Sulfur-Containing Homo- and Methanofullerenes: Synthesis and Study of Tribological Properties †
Abstract
:1. Introduction
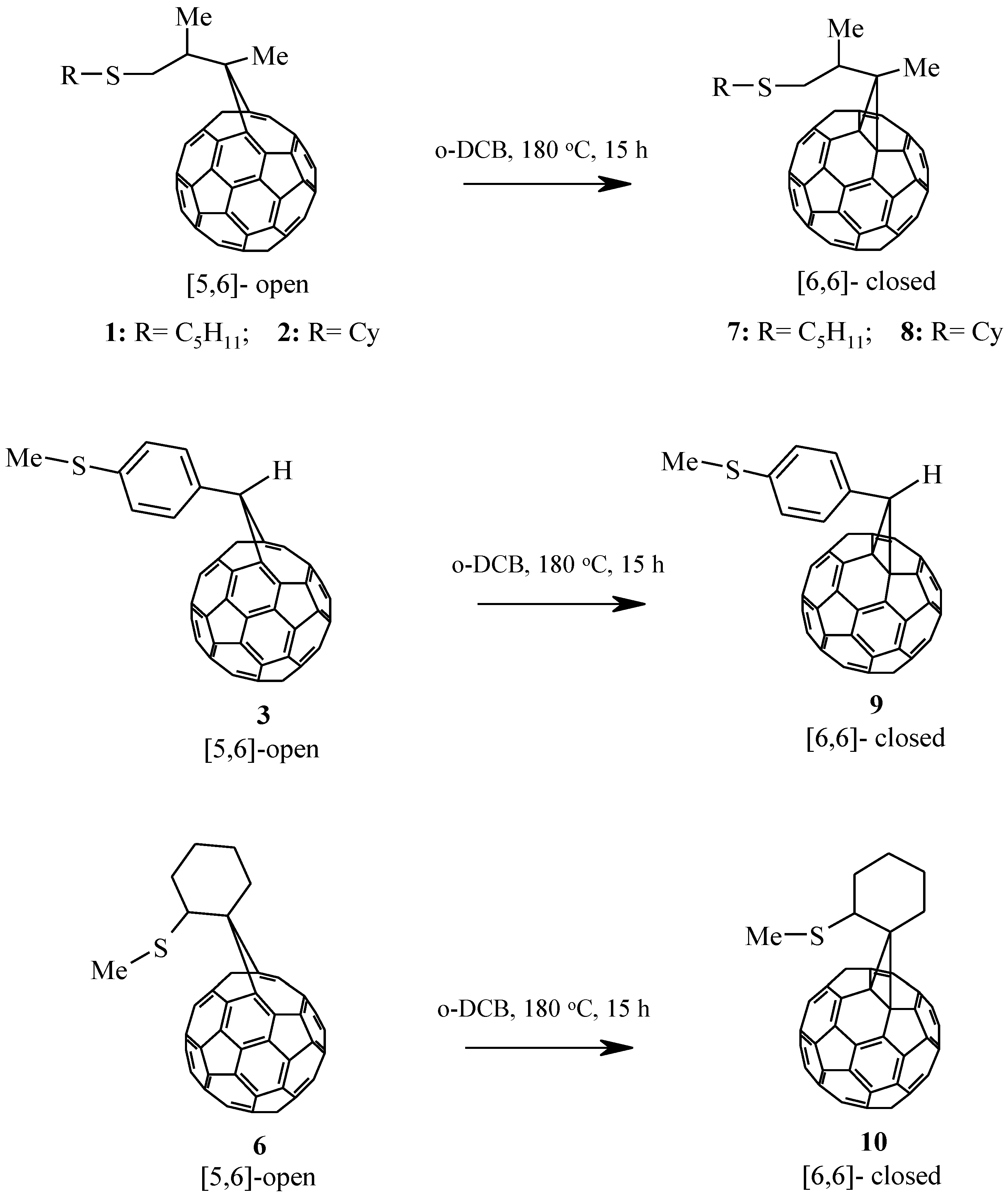
2. Results and Discussion
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Takaguchi, Y.; Katayose, Y.; Yanagimoto, Y.; Motoyoshiya, J.; Aoyama, H.; Wakahara, T.; Maeda, Y.; Akasaka, T. Photoinduced Dithiolation of Fullerene[60] with Dendrimer Disulfide. Chem. Lett. 2003, 32, 1124–1125. [Google Scholar] [CrossRef]
- Isobe, H.; Mashima, H.; Yorimitsu, H.; Nakamura, E. Synthesis of Fullerene glycoconjugates through sulfide connection in aqueous media. Org. Lett. 2003, 5, 4461–4463. [Google Scholar] [CrossRef] [PubMed]
- Boutorine, A.S.; Tokuyama, H.; Takasugi, M.; Isobe, H.; Nakamura, E.; Helene, C. Fullerene-Oligonucleotide Conjugates: Photoinduced Sequence-Specific DNA Cleavage. Angew. Chem. Int. Ed. Engl. 1994, 33, 2462–2465. [Google Scholar] [CrossRef]
- An, Y.-Z.; Chen, C.-H.B.; Anderson, J.L.; Sigman, D.S.; Foote, C.S.; Rubin, Y. Sequence-specific modification of quanosine in DNA by a C60-linked deoxyoligonucleotide: Evidence for a non-singlet oxygen mechanism. Tetrahedron 1996, 52, 5179–5189. [Google Scholar] [CrossRef]
- Allard, E.; Cousseau, J.; Orduna, J.; Garin, J.; Luo, H.; Araki, Y.; Ito, O. Photoinduced electron-transfer processes in C60-tetrathiafulvalene dyads containing a short or long flexible spacer. Phys. Chem. Chem. Phys. 2002, 4, 5944–5951. [Google Scholar] [CrossRef]
- Schulze, B.K.; Uhrich, C.; Schlippel, R.; Leo, K.; Pfeiffer, M.; Brier, E.; Reinold, E.; Bauerle, P. Efficient vacuum-deposited organic solar cells based on a new low-bandgapoligothiophene and fullerene C60. Adv. Mater. 2006, 18, 2872–2875. [Google Scholar] [CrossRef]
- Cravino, A.; Leriche, P.; Aleveque, O.; Roquet, S.; Roncali, J. Light-Emitting Organic Solar Cells Based on a 3D Conjugated System with internal charge transfer. Adv. Mater. 2006, 18, 3033–3037. [Google Scholar] [CrossRef]
- Guldi, D.M.; Gonzalez, S.; Martin, N.; Anton, A.; Garin, J.; Orduna, J. Efficient Charge Separation in C60-Based Dyads: Triazolino[4′,5′:1,2][60]fullerenes. J. Org. Chem. 2000, 65, 1978–1983. [Google Scholar] [CrossRef]
- Ge, Z.; Li, Y.; Guo, Z.; Shi, Z.; Zhu, D. Synthesis of a New Crown Ether-Bearing [60]-Fulleropyrrolidine Containing a BenzothiazoliumStyryl Dye. Tetrahedron Lett. 1999, 40, 5759–5762. [Google Scholar] [CrossRef]
- Benincori, T.; Brenna, E.; Sannicolo, F.; Trimarco, L.; Zotti, G.; Sozzani, P. Ein elektrisch leitfähiges Polythiophen mit kovalent gebundenen Fullerenresten. Angew. Chem. Int. Ed. Engl. 1996, 35, 718–720. [Google Scholar] [CrossRef]
- Meijer, M.D.; Mulder, B.; van Klink, G.P.M.; van Koten, G. Synthesis of C60-attached SCS pincer palladium(II) complexes. Inorg. Chim. Acta 2003, 352, 247–252. [Google Scholar] [CrossRef]
- Tuktarov, A.R.; Dzhemilev, U.M. Diazo compounds in the chemistry of fullerenes. Russ. Chem. Rev. 2010, 79, 585. [Google Scholar] [CrossRef]
- Tuktarov, A.R.; Akhmetov, A.R.; Kamalov, R.F.; Khalilov, L.M.; Pudas, M.; Ibragimov, A.G.; Dzhemilev, U.M. Catalytic [2+1]-cycloaddition of ethyl diazoacetate to fullerene [60]. Russ. J. Org. Chem. 2009, 45, 1168–1174. [Google Scholar] [CrossRef]
- Tuktarov, A.R.; Akhmetov, A.R.; Sabirov, D.S.; Khalilov, L.M.; Ibragimov, A.G.; Dzhemilev, U.M. Catalytic [2+1]-cycloaddition of diazo compounds to fullerene[60]. Russ. Chem. Bull. 2009, 8, 1724–1730. [Google Scholar] [CrossRef]
- Tuktarov, A.R.; Korolev, V.V.; Tulyabaev, A.R.; Yanybin, V.M.; Khalilov, L.M.; Dzhemilev, U.M. Cycloaddition of cyclic diazo compounds to C60 fullerene in the presence of a Pd-containing complex catalyst. Russ. Chem. Bull. 2010, 5, 977–993. [Google Scholar] [CrossRef]
- Tuktarov, A.R.; Korolev, V.V.; Khalilov, L.M.; Ibragimov, A.G.; Dzhemilev, U.M. Catalytic cyclopropanation of fullerene[60] with diazomethane. Russ. J. Org. Chem. 2009, 45, 1594. [Google Scholar]
- Tuktarov, A.R.; Korolev, V.V.; Dzhemilev, U.M. Catalytic cycloaddition of diazoalkanes generated in situ to C60-fullerene. Russ. J. Org. Chem. 2010, 46, 588–589. [Google Scholar] [CrossRef]
- Tuktarov, A.R.; Korolev, V.V.; Tulyabaev, A.R.; Yanybin, V.M.; Khalilov, L.M.; Dzhemilev, U.M. Cycloaddition of diazocycloalkanes to [60]fullerene in the presence of Pd-containing complex catalyst. Russ. Chem. Bull. 2010, 59, 977–983. [Google Scholar] [CrossRef]
- Tuktarov, A.R.; Akhmetov, A.R.; Khalilov, L.M.; Dzhemilev, U.M. Cycloaddition of diazoketones to [60]fullerene in the presence of the catalytic system Pd(acac)2—PPh3—Et3Al. Russ. Chem. Bull. 2010, 59, 611–614. [Google Scholar] [CrossRef]
- Tuktarov, A.R.; Akhmetov, A.R.; Khasanova, L.L.; Khalilov, L.M.; Dzhemilev, U.M. Cycloaddition of diazoacetates to C60 fullerene catalysed by Pd complexes. Russ. Chem. Bull. 2010, 59, 1959–1963. [Google Scholar] [CrossRef]
- Tuktarov, A.R.; Akhmetov, A.R.; Korolev, V.V.; Khuzin, A.A.; Khasanova, L.L.; Popod’ko, N.R.; Khalilov, L.M. Palladium-catalyzed selective cycloaddition of diazo compounds to [60]fullerene. Arkivoc 2011, 8, 54–66. [Google Scholar] [CrossRef]
- Tuktarov, A.R.; Khuzin, A.A.; Korolev, V.V.; Dzhemilev, U.M. Catalytic cycloaddition of diazoalkanes with heterocyclic substituents to fullerene C60. Russ. J. Org. Chem. 2012, 48, 99–103. [Google Scholar] [CrossRef]
- Smith, A.B., III; Strongin, R.M.; Brard, L.; Furst, G.T.; Romanov, W.J.; Owens, K.G.; Goldschmidt, R.J.; King, R.C. Synthesis of Prototypical Fullerene Cyclopropanes and Annulenes. Isomer Differentiation via NMR and UV Spectroscopy. J. Am. Chem. Soc. 1995, 117, 5492–5502. [Google Scholar] [CrossRef]
- Tuktarov, A.R.; Khuzin, A.A.; Popod’ko, N.R.; Dzhemilev, U.M. Cycloaddition of diazothioates to [60]fullerene. Tetrahedron Lett. 2012, 53, 3123–3125. [Google Scholar] [CrossRef]
- Tuktarov, A.R.; Khuzin, A.A.; Popod’ko, N.R.; Dzhemilev, U.M. Synthesis and tribological properties of sulfur-containing methanofullerenes. Fuller. Nanotub. Carbon Nanostruct. 2014, 22, 397–403. [Google Scholar] [CrossRef]













| № | Test Samples | Pcr, kgf | Pw, kgf | dws, mm |
|---|---|---|---|---|
| 1 | Industrial oil + 5 wt% STP * + 0.005 wt% of compound 2′ | 71 | 596 | 0.73 |
| 2 | Industrial oil + 5 wt% STP + 0.005 мас.% of compound 12 | 75 | >1000 | 0.76 |
| 3 | Industrial oil + 5 wt% STP + 0.005 мас.% of compound 21 | 71 | >1000 | 0.74 |
| 4 | Industrial oil + 5 wt% STP + 0.005 мас.% of compound 30 | 71 | >1000 | 0.51 |
| 5 | Industrial oil + 5 wt% of sulfidized tetramers of propylene (STP) (Control sample) | 79 | 398 | 0.93 |
| 6 | Industrial oil + 40 wt% of sulfidized tetramers of propylene (STP) (Control sample) | 100 | 596 | 0.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khuzin, A.A.; Tuktarov, A.R.; Dzhemilev, U.M. Sulfur-Containing Homo- and Methanofullerenes: Synthesis and Study of Tribological Properties. Chem. Proc. 2022, 12, 25. https://doi.org/10.3390/ecsoc-26-13537
Khuzin AA, Tuktarov AR, Dzhemilev UM. Sulfur-Containing Homo- and Methanofullerenes: Synthesis and Study of Tribological Properties. Chemistry Proceedings. 2022; 12(1):25. https://doi.org/10.3390/ecsoc-26-13537
Chicago/Turabian StyleKhuzin, Artur A., Airat R. Tuktarov, and Usein M. Dzhemilev. 2022. "Sulfur-Containing Homo- and Methanofullerenes: Synthesis and Study of Tribological Properties" Chemistry Proceedings 12, no. 1: 25. https://doi.org/10.3390/ecsoc-26-13537
APA StyleKhuzin, A. A., Tuktarov, A. R., & Dzhemilev, U. M. (2022). Sulfur-Containing Homo- and Methanofullerenes: Synthesis and Study of Tribological Properties. Chemistry Proceedings, 12(1), 25. https://doi.org/10.3390/ecsoc-26-13537






