fatty Acids (FFAs)
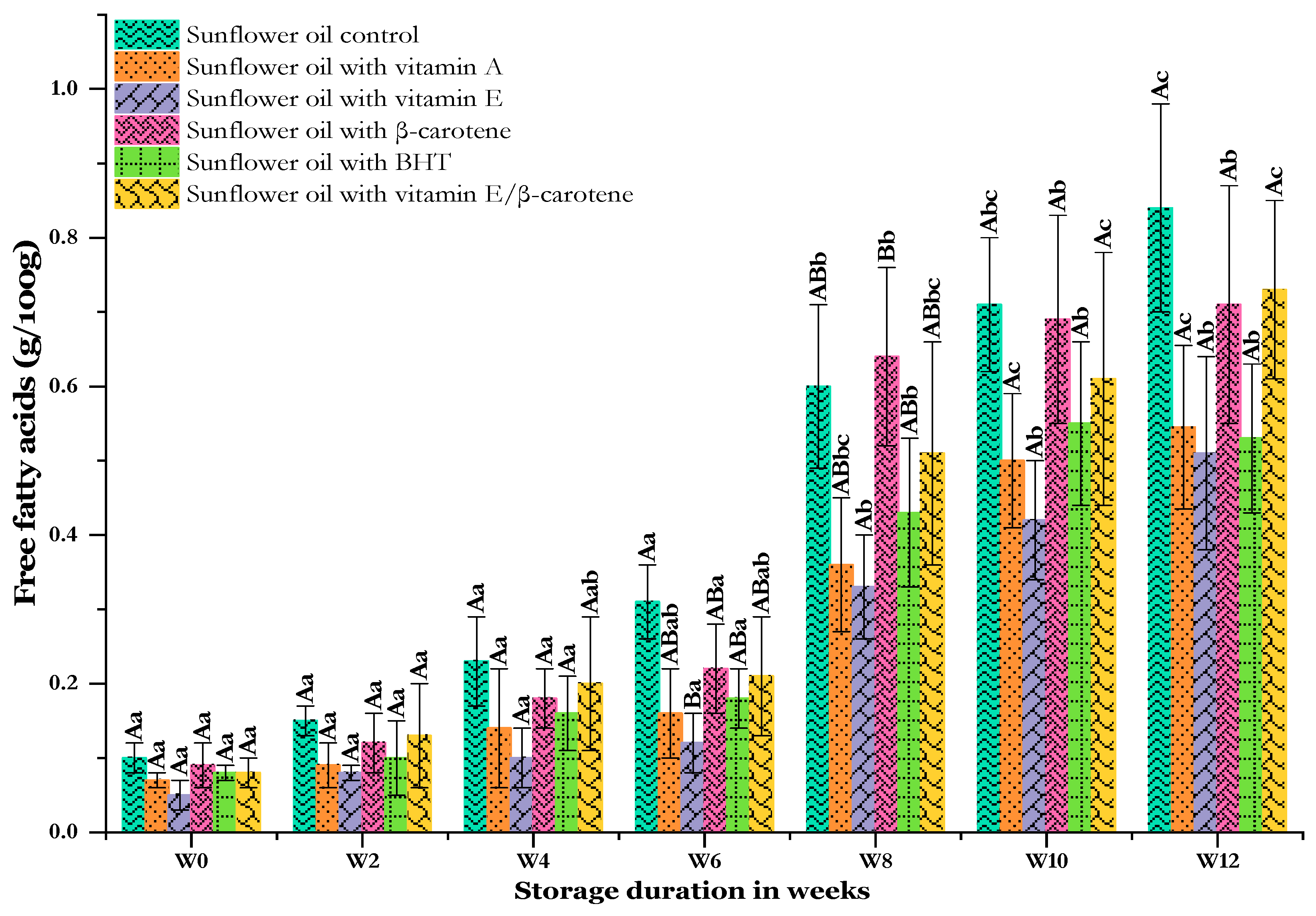
The hydrolytic degradation of oils and fats leads to the formation of free fatty acids (FFAs), which are an important indicator of their rancidity [2]. FFAs are formed by the hydrolysis of triacylglycerols and the decomposition of hydroperoxides into carboxyl groups [33]. Figure 1 shows the influence of antioxidants on the FFA content, which is measured in grams per 100 g (g/100 g). The results revealed that the FFA values increased linearly with storage time, an effect that was particularly noticeable after 8 weeks in all samples. FFA generation rates were low during the initial period. The control samples (SF-Cs) had the highest FFA values (from 0.10 ± 0.02 to 0.84 ± 0.14 g/100 g). Significant differences (p < 0.05) were observed between the various samples at 6 and 8 weeks. Notably, the oil samples containing synthetic antioxidants demonstrated lower FFA values than the SF-Cs at all concentrations studied. Lower levels of FFAs during storage mean that less oxidative degradation has occurred [34]. The FFA values in oil samples with vitamin A (SF-VA), vitamin E (SF-VE), β-carotene (SF-βC), BHT (SF-BHT), and a mixture of vitamin E and β-carotene (SF-VE + βC) at the end of storage were 0.55 ± 0.09, 0.51 ± 0.13, 0.71 ± 0.16, 0.53 ± 0.10, and 0.73 ± 0.12 g/100 g, respectively. However, no significant differences (p < 0.05) were observed between the different samples at the end of the storage period. The incorporation of synthetic antioxidants slowed down the rate of FFA formation at 60 °C. The SF-VE treatment was the most effective in preventing FFA formation, with lower FFA values than in the SF-Cs and following other sample treatments. Among the antioxidants studied, the SF-βC and SF-VE + βC samples had slightly higher FFA values. In general, at to end of storage, the antioxidant power against FFA liberation was as follows in ascending order: SF-VE > SF-BHT > SF-VA > SF-βC > SF-VE + βC > SF-C. Applying antioxidants reduced the rate of FFA formation in the oil.

Figure 1.
Changes in free fatty acids in sunflower oil stored at 60 °C for 12 weeks. Distinct letters indicate significant differences (p < 0.05) between oils. Uppercase letters express differences between treatments for the same week of storage, while lowercase letters express the differences for each treatment over the different weeks of storage (from W0 to W12).
The present results were also consistent with those of Chen et al. [35], who revealed an increase in FFA content during sunflower oil storage at 60 °C after the addition of synthetic antioxidants (BHA, BHT, and TBHQ) and rosemary extracts. Additionally, it is evident that the TBHQ showed the smallest upward trend (p < 0.05) in sunflower oil over six frying periods [36]. According to Hussain et al. [2], sunflower oil containing the synthetic antioxidant BHT has a lower FFA content than oil without antioxidants. Ling et al. [37] reported that BHA and α-tocopherol at 200 ppm have similar inhibitory effects in preventing the triglyceride hydrolysis of sunflower oil over 24 days of accelerated storage (65 °C). Adiiba et al. [38] revealed that phenolic antioxidants (TBHQ, BHT, and BHA) did not interfere with or enhance FFA production and preserved carotene and tocopherols during the enzymatic hydrolysis of crude palm oil as follows: TBHQ > BHT > BHA. In a study conducted on the stability of chia oil (Salvia hispanica L.), Bodoira et al. [39] showed no significant increase in acidity over time (p ≤ 0.05) for oil containing TBHQ as synthetic antioxidant compared to natural antioxidants (tocopherol, ascorbyl palmitate, and rosemary extract) in fluorescent lighting (800 Lux) or in the dark for 300 days.
3.2. Primary Oxidation
3.2.1. Effect of Thermal Storage on Peroxide Value (PV)
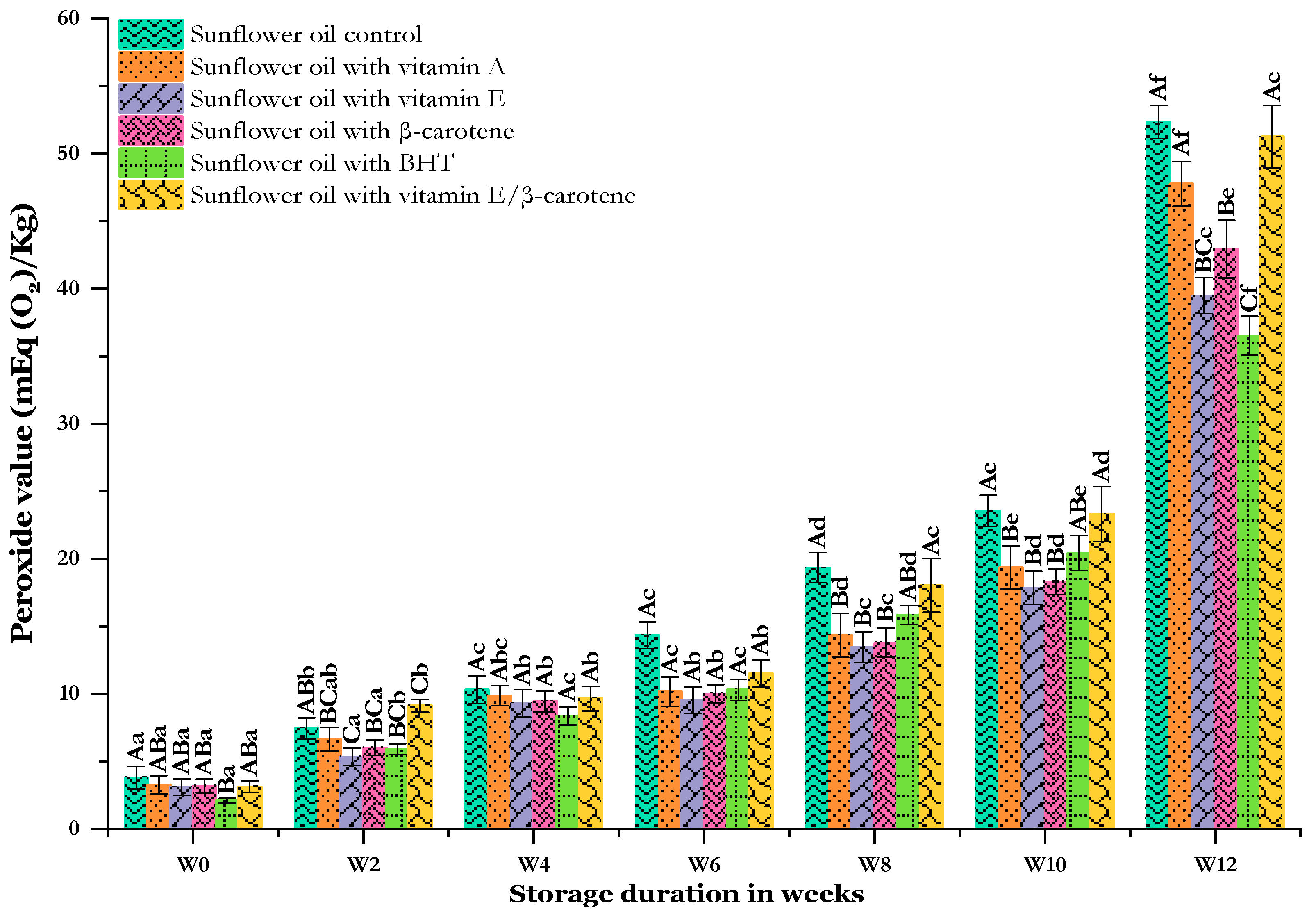
The peroxide value (PV) is among the leading tests for measuring peroxides and the rancidity of oils and fats. This is a revealer of the primary oxidation of oils, which produces colorless, odorless hydroperoxides [11]. A higher PV reflects less lipid chemical stability [40]. Influences of antioxidants on the PV of sunflower oil samples during storage are shown in Figure 2. All oil samples up to week 10 showed a slight increase in the PV, although levels at the end of the storage week were higher. Notably, the PV is greater in the SF-Cs (3.78 ± 0.85 to 52.34 ± 1.22 mEq (O2)/Kg) compared to the other enriched oils. Initially, the PV were comparable between samples, although there was a difference between the SF-Cs and SF-BHT. The use of antioxidants led to PVs aligning with the standard oil value as per Codex Alimentarius [41] after 6 weeks except for vitamin E after 8 weeks, while the control exceeded its limit after 2 weeks of storage. Antioxidants exhibited better protective properties against hydroperoxides than the SF-Cs. Arabsorkhi et al. [42] reported that the rate of oxidation increased in the absence of antioxidants.

Figure 2.
Changes in the peroxide value of sunflower oil stored at 60 °C for 12 weeks. Distinct letters indicate significant differences (p < 0.05) between oils. Uppercase letters express differences between treatments for the same week of storage, while lowercase letters express the differences for each treatment over different weeks of storage (from W0 to W12).
Of the various synthetic antioxidants, PV found that the oil with the blend (SF-VE + βC) added had less antioxidant activity than other enriched oils. SF-VE and SF-βC showed similar changes throughout the storage time. Similarly, it has been shown that 50 µg/g of α-tocopherol in butter oil shows excellent results in terms of PV after 32 days storage at 60 °C [43]. We found that there was a significant difference between the combination of vitamin E and β-carotene compared with when it was used separately from week 8 onwards, so that SF-VE + βC had a weaker protective effect than SF-VE and SF-βC. Likewise, Smyk et al. [44] showed that in the presence of β-carotene, tocopherols were less effective in protecting vegetable oils than in the absence of β-carotene. According to Kehili et al. [45], antioxidants such as β-carotene and vitamin E have pro-oxidant properties when used in high concentrations. However, it has been proven that oil containing a combination of TBHQ and BHA demonstrated PVs similar to those of oils containing TBHQ and higher than those of oils containing BHA during 22 days of accelerated storage [46]. At the end of storage, BHT was found to have the greatest inhibitory effect on the PV, followed by vitamin E and β-carotene, with values of 36.52 ± 1.44, 39.48 ± 1.34, and 42.93 ± 2.14 mEq (O2)/Kg, respectively. Keramat et al. [47] showed that virgin olive oil samples incorporating β-carotene exhibited higher PVs than those with BHT at storage at 60 °C. Differently from our study, Ling et al. [37] revealed that a significant difference (p < 0.05) existed in the PVs of sunflower oils containing BHA and α-tocopherol at the end of storage at 60 °C.
The treatment of samples with antioxidants was effective in reducing the PV during storage. This is consistent with the results obtained in previous studies conducted by [35,48]. Mohanan et al. [49] reported that a higher temperature produces a considerably greater rate of peroxide formation in flaxseed oil as compared to the ability of the antioxidants present in the flaxseed oil to neutralize it, resulting in a higher level of oxidation. In general, incorporating a synthetic antioxidant into an oil resulted in the highest antioxidant activity against primary oxidation. Similarly, as evidenced by the results of a study conducted by Yang et al. [50], the PVs of virgin soybean and rice bran oils were significantly (p < 0.05) greater than those of their counterparts incorporating rosemary extract, BHA, and BHT, during storage for 24 days at 62 °C. The addition of BHA to soybean oil showed a lower PV than the control over the first 12 days at 60 °C [51].
3.2.2. Effect of Thermal Storage on K232 (Conjugated Diene) Value
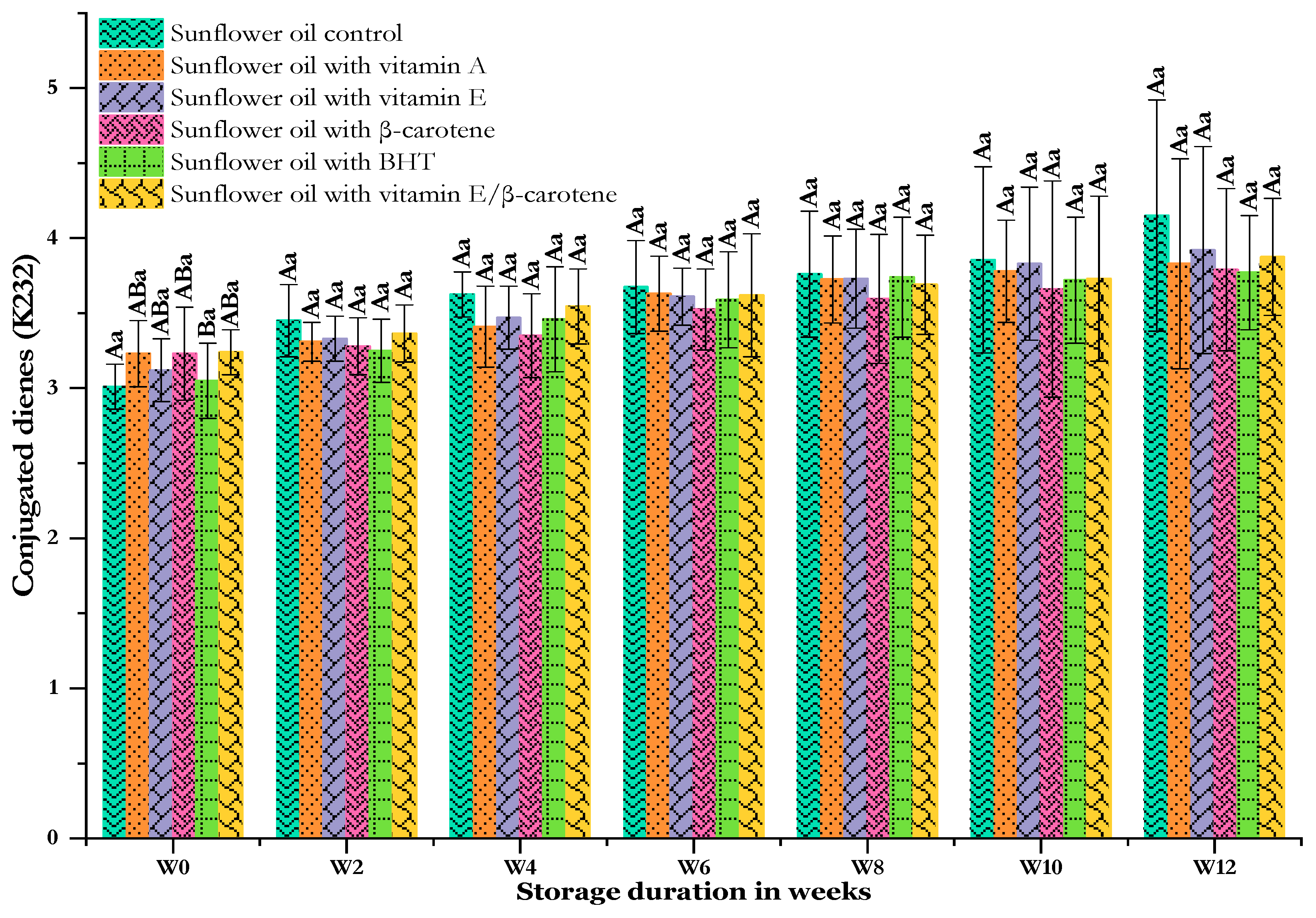
Another quality indicator for assessing primary oxidation products is the extinction coefficient K232. Indeed, at 232 nm, a maximum absorbance is reached for hydroperoxide (conjugated dienes), the product of the primary oxidation phase [52]. Figure 3 indicates that storage time impacted the K232 values, with an increase observed across all samples and antioxidants, in agreement with [45]. Over the full study duration, excluding the initial week, the increase in K232 for both the control and antioxidant-supplemented oils was not significant (p > 0.05) over time. However, notably higher values of K232 were found for the SF-Cs that ranged from 3.01 ± 0.15 to 4.15 ± 0.97 after 12 weeks of storage at 60 °C. In parallel, Günal and Turan [53] observed an increase in the K232 value of sunflower oil from 3.54 to 17.39 after 12 days and up to 98.25 at 21 days at 60 °C. Oils containing synthetic antioxidants exhibit a slight reduction in K232 values, which ranged from 3.05 ± 0.25 to 3.77 ± 0.38 for the SF-BHT, from 3.23 ± 0.31 to 3.79 ± 0.54 for the SF-βC, from 3.23 ± 0.22 to 3.83 ± 0.70 for the SF-VE + βC, from 3.24 ± 0.15 to 3.88 ± 0.39 for the SF-VE + βC, and from 3.12 ± 0.21 to 3.92 ± 0.69 for the SF-VE. The conjugated diene content formed during heating increases as the content of polyunsaturated fatty acids (PUFAs) in the oils increases [54]. Kehili et al. [49] showed comparable K232 values between a sunflower oil control sample and sunflower oil supplemented with BHT (200 µg/g) during accelerated shelf-life storage. In agreement with previous findings regarding the PV, the K232 results show that synthetic antioxidants lower the production of primary oxidation products. This aligns with observations that the application of the synthetic antioxidant TBHQ in soybean oil reduced the rate of oxidation product formation, as evidenced by Tavakoli et al. [55]. A low conjugated diene content reflects good oxidative stability [52].

Figure 3.
Changes in the conjugated diene contents of sunflower oil stored at 60 °C for 12 weeks. Distinct letters indicate significant differences (p < 0.05) between oils. Uppercase letters express differences between treatments for the same week of storage, while lowercase letters express the differences for each treatment over the different weeks of storage (from W0 to W12).
3.3. Secondary Oxidation
3.3.1. Effect of Thermal Storage on p-Anisidine Value (p-AV)
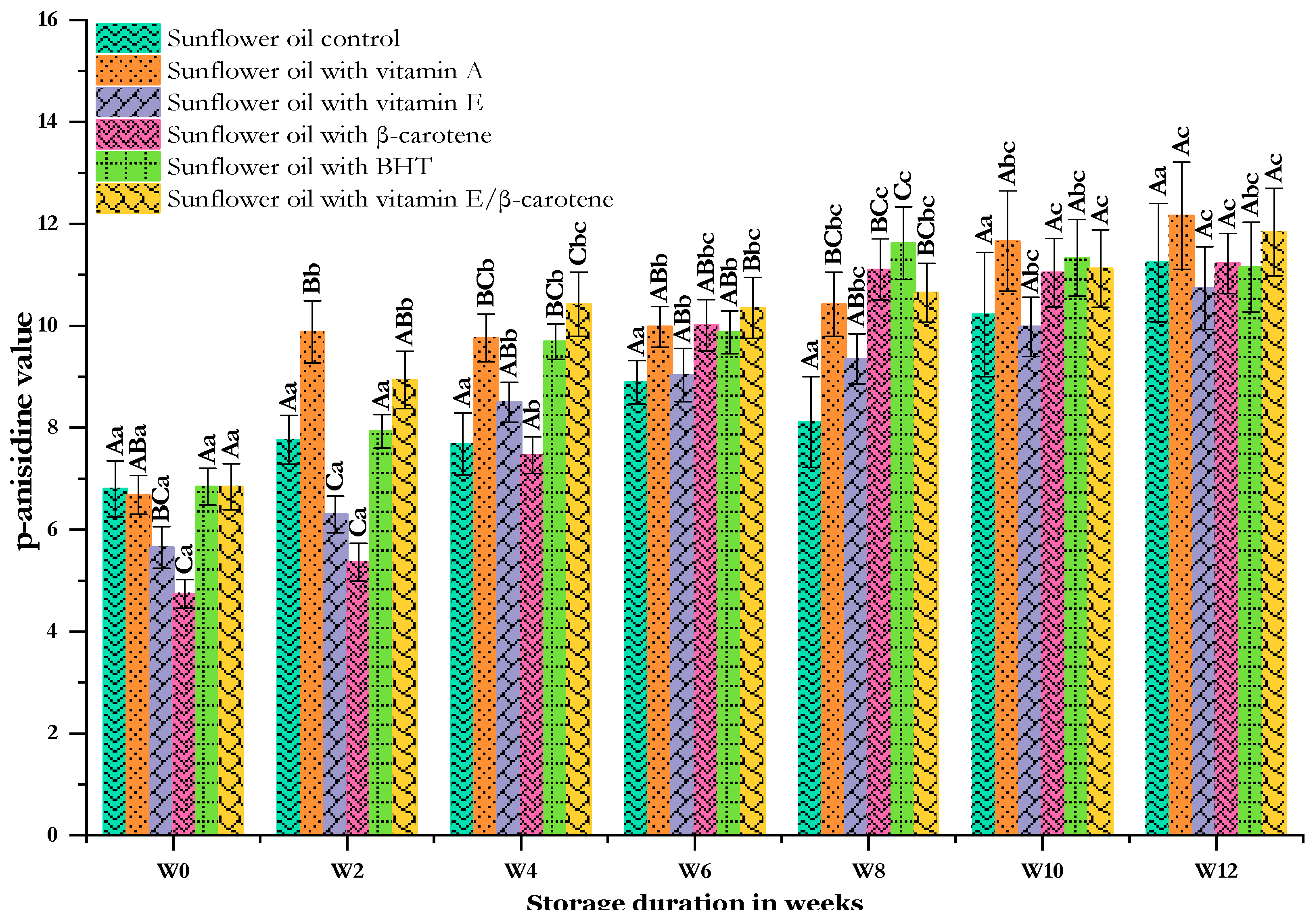
A low PV does not necessarily indicate good oil quality during storage as levels of hydroperoxides decrease during the secondary phase of oxidation. Therefore, it is crucial to supplement this indicator by measuring secondary oxidation products in order to accurately assess the state of oxidation of an oil [56]. This comprehensive evaluation is achieved through the measurement of the p-anisidine value (p-AV), detecting the occurrence of non-volatile aldehydic compounds formed through hydroperoxide decomposition, as noted by Sidhu et al. [57]. Figure 4 presents information on the quantities of secondary lipid oxidation products measured using the p-AV in the different sunflower oils evaluated. The initial p-AV of the control sunflower oil (SF-C) was determined to be 6.80 ± 0.55, revealing no significant differences compared to the SF-VA, SF-BHT, and SF-VE + βC (p < 0.05) samples except for sunflower oils enriched with VE (SF-VEs) and β-carotene (SF-βC), both of which had the second-lowest (5.65 ± 0.41) and lowest (4.74 ± 0.28) levels, respectively. After storing the samples at 60 °C for up to 2 weeks, all samples demonstrated an increased p-AV, which occurred most significantly in sunflower oil supplemented with the vitamin E and β-carotene mixture (SF-VE + βC), with an increase of 47% (9.88 ± 0.61), closely followed by the SF-VE + βC with an increase of 31% (8.94 ± 0.56). As depicted in Figure 4, these two samples showed the highest secondary oxidation rates by the end of storage. However, the p-AVs of the other samples remained relatively low for the first 2 weeks and then steadily increased, particularly in the SF-BHT and SF-βC, reaching 11.62 ± 0.71 (almost three times the initial value) and 11.10 ± 0.60 (a 70% increase from the initial value) by 8 weeks. During this storage period, the increased rates caused the sunflower oil supplemented with the BHT antioxidant to score significantly higher than the other samples, which were themselves significantly higher than the control. Subsequently, the p-AVs of the samples became quite similar, with the sunflower oil supplemented with vitamin A displaying the highest value and the sunflower oil supplemented with vitamin E showing a slower dynamic of oxidation throughout the storage period. The remaining samples fell in between. By the end of storage, the difference between the samples was non-significant (p < 0.05). The recommended p-AV for good-quality oil is typically less than 10, as suggested by Aissa et al. [58]. However, in our study, all samples exhibited a substantial increase in the p-AV as a result of exposure to higher temperatures.

Figure 4.
Changes in the p-anisidine value of sunflower oil stored at 60 °C for 12 weeks. Distinct letters indicate significant differences (p < 0.05) between oils. Uppercase letters express differences between treatments for the same week of storage, while lowercase letters express the differences for each treatment over the different weeks of storage (from W0 to W12).
Our findings clearly illustrate that the prolonged exposure of oil to heat leads to a significant rise in this parameter, primarily due to the combined effects of heat and other factors on the oxidation of sunflower oil. As sunflower oil is known for its high content of UFA, is particularly susceptible to oxidation under such conditions. The authors Kiralan et al. [59] noted a significant increase in secondary products like aldehydes in vegetable oils stored at 60 °C, leading to a notable rise in the p-AV, which is consistent with our observations. Furthermore, Athanasiadis et al. [60], found that BHT (200 ppm), as well as mixtures of tocopherols (α-T/δ-T) in ratios of 4:1, were slightly more effective than a control in preserving sunflower and olive pomace oils under Rancimat incubation for 24 h. Similarly, Chong et al. [61] demonstrated the efficacy of synthetic BHA (200 ppm) and α-tocopherol (200 ppm) in reducing p-AV levels in sunflower oils under accelerated storage conditions (65 °C) for 24 days. In another study by Sahunie [62], the supplementation of sunflower oil with phenolic extracts and BHA resulted in a greater decrease in the p-AV compared to the control during 6 months of storage in both dark and light conditions. They also observed a significant increase in the p-AV when sunflower oil underwent repeated heating for 4 weeks, highlighting the impact of processing conditions on oil stability. These latter authors claimed that the variations observed between the results and the literature may be attributed to differences in heating cycles and temperatures utilized during experimentation.
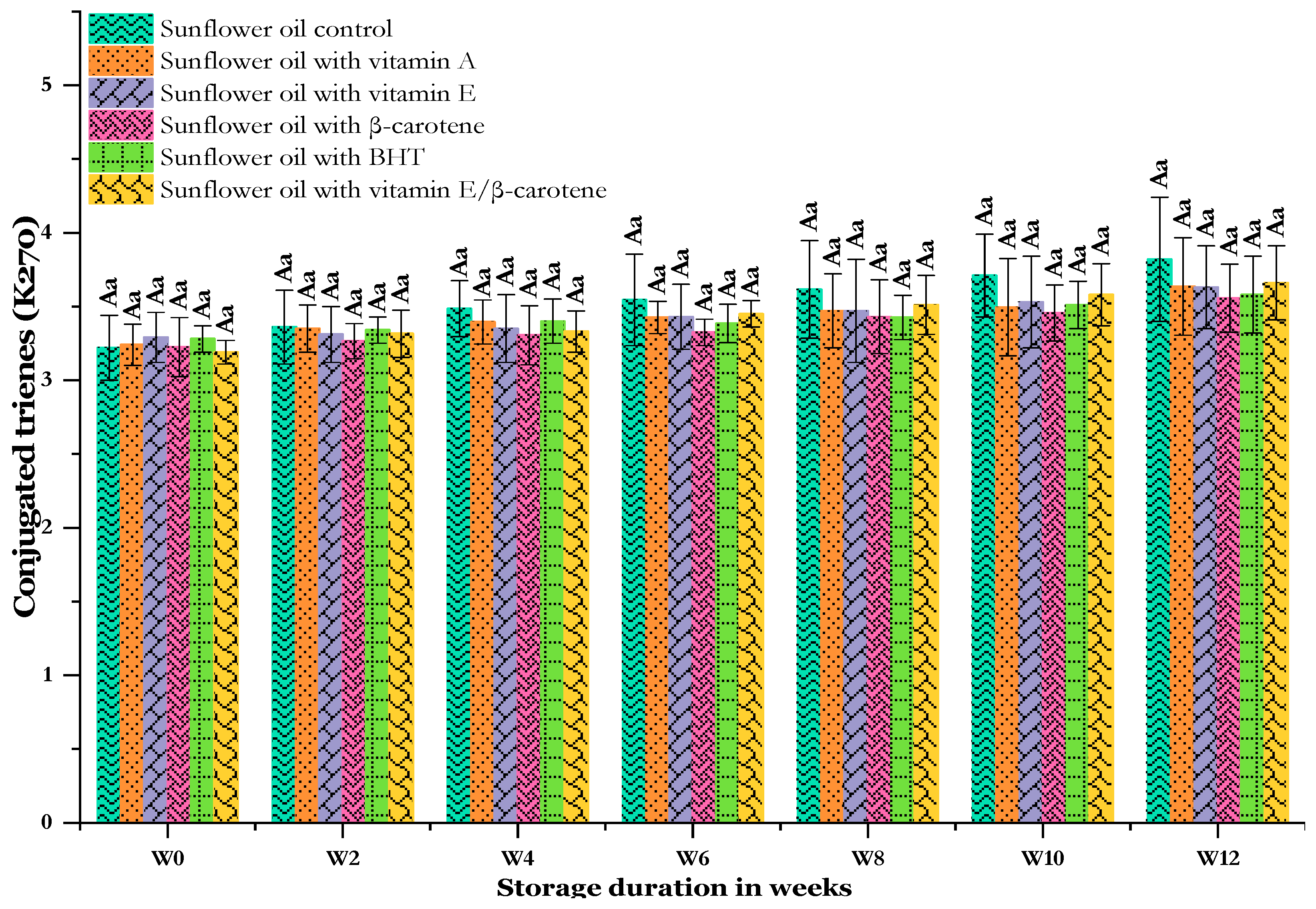
3.3.2. Effect of Thermal Storage on K270 (Conjugated Triene) Value
UV absorbance variations at 270 nm (K270) are used for the relative quantification of oxidation [52]. Secondary oxidation compounds, such as small-chain fatty acids, aldehydes, and ketones, are absorbed at 270 nm [63]. Over time, the K270 rises (Figure 5), indicating increased production of secondary oxidation compounds. The initial K270 value in the SF-VE sample was the highest among the oils at 3.29 ± 0.17. The SF-C recorded high K270 values after two weeks and right up to the end. The results are in line with Kiralan et al.’s [59] results; they found that the K270 value of sunflower oil increased from an initial value of 3.49 to 4.69 after 16 days of storage at 60 °C. Statistically, the SF-C, SF-VA, SF-VE, SF-βC, SF-BHT, and SF-VE + βC samples showed comparable secondary oxidation products (K270) that were not significant at all time weeks. However, the addition of antioxidants to the oil slightly reduced their K270 during storage. β-carotene (SF-βC) showed the lowest K270 values, ranging from 3.22 ± 0.20 at W0 to 3.56 ± 0.23 at W12. Generally, the amount of conjugated trienes in SF-BHT was lower in the SF-VA. When β-carotene (100 mg/Kg) was combined with vitamin E (100 mg/Kg), the mixture showed little additional protective effect against secondary peroxides compared with the use of either alone. At the end of the storage period, for the fortified oils, the SF-VE + βC oil had the highest conjugated triene value (3.66 ± 0.25), followed by the SF-VA (3.64 ± 0.33) oil and SF-VE (3.63 ± 0.28) oil. Our results, in concordance with Tavakoli et al. [55], revealed that soybean oil containing the synthetic antioxidant TBHQ had the least conjugated triene products compared with the control oil. Compared to other oils, the evolution of the K270 value of the SF-C is not perfectly identical to that observed for the p-AV. According to Fadda et al. [64], the K270 measurement is not at all similar to that of the p-AV as ketones are not detected during the determination of the latter.

Figure 5.
Changes in the conjugated trienes of sunflower oil stored at 60 °C for 12 weeks. Distinct letters indicate significant differences (p < 0.05) between oils. Uppercase letters express differences between treatments for the same week of storage, while lowercase letters express the differences for each treatment over the different weeks of storage (from W0 to W12).
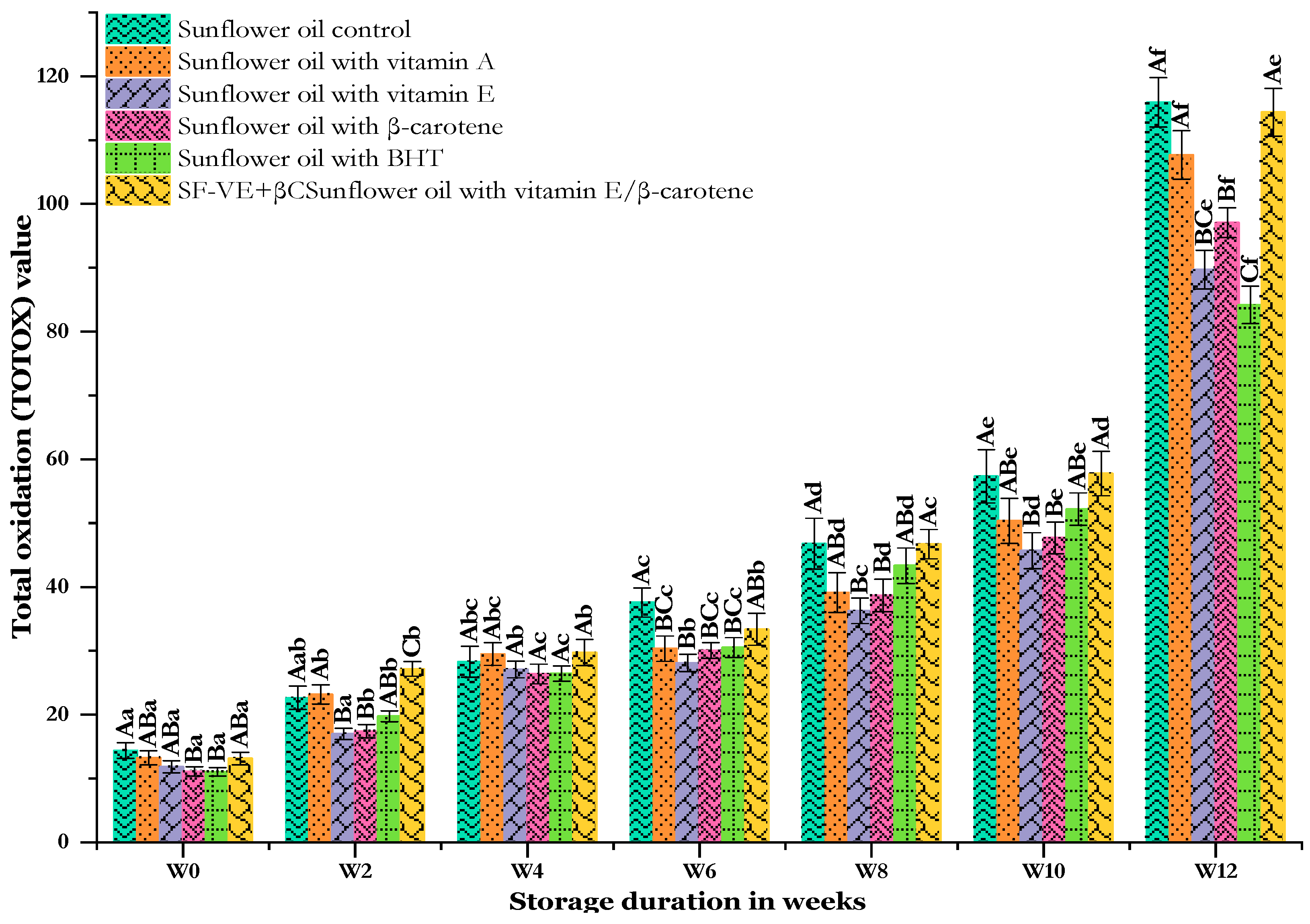
3.4. Effect of Thermal Storage on Total Oxidation (TOTOX) Value
The total oxidation value or TOTOX is a measure of oxidative degradation that simultaneously reflects the values of primary as well as secondary oxidation products [65]. This parameter reflects both the oxidation history and the rancidity potential of oils [40]. A TOTOX value less than 10 indicates better quality [64]. The initial total oxidation values of the oils analyzed were quite similar, ranging from 11.04 ± 0.69 (SF-BHT) to 14.36 ± 1.22 (SF-C) (Figure 6). As a result of the accelerated storage, this parameter demonstrated a progressive increase in all analyzed oils. At first, the increase advanced in parallel at a slow rate up to 6 weeks. However, after this period, the increase systematically gathered considerable speed, with all values twice their previous levels. The control sunflower oil (SF-C) consistently demonstrated the highest oxidation rate throughout the storage period, reaching a peak value of 115.91 ± 1.22. Interestingly, the sunflower oil enriched with mixed vitamin E and β-carotene (SF-VE + βC) closely followed the control, demonstrating a value of 114.36 ± 3.72 by the end of storage. It is worth noting that this oil (SF-VE + βC) showed an oxidation rate comparable to that of the control from early to late in the storage period. In the same period, sunflower oils enriched with BHT (SF-BHT) and vitamin E (SF-VE) possessed the lowest levels of total oxidation value, culminating in values of 84.19 ± 2.93 and 89.69 ± 3.03, respectively. The remaining oils (SF-βC and SF-VA) fell in between.

Figure 6.
Changes in the total oxidation of sunflower oil stored at 60 °C for 12 weeks. Distinct letters indicate significant differences (p < 0.05) between oils. Uppercase letters express differences between treatments for the same week of storage, while lowercase letters express the differences for each treatment over the different weeks of storage (from W0 to W12).
Our results are in line with the existing literature, which highlights the impact of additives on reducing oil oxidation. For instance, in a study conducted by Hussain et al. [2], the oxidative parameters of sunflower oil were significantly higher than those of samples containing a sesame seed extract and BHT following 24 days of storage at 62 °C. Likewise, Yildiz et al. [66] conducted a study in which natural phenolic compounds such as thymol, carvacrol, and thymoquinone exhibited comparable oxidative stability to commercial antioxidants like α-tocopherol, BHT, and BHA. However, their effectiveness was slightly lower than that of BHT in refined and stripped corn oils under 60 °C conditions, as indicated by various tests assessing oxidative stability during accelerated storage. Additionally, their various tests revealed that BHT improved oxidative stability to a greater extent compared to α-tocopherol and BHA during accelerated storage at 60 °C. Thus, this parameter was effectively reduced in oil samples supplemented with antioxidant compounds. Nid Ahmed et al. [30], similarly, demonstrated the enhanced antioxidant efficacy of tocobiol as a synthetic antioxidant in preventing oxidation compared with unpurified sunflower oil stored at 60 °C for 4 months. Under same storage conditions, Karabulut [43], demonstrated that in purified butter oil, the combination of α-tocopherol (50 ppm), ascorbyl palmitate (50 ppm), and β-carotene (5 ppm) was better at retarding oxidation than that of a bi-mixture of α-tocopherol and ascorbyl palmitate or β-carotene. Notably, data revealed by Marinova et al. [67] support the synergistic effect of α-tocopherol and myricetin at concentrations (50–250 ppm) in sunflower oil at 100 °C, most notably with an equimolar mixture at total concentrations below 100 Mm.
3.5. Effect of Thermal Storage on Fatty Acid Composition (FAC)
Fatty acids are compounds that provide the human body with a source of energy [68]. It should be noted that the properties, nutritional benefits, and stability of a particular vegetable oil are highly dependent on its fatty acid composition (FAC) [69]. Sunflower oil is considered nutritious owing to its rich levels of polyunsaturated fatty acids (PUFAs). The latter are widely considered to be nutritionally beneficial in terms of human health [70]. The FAC varied significantly (p < 0.05) among the different samples (Table 1, Table 2 and Table 3). The initial FAC analysis further elucidated the distinct properties of the SF-C, SF-VA, SF-VE, SF-βC, SF-BHT, and SF-VE + βC samples. Polyunsaturated fatty acids (PUFAs) dominate the total fatty acids in all samples, ranging from 48.72 ± 0.19 (SF-βC) to 51.35 ± 0.28 g/100 g (SF-VA), followed by monounsaturated fatty acids (MUFAs), ranging from 36.27 ± 0.17 (SF-C) to 39.44 ± 0.19 g/100 g (SF-VE), and saturated fatty acids (SFAs), ranging from 11.47 ± 0.27 (SF-VE + C) to 12.52 ± 0.14 g/100 g (SF-VA). The SF-VA sample displayed a notable abundance of linoleic acid (C18:2) (51.3 ± 0.11 g/100 g), comprising over half of all fatty acids. Oleic acid (C18:1) was the second most abundant UFA, comprising approximately 39.2 ± 0.20 g/100 g of the SF-βC. In contrast, the fatty acid composition of the SF-VA showed a higher content of SFAs. Palmitic acid (C16:0) was the majority fatty acid, constituting around 7.72 ± 0.09 g/100 g of the composition. The variation in the FAC percent of sunflower oil might be due to the synthetic antioxidants and processing treatment employed during its enhancement. Generally, no significant differences were observed among the sunflower oil samples, and this enriched in terms of the FAC over one month of storage at 60 °C; this was already reported by El Bernoussi et al. [71]. In contrast, there is a significant difference between the first month and the third month. The amounts of PUFAs decreased by storage days in control and all enriched oil samples; this degradation can be explained by the sensitivity of PUFAs to heat [72]. Similarly, after 90 days storage, the oil samples showed significant decreases in their UFA contents. However, the SFA content increased between the initial and final stages, which was also reported by Meng et al. [73]. The results obtained, irrespective of the variations observed, always comply with the maximum levels authorized by [41].

Table 1.
Changes in the fatty acid composition and oxidizability value of a sunflower oil control and sunflower oil with vitamin A stored at 60 °C for 12 weeks.

Table 2.
Changes in fatty acid composition and oxidizability value of sunflower oil with vitamin E and with β-carotene stored at 60 °C for 12 weeks.

Table 3.
Changes in fatty acid composition and oxidizability value of sunflower oil with BHT and with vitamin E/β-carotene stored at 60 °C for 12 weeks.
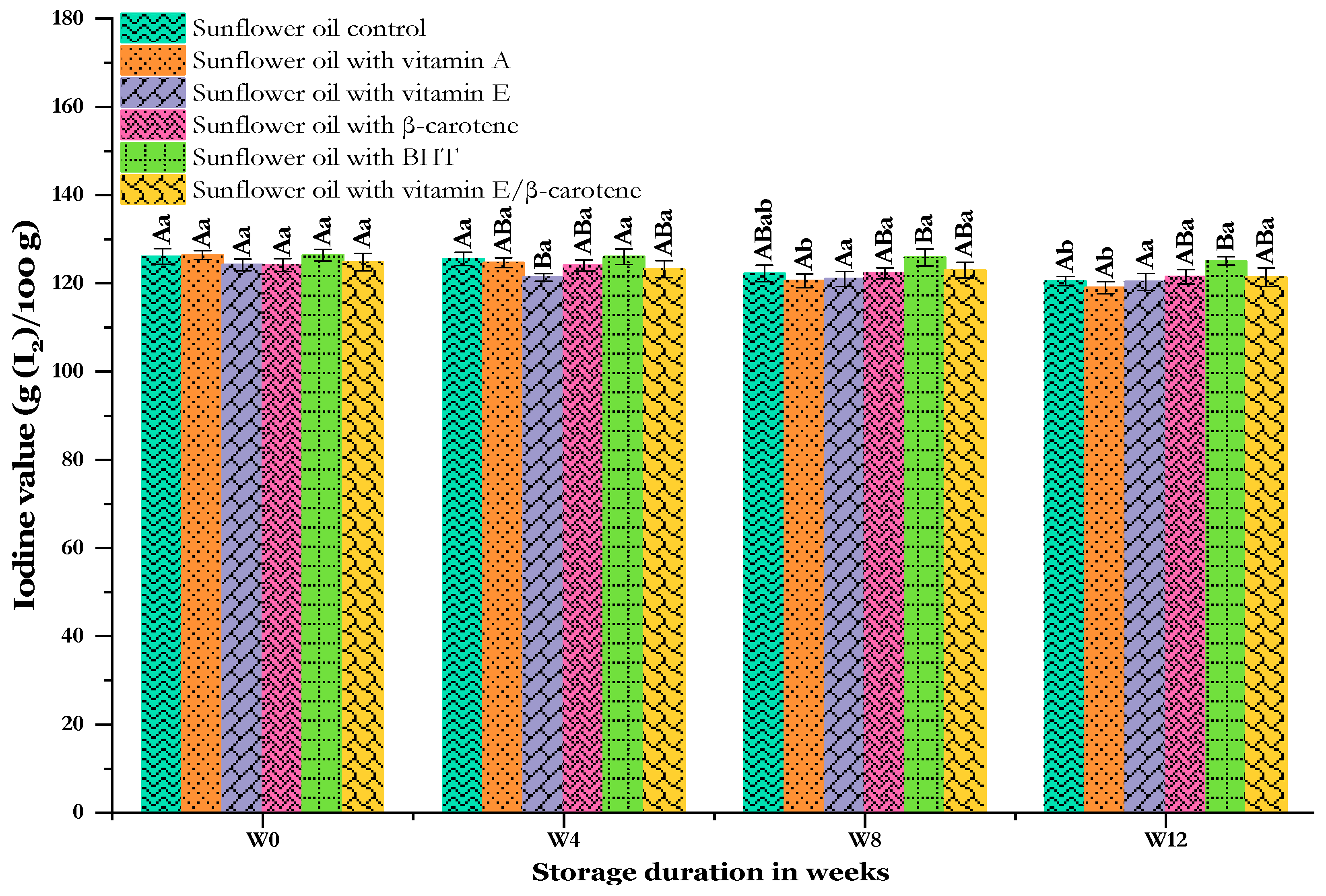
3.6. Effect of Thermal Storage on Iodine Value (IV)
The iodine value (IV) is indeed a measure of the UFAs present in vegetable oil. The IV is used to assess the oxidation stability of oils and greases [74]. Consecutive changes in the IVs of sunflower oils evaluated during accelerated storage are shown in Figure 7. The initial IVs measured at the start of the experiment (W0) were quite similar, starting with the highest IV for the SF-BHT sample (126.38 ± 1.33 g (I2)/100 g) and the lowest for SF-VE (124.18 ± 1.32 g (I2)/100 g). However, the results did not show any significant differences (p < 0.05) in the initial IVs of the samples, all of which were over 120 g (I2)/100 g. A high IV indicates a high content of UFAs in the oil, making it more prone to oxidation [75]. Previous research, such as that by Romanić et al. [76], has shown that adding supplements to sunflower oil did not significantly affect its iodine value, which is consistent with our findings. Over the 12-week storage period at 60 °C, the IV parameter started to decrease slightly in all samples. The control sample experienced a significant decrease in IV, dropping from 126.05 ± 1.77 to 120.49 ± 1.05 g (I2)/100 g by the end of storage. The sunflower oil supplemented with vitamin A failed to maintain the stability of its IV, showing a significant decrease from an initial value of 126.42 ± 0.98 to 119.01 ± 1.34 g (I2)/100 g. On the other hand, sunflower oils with other antioxidant additions were able to maintain the iodine value to a greater (SF-BHT) or lesser extent (SF-VE). However, the latest results still fall within the acceptable range compared to the Codex standard value, which typically ranges from 110 to 145 g (I2) /100 g for sunflower oil [41].

Figure 7.
Changes in the iodine value of sunflower oil stored at 60 °C for 12 weeks. Distinct letters indicate significant differences (p < 0.05) between oils. Uppercase letters express differences between treatments for the same week of storage, while lowercase letters express the differences for each treatment over the different weeks of storage (from W0 to W12).
A decrease in the IV is consistent with a reduction in double bonds as an oil becomes oxidized. This decrease is a result of oxidation, which involves a complex chain of chemical reactions leading to a decline in unsaturation rates. Specifically, this process involves the elimination of adjacent hydrogen atoms from double bonds, leading to the formation of free radicals [77]. These findings are expected due to the high concentration of PUFAs present in sunflower oil, accounting for more than 70%, as reported by Chong et al. [61]. The abundance of PUFAs makes sunflower oil particularly sensitive to oxidation reactions. In storage, the double bonds within these PUFAs are vulnerable to attack by free radicals, resulting in the creation of conjugated bonds, as also noted by Farahmandfar et al. [78]. In line with our findings, Nogales-Delgado et al. [79] investigated the oxidative stability of sunflower oils with added BHA and TBHQ, concluding that these antioxidants effectively protect against lipid oxidation, showing superior results compared to a control sample. Similarly, Chong et al. [61] reported lower iodine values in oil samples containing mangosteen peel extracts (100 ppm and 200 ppm), α-tocopherol, and BHA compared to a control during 24 days of accelerated storage at 65 °C.
3.7. Effect of Thermal Storage on Oxidizability Value (COX)
The oxidizability value (COX) is an indicator of an oil’s susceptibility to oxidation; as the COX value increases, the oil’s stability decreases [80]. The final COX value of the sunflower oil control was significantly lower than that of the enriched oil with the exemption of the SF-VA (SF-VA < SF-C < SF-VE + βC < SF-VE < SF-βC < SF-BHT) (Table 1, Table 2 and Table 3). This means that the FAC of the oil enriched with BHT should be more prone to oxidation than that of the control and the other enriched oils. The COX values decreased slightly during storage; hence, the stability increased. The COX values ranged from 5.45 (SF-VE and SF-βC) to 5.69 (SF-C and SF-VA) and from 5.17 (SF-VA) to 5.58 (SF-BHT) before storage and after 90 days of storage, respectively. These COX values of control and enriched oils are better than some other oils such as Opuntia ficus-indica seed oil (6.5), Prunus spinosa L. oil (6.97 ± 0.35), Prunus avium L. oil (9.02 ± 0.39), and Perilla seed oil (14.83) [80,81,82].
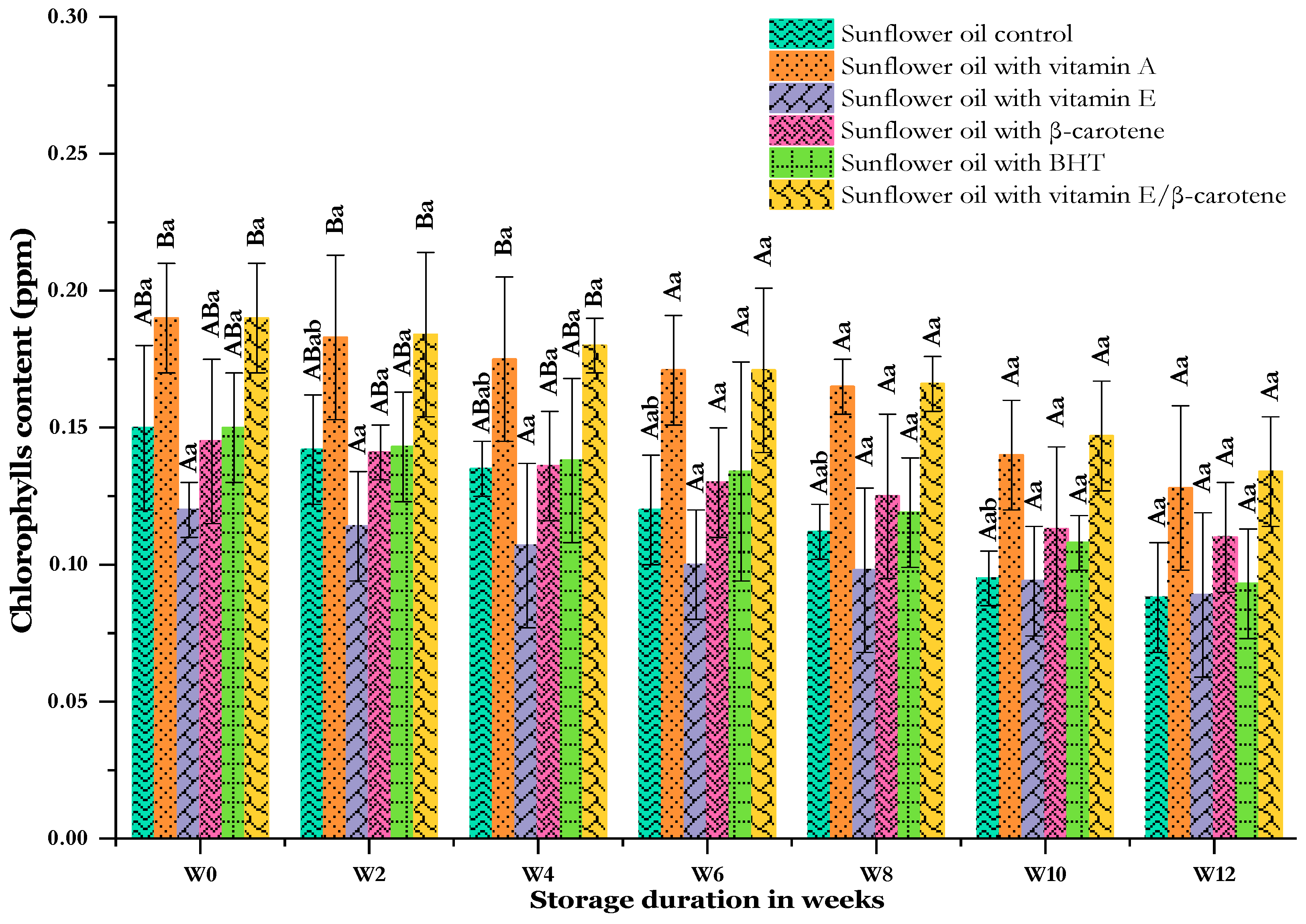
3.8. Effect of Thermal Storage on Pigment Content
The greenish color of crude vegetable oils is attributable to the chlorophyll and carotenoid pigments contained in the oil. This color is considered to be an important element in the sensory properties assessed by consumers and a crucial indicator of quality and antioxidant properties. Until now, several natural pigments have been used as supplements in food products, providing nutritional benefits as well as an attractive color combined with excellent functional properties, especially antioxidant effects [83]. However, pigments are also known to contribute to the appearance of a bad taste and can act as photosensitizers, catalyzing the oxidation of vegetable oil under severe conditions [84]. The initial values as well as changes in pigment contents in the analyzed sunflower oils during storage at 60 °C are presented in Figure 8 and Figure 9.

Figure 8.
Changes in the chlorophyll content of sunflower oil stored at 60 °C for 12 weeks. Distinct letters indicate significant differences (p < 0.05) between oils. The uppercase letters correspond to different treatments for each storage period, while the lowercase letters correspond to the storage periods (from W0 to W12) for each treatment.

Figure 9.
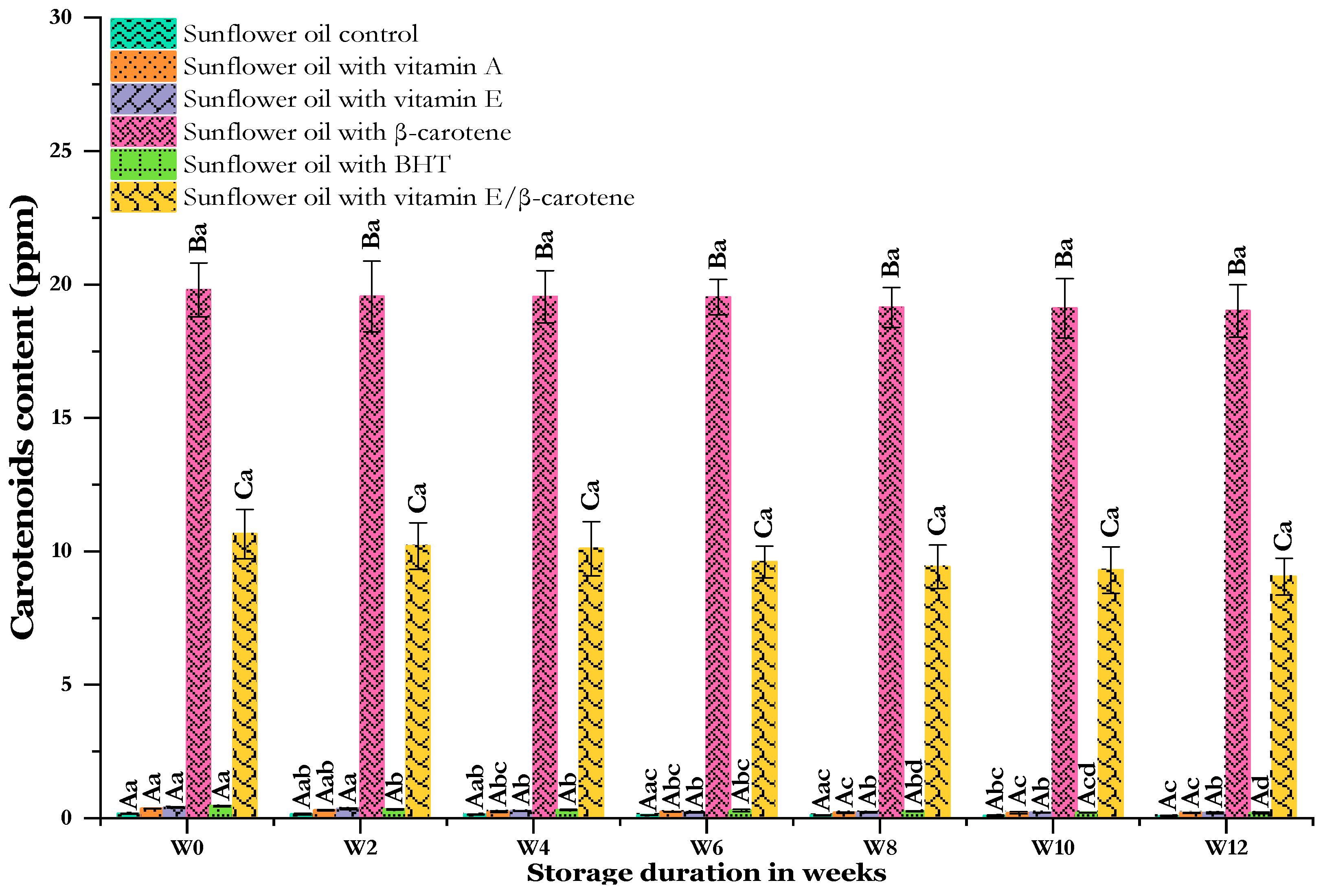
Changes in the carotenoid content of sunflower oil stored at 60 °C for 12 weeks. Distinct letters indicate significant differences (p < 0.05) between oils. Uppercase letters express differences between treatments for the same week of storage, while lowercase letters express the differences for each treatment over the different weeks of storage (from W0 to W12).
Figure 8 illustrates the evolution of chlorophylls among the samples. The chlorophyll values registered at the start of the assay were quite similar, with the highest value of 0.19 recorded in both the SF-VA and SF-VE + βC samples, followed by the SF-C and SF-BHT samples with values of 0.15 each. The last two values were 0.14 and 0.12, recorded in the SF-βC and SF-VE samples, respectively. Our results are almost lower than the chlorophyll content in sunflower oils reported in [85]. In a study by Ghaliaoui et al. [83], the chlorophyll content of sunflower and soybean oils fortified with the pigment extract of Phyllaria reniformis increased compared to a control and BHA samples. The concentrations of chlorophylls gradually decreased over time, with a notable decrease observed at the end of storage. The control sunflower oil (SF-C) experienced the most significant loss, with a decrease of almost 41% compared to the initial value. In contrast, the other samples containing additive antioxidants showed lower losses than the control, with reductions of approximately 38, 32, 30, and 26% for SF-BHT, SF-VA, SF-VE + βC, and SF-VE, respectively. The sample with the least loss was sunflower oil with the addition of β-carotene (SF-βC), which experienced a reduction of 24.14% compared to its initial value. Similar to our findings, Ghaliaoui et al. [83] assessed the chlorophyll content in soybean and sunflower oils with the addition of BHA, comparing it with 200 and 1000 ppm of Phyllaria reniformis pigment extract. Their study revealed that supplementing with the synthetic antioxidant BHA did not lead to significant changes compared to the control group for both analyzed oils. However, In the presence of light, temperature, or other factors contributing to oxidation during storage, chlorophylls undergo thermal decomposition into another pigment called pheophytin. This transformation results in the oil acquiring a dull, dark color. The chlorophyll and pheophytin profile may experience further modifications, with pheophytinization leading to a decrease in chlorophyll levels [86]. Bijla et al. claimed that enrichment with 0.01, 0.02, and 0.03% vitamin E led to a reduction in chlorophyll levels by approximately 45% over four months of accelerated storage [24].
Carotenoids, meanwhile, are tetra-terpenoids that can give orange, red, and yellow hues, playing a vital role in the coloration of oils. Carotenoids are also powerful antioxidants which prevent the oxidation of oil by inhibiting 1O2, which can inhibit its generation by neutralizing activated sensitizers [87,88]. In contrast to chlorophylls, the carotenoid content increased after supplementation (Figure 9). The initial values observed in the samples with additives were consistently higher compared to that of the control. Among the analyzed samples, the sunflower oil with added β-carotene (SF-βC) demonstrated the highest initial value at 19.80 ± 1.01 mg/Kg, which was 116 times higher than the lowest value recorded in the samples (0.17 ± 0.02 mg/Kg). The sunflower oil enriched with mixed antioxidants (SF-VE + βC) held the second-highest level at 10.65 ± 0.92 mg/Kg; both of these samples had significantly higher levels than the others. The sunflower oil enriched with BHT held the third position with a concentration of 0.45 ± 0.03 mg/Kg. The sunflower oil samples enriched with vitamin E (SF-VE) and vitamin A (SF-VA) had the lowest enrichment levels, with concentrations of 0.4 ± 0.02 and 0.36 ± 0.01 mg/Kg, respectively.
The carotenoid content evolved in the same way as the chlorophyll content during accelerated storage. According to the literature, the rapid decline in carotenoids observed in sunflower oil, which is rich in PUFAs, indicates that unsaturated lipids are oxidizing quickly. This oxidation produces radicals that can attack carotenoids, as mentioned by Ghendov-Mosanu et al. [89]. However, despite this decline, our study noted a modest decrease, suggesting that carotenoids may still have a positive effect as antioxidants in slowing down this oxidation process. Their efficacy is such that a single carotenoid molecule can neutralize approximately 1000 singlet oxygen molecules. Mishra et al. [10] demonstrated the enhanced performance of α-tocopherol in preserving the oxidative stability of purified triacylglycerols from sunflower oil under accelerated oxidation conditions in darkness for 20 h across three different concentrations. In contrast, El Bernoussi et al. [71] investigated the carotenoid and chlorophyll content in oils from sweet and bitter almonds supplemented with BHA, noting a reduction of over 80% in pigment content, which is significantly higher than the reduction observed in our study.
4. Conclusions
The current study suggests that during thermal storage at 60 °C, the oxidation parameters of pure sunflower oil increase, leading to a decrease in its relative stability. However, when comparing this oil with oil supplemented with antioxidants, the evolution of these analyzed parameters can be inhibited, demonstrating a significant protective effect of antioxidants against oxidative deterioration. Significant differences were found between sunflower oil enriched with antioxidants and pure sunflower oil with regard to some of the parameters studied (FFAs, the PV, the p-AV, and the IV). Consequently, the addition of antioxidants significantly increased the antioxidant capacity of sunflower oil. However, sunflower oil enriched with β-carotene combined with vitamin E does not provide the best antioxidant effect. In addition, the antioxidants incorporated into the oil inhibit its degradation up on exposure to temperature by promoting hydrolytic stability and limiting the depletion of polyunsaturated fatty acids (PUFAs). The incorporation of synthetic antioxidants in sunflower oil effectively inhibits its oxidation processes, considerably extending its shelf life. However, these synthetic antioxidants have health risks due to carcinogenesis, leading to their removal from the list of items generally recognized as safe (GRAS). As a result, as an alternative to using synthetic antioxidants to prevent the oxidative degradation of oil, a recourse to natural antioxidants obtained from agricultural by-products has been called into question.
Author Contributions
M.N.A.: conceptualization, investigation, and writing—original draft; J.G.: writing—review and editing; A.A.: visualization; O.H.: writing—review and editing; L.A.: methodology and writing—original draft; A.M.G.: supervision, validation, and writing—review and editing; K.M.: data curation and validation; S.G.: methodology, resources, validation, and writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The data are contained within the article.
Acknowledgments
We extend our heartfelt thanks to the authors for their invaluable support and contribution to this study and to the “Huileries du Souss Belhassan (HSB)” for supporting this work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Syed, A. Oxidative Stability and Shelf Life of Vegetable Oils. In Oxidative Stability and Shelf Life of Foods Containing Oils and Fats; Hu, M., Jacobsen, C., Eds.; AOCS Press: Champaign, IL, USA, 2016; pp. 187–207. ISBN 978-1-63067-056-6. [Google Scholar]
- Hussain, S.A.; Hameed, A.; Ajmal, I.; Nosheen, S.; Suleria, H.A.R.; Song, Y. Effects of Sesame Seed Extract as a Natural Antioxidant on the Oxidative Stability of Sunflower Oil. J. Food Sci. Technol. 2018, 55, 4099–4110. [Google Scholar] [CrossRef] [PubMed]
- Kozłowska, M.; Gruczyńska, E. Comparison of the Oxidative Stability of Soybean and Sunflower Oils Enriched with Herbal Plant Extracts. Chem. Pap. 2018, 72, 2607–2615. [Google Scholar] [CrossRef] [PubMed]
- Rauf, S.; Jamil, N.; Tariq, S.A.; Khan, M.; Kausar, M.; Kaya, Y. Progress in Modification of Sunflower Oil to Expand Its Industrial Value. J. Sci. Food Agric. 2017, 97, 1997–2006. [Google Scholar] [CrossRef] [PubMed]
- Rauf, S.; Ortiz, R.; Shehzad, M.; Haider, W.; Ahmed, I. The Exploitation of Sunflower (Helianthus annuus L.) Seed and Other Parts for Human Nutrition, Medicine and the Industry. Helia 2020, 43, 167–184. [Google Scholar] [CrossRef]
- Silva, H.R.P.; Iwassa, I.J.; Marques, J.; Postaue, N.; Stevanato, N.; Silva, C. Enrichment of Sunflower Oil with Β-carotene from Carrots: Maximization and Thermodynamic Parameters of the Β-carotene Extraction and Oil Characterization. J. Food Process. Preserv. 2020, 44, e14399. [Google Scholar] [CrossRef]
- Shahidi, F.; Ambigaipalan, P. Phenolics and Polyphenolics in Foods, Beverages and Spices: Antioxidant Activity and Health Effects—A Review. J. Funct. Foods 2015, 18, 820–897. [Google Scholar] [CrossRef]
- Yıldız, Ş.; Dilmen, Ş.; Turan, S.; Kıralan, M.; Ramadan Hassanien, M.F. Effects of Natural Phenolics and Synthetic Antioxidants on the Oxidative Thermal Stability of Refined and Purified Sunflower Oils. Riv. Ital. Delle Sostanze Grasse 2021, 98, 93–103. [Google Scholar]
- Upadhyay, R.; Mishra, H.N. Effect of Relative Humidity and Light Conditions on the Oxidative Stability of Sunflower Oil Blends Stabilised with Synthetic and Natural Antioxidants. Int. J. Food Sci. Technol. 2016, 51, 293–299. [Google Scholar] [CrossRef]
- Mishra, S.K.; Belur, P.D.; Iyyaswami, R. Use of Antioxidants for Enhancing Oxidative Stability of Bulk Edible Oils: A Review. Int. J. Food Sci. Technol. 2021, 56, 1–12. [Google Scholar] [CrossRef]
- Zhang, Y.-Y.; Zhang, F.; Thakur, K.; Ci, A.-T.; Wang, H.; Zhang, J.-G.; Wei, Z.-J. Effect of Natural Polyphenol on the Oxidative Stability of Pecan Oil. Food Chem. Toxicol. 2018, 119, 489–495. [Google Scholar] [CrossRef]
- Viana Da Silva, M.; Santos, M.R.C.; Alves Silva, I.R.; Macedo Viana, E.B.; Dos Anjos, D.A.; Santos, I.A.; Barbosa De Lima, N.G.; Wobeto, C.; Jorge, N.; Lannes, S.C.D.S. Synthetic and Natural Antioxidants Used in the Oxidative Stability of Edible Oils: An Overview. Food Rev. Int. 2022, 38, 349–372. [Google Scholar] [CrossRef]
- Nayak, P.K.; Dash, U.; Rayaguru, K.; Krishnan, K.R. Physio-Chemical Changes During Repeated Frying of Cooked Oil: A Review: Deep Frying of Oil. J. Food Biochem. 2016, 40, 371–390. [Google Scholar] [CrossRef]
- Yang, J.H.; Tran, T.T.T.; Le, V.V.M. Effects of Natural Antioxidants on the Palm Olein Quality during the Heating and Frying. Food Meas. 2020, 14, 2713–2720. [Google Scholar] [CrossRef]
- Redondo-Cuevas, L.; Castellano, G.; Torrens, F.; Raikos, V. Revealing the Relationship between Vegetable Oil Composition and Oxidative Stability: A Multifactorial Approach. J. Food Compos. Anal. 2018, 66, 221–229. [Google Scholar] [CrossRef]
- Van Der Westhuizen, I.; Focke, W.W. Stabilizing Sunflower Biodiesel with Synthetic Antioxidant Blends. Fuel 2018, 219, 126–131. [Google Scholar] [CrossRef]
- Brewer, M.S. Natural Antioxidants: Sources, Compounds, Mechanisms of Action, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Aladedunye, F.; Przybylski, R.; Matthaus, B. Performance of Antioxidative Compounds under Frying Conditions: A Review. Crit. Rev. Food Sci. Nutr. 2017, 57, 1539–1561. [Google Scholar] [CrossRef] [PubMed]
- Akbarirad, H.; Gohari Ardabili, A.; Kazemeini, S.m.; Khaneghah, M. An Overview on Some of Important Sources of Natural Antioxidants. Int. Food Res. J. 2016, 23, 928–933. [Google Scholar]
- Munteanu, I.G.; Apetrei, C. Analytical Methods Used in Determining Antioxidant Activity: A Review. Int. J. Mol. Sci. 2021, 22, 3380. [Google Scholar] [CrossRef]
- Turan, S.; Köroğlu, D.G. Oxidative Stability of Soybean Oil Enriched with Ethyl Acetate Extract of Olive By-Products. Turk. J. Agric. Food Sci. Technol. 2020, 8, 1373–1379. [Google Scholar] [CrossRef]
- Gunal Köroglu, D.; Turan, S.; Kiralan, M.; Ramadan Hassanien, M.F. Enhancement of Sunflower Oil Stability during Deep-Frying Using Extracts from Olive Oil by-Products and Soy Lecithin. Int. Food Res. J. 2019, 4, 1269–1277. [Google Scholar]
- Shadyro, O.I.; Sosnovskaya, A.A.; Edimecheva, I.P. Flaxseed Oil Stabilization Using Natural and Synthetic Antioxidants. Eur. J. Lipid Sci. Technol. 2017, 119, 1700079. [Google Scholar] [CrossRef]
- Bijla, L.; Hmitti, A.; Fadda, A.; Oubannin, S.; Gagour, J.; Aissa, R.; Laknifli, A.; Sakar, E.H.; Gharby, S. Valorization of Spent Coffee Ground as a Natural Antioxidant and Its Use for Sunflower Oil Shelf-Life Extension. Eur. J. Lipid Sci. Technol. 2024, 126, 2300115. [Google Scholar] [CrossRef]
- ISO 660; Animal and Vegetable Fats and Oils—Determination of Acid Value and Acidity. ISO: Geneva, Switzerland, 2020. Available online: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/07/55/75594.html (accessed on 7 October 2022).
- ISO 3960; Animal and Vegetable Fats and Oils—Determination of Peroxide Value—Iodometric (Visual) Endpoint Determination. ISO: Geneva, Switzerland, 2017. Available online: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/07/12/71268.html (accessed on 7 October 2022).
- ISO 3656; Animal and Vegetable Fats and Oils—Determination of Ultraviolet Absorbance Expressed as Specific UV Extinction. ISO: Geneva, Switzerland, 2011. Available online: https://www.iso.org/standard/51008.html (accessed on 16 February 2023).
- Gagour, J.; Hallouch, O.; Asbbane, A.; Laknifli, A.; Sakar, E.H.; Majourhat, K.; Gharby, S. Physicochemical Characterization of ‘Moroccan Picholine’ Olive (Olea europaea L.) Oil Produced in Southern Morocco Using Multivariate Statistical Analysis. Analytica 2024, 5, 119–138. [Google Scholar] [CrossRef]
- ISO 6885; Animal and Vegetable Fats and Oils—Determination of Anisidine Value. ISO: Geneva, Switzerland, 2016. Available online: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/06/95/69593.html (accessed on 7 October 2022).
- Nid Ahmed, M.; Abourat, K.; Gagour, J.; Sakar, E.H.; Majourhat, K.; Koubachi, J.; Gharby, S. Valorization of Saffron (Crocus sativus L.) Stigma as a Potential Natural Antioxidant for Soybean (Glycine max L.) Oil Stabilization. Heliyon 2024, 10, e25875. [Google Scholar] [CrossRef]
- ISO 12966-2; Animal and Vegetable Fats and Oils—Gas Chromatography of Fatty Acid Methyl Esters—Part 2: Preparation of Methyl Esters of Fatty Acids. ISO: Geneva, Switzerland, 2017. Available online: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/07/21/72142.html (accessed on 7 October 2022).
- Amini, M.; Golmakani, M.T.; Abbasi, A.; Nader, M. Effects of Sesame Dehulling on Physicochemical and Sensorial Properties of Its Oil. Food Sci. Nutr. 2023, 11, 6596–6603. [Google Scholar] [CrossRef]
- Aydenız, B.; Yilmaz, E. Performance of Different Natural Antioxidant Compounds in Frying Oil. Food Technol. Biotechnol. 2016, 54, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Rajab, Z.M.; Kareem, A.A. Fortification of Vegetable Oils—A Review. IOP Conf. Ser. Earth Environ. Sci. 2023, 1262, 062013. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Zu, Y.; Yang, L.; Lu, Q.; Wang, W. Antioxidant Effects of Rosemary Extracts on Sunflower Oil Compared with Synthetic Antioxidants. Int. J. Food Sci. Technol. 2014, 49, 385–391. [Google Scholar] [CrossRef]
- Alizadeh, L.; Nayebzadeh, K.; Mohammadi, A. A Comparative Study on the in Vitro Antioxidant Activity of Tocopherol and Extracts from Rosemary and Ferulago Angulata on Oil Oxidation during Deep Frying of Potato Slices. J. Food Sci. Technol. 2016, 53, 611–620. [Google Scholar] [CrossRef]
- Ling, S.S.C.; Chang, S.K.; Sia, W.C.M.; Yim, H.S. Antioxidant Effcacy of Unripe Banana (Musa Acuminata Colla) Peel Extracts in Sunflower Oil during Accelerated Storage. Acta Sci. Pol. Technol. Aliment. 2015, 14, 343–356. [Google Scholar] [CrossRef] [PubMed]
- Adiiba, S.H.; Song, C.P.; Lee, Y.Y.; Amelia; Chang, M.Y.; Chan, E.-S. Effects of Water-Soluble Secondary Antioxidants on the Retention of Carotene and Tocols during Hydrolysis of Crude Palm Oil Catalysed by Eversa® Transform 2.0 for Alcohol-Free Production of Palm Phytonutrients Concentrate. Ind. Crops Prod. 2024, 209, 117929. [Google Scholar] [CrossRef]
- Bodoira, R.M.; Penci, M.C.; Ribotta, P.D.; Martínez, M.L. Chia (Salvia hispanica L.) Oil Stability: Study of the Effect of Natural Antioxidants. LWT 2017, 75, 107–113. [Google Scholar] [CrossRef]
- Koohikamali, S.; Alam, M.S. Improvement in Nutritional Quality and Thermal Stability of Palm Olein Blended with Macadamia Oil for Deep-Fat Frying Application. J. Food Sci. Technol. 2019, 56, 5063–5073. [Google Scholar] [CrossRef] [PubMed]
- FAO. Standard for Named Vegetable Oils CXS 210-1999. Available online: https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXS%2B210-1999%252FCXS_210e.pdf (accessed on 17 January 2024).
- Arabsorkhi, B.; Pourabdollah, E.; Mashadi, M. Investigating the Effect of Replacing the Antioxidants Ascorbyl Palmitate and Tocopherol Instead of TBHQ on the Shelf Life of Sunflower Oil Using Temperature Accelerated Method. Food Chem. Adv. 2023, 2, 100246. [Google Scholar] [CrossRef]
- Karabulut, I. Effects of α-Tocopherol, β-Carotene and Ascorbyl Palmitate on Oxidative Stability of Butter Oil Triacylglycerols. Food Chem. 2010, 123, 622–627. [Google Scholar] [CrossRef]
- Smyk, B. Singlet Oxygen Autoxidation of Vegetable Oils: Evidences for Lack of Synergy between β-Carotene and Tocopherols. Food Chem. 2015, 182, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Kehili, M.; Choura, S.; Zammel, A.; Allouche, N.; Sayadi, S. Oxidative Stability of Refined Olive and Sunflower Oils Supplemented with Lycopene-Rich Oleoresin from Tomato Peels Industrial by-Product, during Accelerated Shelf-Life Storage. Food Chem. 2018, 246, 295–304. [Google Scholar] [CrossRef] [PubMed]
- Yin, F.; Sun, X.; Zheng, W.; Luo, X.; Zhang, Y.; Yin, L.; Jia, Q.; Fu, Y. Screening of Highly Effective Mixed Natural Antioxidants to Improve the Oxidative Stability of Microalgal DHA-Rich Oil. RSC Adv. 2021, 11, 4991–4999. [Google Scholar] [CrossRef]
- Keramat, M.; Golmakani, M.-T.; Aminlari, M.; Shekarforoush, S.S. Comparative Effect of Bunium Persicum and Rosmarinus Officinalis Essential Oils and Their Synergy with Citric Acid on the Oxidation of Virgin Olive Oil. Int. J. Food Prop. 2016, 19, 2666–2681. [Google Scholar] [CrossRef]
- Cizkova, H. A New Approach for Stabilization of Gac Oil by Natural Antioxidants. Curr. Appl. Sci. Technol. 2021, 21, 431444. [Google Scholar] [CrossRef]
- Mohanan, A.; Nickerson, M.T.; Ghosh, S. Oxidative Stability of Flaxseed Oil: Effect of Hydrophilic, Hydrophobic and Intermediate Polarity Antioxidants. Food Chem. 2018, 266, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Song, X.; Sui, X.; Qi, B.; Wang, Z.; Li, Y.; Jiang, L. Rosemary Extract Can Be Used as a Synthetic Antioxidant to Improve Vegetable Oil Oxidative Stability. Ind. Crops Prod. 2016, 80, 141–147. [Google Scholar] [CrossRef]
- Ye, L.; Wang, H.; Duncan, S.E.; Eigel, W.N.; O’Keefe, S.F. Antioxidant Activities of Vine Tea (Ampelopsis Grossedentata) Extract and Its Major Component Dihydromyricetin in Soybean Oil and Cooked Ground Beef. Food Chem. 2015, 172, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Aşkın, B.; Kaya, Y. Effect of Deep Frying Process on the Quality of the Refined Oleic/Linoleic Sunflower Seed Oil and Olive Oil. J. Food Sci. Technol. 2020, 57, 4716–4725. [Google Scholar] [CrossRef] [PubMed]
- Günal, D.; Turan, S. Effects of Olive Wastewater and Pomace Extracts, Lecithin, and Ascorbyl Palmitate on the Oxidative Stability of Refined Sunflower Oil. J. Food Process. Preserv. 2018, 42, e13705. [Google Scholar] [CrossRef]
- Gharby, S.; Harhar, H.; Matthäus, B.; Bouzoubaa, Z.; Charrouf, Z. The Chemical Parameters and Oxidative Resistance to Heat Treatment of Refined and Extra Virgin Moroccan Picholine Olive Oil. J. Taibah Univ. Sci. 2016, 10, 100–106. [Google Scholar] [CrossRef]
- Tavakoli, A.; Sahari, M.A.; Barzegar, M. Antioxidant Activity of Berberis Integerrima Seed Oil as a Natural Antioxidant on the Oxidative Stability of Soybean Oil. Int. J. Food Prop. 2017, 20, S2914–S2925. [Google Scholar] [CrossRef]
- Gagour, J.; Ahmed, M.N.; Bouzid, H.A.; Oubannin, S.; Bijla, L.; Ibourki, M.; Hajib, A.; Koubachi, J.; Harhar, H.; Gharby, S. Proximate Composition, Physicochemical, and Lipids Profiling and Elemental Profiling of Rapeseed (Brassica napus L.) and Sunflower (Helianthus annuus L.) Grown in Morocco. Evid. -Based Complement. Altern. Med. 2022, 2022, 1–12. [Google Scholar] [CrossRef]
- Sidhu, A.R.; Naz, S.; Mahesar, S.A.; Kandhro, A.A.; Khaskheli, A.R.; Ali, Z.; Memon, H.D.; Shoaib, H.; Ur Rehman Mahesar, H. Effect of Storage at Elevated Temperature on the Quality and Stability of Different Almond Oils: A Comprehensive Study. Food Mater. 2023, 3, 30. [Google Scholar] [CrossRef]
- Aissa, R.; Asbbane, A.; Oubannin, S.; Bijla, L.; Bousaid, Z.; Hallouch, O.; El Harkaoui, S.; Matthäus, B.; Sakar, E.H.; Gharby, S. Oxidative Stability of Virgin and Refined Argan [Argania spinosa L. (Skeels)] Oil under Accelerated Aging Conditions and Shelf-Life Prediction at Room Temperature: A Comparative Study. Analytica 2023, 4, 500–512. [Google Scholar] [CrossRef]
- Kiralan, M.; Ulaş, M.; Özaydin, A.; Özdemır, N.; Özkan, G.; Bayrak, A.; Ramadan, M.F. Blends of Cold Pressed Black Cumin Oil and Sunflower Oil with Improved Stability: A Study Based on Changes in the Levels of Volatiles, Tocopherols and Thymoquinone during Accelerated Oxidation Conditions: Blends of Cold Pressed Black Cumin Oil and Sunflower Oil. J. Food Biochem. 2017, 41, e12272. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Chatzimitakos, T.; Kalompatsios, D.; Palaiogiannis, D.; Makrygiannis, I.; Bozinou, E.; Lalas, S.I. Evaluation of the Efficacy and Synergistic Effect of α- and δ-Tocopherol as Natural Antioxidants in the Stabilization of Sunflower Oil and Olive Pomace Oil during Storage Conditions. Int. J. Mol. Sci. 2023, 24, 1113. [Google Scholar] [CrossRef] [PubMed]
- Chong, Y.M.; Chang, S.K.; Sia, W.C.M.; Yim, H.S. Antioxidant Efficacy of Mangosteen (Garcinia Mangostana Linn.) Peel Extracts in Sunflower Oil during Accelerated Storage. Food Biosci. 2015, 12, 18–25. [Google Scholar] [CrossRef]
- Sahunie, A. Effect of Rosmarinus Officinalis and Origanum Majorana Extracts on Stability of Sunflower Oil during Storage and Repeated Heating. Oil Crop Sci. 2024, 9, 29–37. [Google Scholar] [CrossRef]
- Sousa, G.; Alves, M.I.; Neves, M.; Tecelão, C.; Ferreira-Dias, S. Enrichment of Sunflower Oil with Ultrasound-Assisted Extracted Bioactive Compounds from Crithmum maritimum L. Foods 2022, 11, 439. [Google Scholar] [CrossRef] [PubMed]
- Fadda, A.; Sanna, D.; Sakar, E.H.; Gharby, S.; Mulas, M.; Medda, S.; Yesilcubuk, N.S.; Karaca, A.C.; Gozukirmizi, C.K.; Lucarini, M.; et al. Innovative and Sustainable Technologies to Enhance the Oxidative Stability of Vegetable Oils. Sustainability 2022, 14, 849. [Google Scholar] [CrossRef]
- Alsufiani, H.; Ashour, W. Effectiveness of the Natural Antioxidant 2,4,4′-Trihydroxychalcone on the Oxidation of Sunflower Oil during Storage. Molecules 2021, 26, 1630. [Google Scholar] [CrossRef]
- Yildiz, S.; Turan, S.; Kiralan, M.; Ramadan, M.F. Antioxidant Properties of Thymol, Carvacrol, and Thymoquinone and Its Efficiencies on the Stabilization of Refined and Stripped Corn Oils. Food Meas. 2021, 15, 621–632. [Google Scholar] [CrossRef]
- Marinova, E.; Toneva, A.; Yanishlieva, N. Synergistic Antioxidant Effect of α-Tocopherol and Myricetin on the Autoxidation of Triacylglycerols of Sunflower Oil. Food Chem. 2008, 106, 628–633. [Google Scholar] [CrossRef]
- El Moudden, H.; El Idrissi, Y.; Belmaghraoui, W.; Belhoussaine, O.; El Guezzane, C.; Bouayoun, T.; Harhar, H.; Tabyaoui, M. Olive Mill Wastewater Polyphenol-based Extract as a Vegetable Oil Shelf Life Extending Additive. J. Food Process. Preserv. 2020, 44, e14990. [Google Scholar] [CrossRef]
- Gharby, S.; Charrouf, Z. Argan Oil: Chemical Composition, Extraction Process, and Quality Control. Front. Nutr. 2021, 8, 804587. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.K.; Chopra, R.; Garg, M. Comparative Study on the Use of Rosemary Bioactive for Enhancing the Oxidative Stability of Blended Perilla Seed Oil: A Multivariant Kinetic Approach. Food Chem. Adv. 2023, 3, 100447. [Google Scholar] [CrossRef]
- El Bernoussi, S.; Boujemaa, I.; Harhar, H.; Belmaghraoui, W.; Matthäus, B.; Tabyaoui, M. Evaluation of Oxidative Stability of Sweet and Bitter Almond Oils under Accelerated Storage Conditions. J. Stored Prod. Res. 2020, 88, 101662. [Google Scholar] [CrossRef]
- Cherif, A.; Slama, A. Stability and Change in Fatty Acids Composition of Soybean, Corn, and Sunflower Oils during the Heating Process. J. Food Qual. 2022, 2022, e6761029. [Google Scholar] [CrossRef]
- Meng, Y.; Yang, H.; Wang, D.; Ma, Y.; Wang, X.; Blasi, F. Improvement for Oxidative Stability and Sensory Properties of Sunflower Oil Flavored by Huai Chrysanthemum × Morifolium Ramat. Essential Oil during Accelerated Storage. Processes 2021, 9, 1199. [Google Scholar] [CrossRef]
- Yılmaz, B.; Şahin, T.Ö.; Ağagündüz, D. Oxidative Changes in Ten Vegetable Oils Caused by the Deep-Frying Process of Potato. J. Food Biochem. 2023, 2023, 1–11. [Google Scholar] [CrossRef]
- Alireza, S.; Tan, C.P.; Hamed, M.; Che Man, Y.B. Effect of Frying Process on Fatty Acid Composition and Iodine Value of Selected Vegetable Oils and Their Blends. Int. Food Res. J. 2010, 17, 295–302. [Google Scholar]
- Romanić, R.; Lužaić, T.; Grgić, K. Examining the Possibility of Improving the Properties of Sunflower Oil in Order to Obtain a Better Medium for the Process of Frying Food. Proceedings 2020, 70, 104. [Google Scholar] [CrossRef]
- Silva de Sousa, L.; Verônica Rodarte de Moura, C.; Miranda de Moura, E. Action of Natural Antioxidants on the Oxidative Stability of Soy Biodiesel during Storage. Fuel 2021, 288, 119632. [Google Scholar] [CrossRef]
- Farahmandfar, R.; Asnaashari, M.; Pourshayegan, M.; Maghsoudi, S.; Moniri, H. Evaluation of Antioxidant Properties of Lemon Verbena (Lippia citriodora) Essential Oil and Its Capacity in Sunflower Oil Stabilization during Storage Time. Food Sci. Nutr. 2018, 6, 983–990. [Google Scholar] [CrossRef] [PubMed]
- Nogales-Delgado, S.; Encinar, J.M.; Guiberteau, A.; Márquez, S. The Effect of Antioxidants on Corn and Sunflower Biodiesel Properties under Extreme Oxidation Conditions. J. Am. Oil Chem. Soc. 2020, 97, 201–212. [Google Scholar] [CrossRef]
- Hassanein, M.M.M.; Al-Amrousi, E.F.; Abo-Elwafa, G.A.; Abdel-Razek, A.G. Characterization of Egyptian Monovarietal Koroneiki Virgin Olive Oil and Its Co-Products. Egypt. J. Chem. 2022, 65, 637–645. [Google Scholar] [CrossRef]
- Athanasiadis, V.; Chatzimitakos, T.; Kotsou, K.; Kalompatsios, D.; Bozinou, E.; Lalas, S.I. Utilization of Blackthorn Plums (Prunus spinosa) and Sweet Cherry (Prunus avium) Kernel Oil: Assessment of Chemical Composition, Antioxidant Activity, and Oxidative Stability. Biomass 2024, 4, 49–64. [Google Scholar] [CrossRef]
- Singh, P.K.; Chopra, R.; Garg, M.; Chauhan, K.; Agarwal, A. Stability of Perilla Seed Oil Based PUFA-Rich Structured Lipids Using Enzymatic Interesterification: A Thermo-Oxidative Kinetic Study. Ind. Crops Prod. 2024, 209, 118029. [Google Scholar] [CrossRef]
- Ghaliaoui, N.; Hazzit, M.; Seridi, H.; Mokrane, H. Oxidative Stability of Soybean And Sunflower Oils Enriched With Pigment Extracts of the Brown Seaweed Phyllaria Reniformis: Stabilisation of Edible Oils by Alga Pigments. J. Microbiol. Biotechnol. Food Sci. 2023, 13, e9290. [Google Scholar] [CrossRef]
- Rhazi, L.; Depeint, F.; Ayerdi Gotor, A. Loss in the Intrinsic Quality and the Antioxidant Activity of Sunflower (Helianthus annuus L.) Oil during an Industrial Refining Process. Molecules 2022, 27, 916. [Google Scholar] [CrossRef] [PubMed]
- Banaś, J.; Maciejaszek, I.; Surówka, K.; Zawiślak, A. Temperature-Induced Storage Quality Changes in Pumpkin and Safflower Cold-Pressed Oils. Food Meas. 2020, 14, 1213–1222. [Google Scholar] [CrossRef]
- Díez-Betriu, A.; Bustamante, J.; Romero, A.; Ninot, A.; Tres, A.; Vichi, S.; Guardiola, F. Effect of the Storage Conditions and Freezing Speed on the Color and Chlorophyll Profile of Premium Extra Virgin Olive Oils. Foods 2023, 12, 222. [Google Scholar] [CrossRef]
- Maoka, T. Carotenoids as Natural Functional Pigments. J. Nat. Med. 2020, 74, 1–16. [Google Scholar] [CrossRef]
- Gharby, S.; Oubannin, S.; Ait Bouzid, H.; Bijla, L.; Ibourki, M.; Gagour, J.; Koubachi, J.; Sakar, E.H.; Majourhat, K.; Lee, L.-H.; et al. An Overview on the Use of Extracts from Medicinal and Aromatic Plants to Improve Nutritional Value and Oxidative Stability of Vegetable Oils. Foods 2022, 11, 3258. [Google Scholar] [CrossRef] [PubMed]
- Ghendov-Mosanu, A.; Popovici, V.; Constantinescu (Pop), C.G.; Deseatnicova, O.; Siminiuc, R.; Subotin, I.; Druta, R.; Pintea, A.; Socaciu, C.; Sturza, R. Stabilization of Sunflower Oil with Biologically Active Compounds from Berries. Molecules 2023, 28, 3596. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).