Abstract
A carboxylic acid azo dye, Dabcyl, was synthesized and evaluated as a colorimetric chemosensor for metal cations with biological and environmental importance. The dye was prepared in high yield and characterized by 1H and 13C NMR and UV–Vis absorption spectroscopies. A preliminary chemosensing study showed that Dabcyl displayed a marked color change from light yellow to pink, for Hg2+, Fe2+, Pd2+, Sn2+ and Al3+ in acetonitrile solution. Consequently, spectrophotometric titrations were carried out for this dye with selected cations, which clearly indicated that Dabcyl has potential application as a chromogenic probe for the cations under study with remarkable sensitivity and with a marked color change.
1. Introduction
Great efforts have been focused on developing selective and sensitive signaling probes for detecting metal ions because they are indispensable in most biological processes but at abnormal levels these analytes may cause severe effects on human health and the environment [1,2,3].
Mercury is considered as one of the most dangerous metal cations in the ecosystem due to its well-known accumulative and toxic features; it is listed as a persistent, bioaccumulative and toxic (PBT) substance [4,5]. Tin is an important vital trace mineral in the human body, and is crucial for many tissues and organs. However, excess accumulation of tin may seriously interfere with respiratory, digestive and nervous systems. In the environment, excess tin may result from the degradation pathway of another PBT, tributyltin chloride [6,7]. Aluminum is extensively released in the environment owing to its wide application in daily life. An excess amount of Al3+ ions can damage the human nervous system and is closely related to many diseases such as Alzheimer’s disease, Parkinson’s disease, anemia and gastrointestinal diseases [8,9]. Therefore, the development of chemosensors capable of accurately and efficiently detecting biologically and environmentally important metal ions is essential.
Among optical chemosensors, azo dyes are widely used as chromogenic probes due to their ease of synthesis, structural variability and tunability of color detection with the naked eye [10,11]. A very popular azo compound is 4-[[4′-(N,N-dimethylamino)phenyl]diazenyl]benzoic acid, known as Dabcyl, a push−pull azobenzene functionalized at the para positions of the phenyl rings [12]. Besides its biomolecular applications as a dark quencher in FRET-based probes, Dabcyl has also been used as a precursor in colorimetric chemosensors for cation recognition [13,14].
Following this group’s interest in the synthesis and evaluation of optical chemosensors for various ions [15,16,17,18], we report herein the synthesis of Dabcyl, as well as its evaluation as a potential colorimetric chemosensor for metal cations through chemosensing studies in the presence of several cations in acetonitrile solutions.
2. Experimental Section
2.1. Instruments and Materials
Nuclear magnetic resonance (NMR) spectra were obtained on a Bruker Avance III 400 (Bruker Corporation, Billerica, MA, USA) at an operating frequency of 400 MHz for 1H and 100.6 MHz for 13C using the solvent peak as an internal reference. The solvents are indicated in parentheses before the chemical shift values (δ relative to tetramethylsilane and given in ppm). Assignments were supported by two-dimensional heteronuclear correlation techniques. Thin-layer chromatography (TLC) analyses were carried out on 0.25 mm-thick silica plates coated with fluorescent indicator F254 (Merck KGaA, Darmstadt, Germany) and spots were visualized in a CN15 viewing cabinet under a UV lamp at 365 nm (Vilber Lourmat, Marne-la-Vallée, France). UV–Vis absorption spectra were obtained using a Shimadzu UV/2501PC spectrophotometer (Shimadzu Europa GmbH, Duisburg, Germany). All reagents were purchased from Acros Organics (Geel, Belgium) and Sigma-Aldrich (St. Louis, MO, USA), and used as received.
2.2. Synthesis of Dabcyl 2
A mixture of 4-aminobenzoic acid (0.500 g, 1 equiv.), aqueous HCl 1 M (7.5 mL) and aqueous HCl 6 M (0.42 mL) was cooled to 0–5 °C in an ice bath. Aqueous NaNO2 (0.251 g, 1 equiv., in 1 mL of water) was added and the reaction mixture was stirred for 30 min. The previously prepared diazonium salt solution was added dropwise to a solution of N,N-dimethylaniline (0.46 mL, 1 equiv.) in a solution of AcOH (1.25 mL) in H2O (0.83 mL). The pH was adjusted to 4 with a saturated aqueous solution of sodium acetate, whereupon precipitation occurred. The precipitated dye was filtered, washed with water and diethyl ether and dried at 40 °C. Compound 2 (Figure 1) was obtained as a dark red solid without additional purification (0.892 g, 91%).
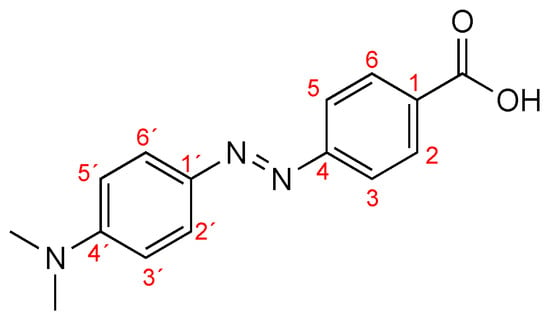
Figure 1.
Structure of Dabcyl 2.
1H NMR (400 MHz, DMSO-d6): δ = 3.06 (6H, s, 2xCH3), 6.83 (2H, dd, J = 2 and 7.2 Hz, H-3’and H-5´), 7.81 (4H, dd, J = 2.8 Hz and 9.2 Hz, H-3, H-5, H-2´and H-6´), 8.05 (2H, dd, J = 2.0 Hz and 7.2 Hz, H-2 and H-6) ppm.
13C NMR (100.6 MHz, DMSO-d6): δ = 39.96 (2xCH3), 111.74 (C3´ + C5´), 121.85 (C3 + C5), 125.42 (C2´ + C6´), 130.65 (C2 + C6), 131.13 (C1), 142.78 (C1´), 153.15 (C4´), 155.15 (C4), 167.12 (C=O) ppm.
2.3. Preliminary Chemosensing Studies and Spectrophotometric Titrations of Dabcyl 2
Evaluation of Dabcyl 2 as a colorimetric chemosensor was performed in the presence of several cations (Ag+, K+, Li+, Na+, Cu+, TBT+, Hg2+, Ca2+, Co2+, Pb2+, Mn2+, Fe2+, Zn2+, Ni2+, Cd2+, Cu2+, Pd2+, Cs2+, Sn2+, Fe3+ and Al3+). Solutions of compound 2 and solutions of these cations were prepared in UV-grade acetonitrile.
A preliminary study was carried out by addition of 20 equivalents of each cation (1 × 10−2 M) to a solution (1 mL, 1 × 10−5 M) of dye 2 and an assessment of the color was evaluated by the naked eye. Given these preliminary results, spectrophotometric titrations of Dabcyl 2 with selected cations were performed by the sequential addition of each cation (5 × 10−3 M) to a solution (3 mL, 1 × 10−5 M) of dye 2, and the absorption spectra were collected until the absorbance plateau was reached.
3. Results and Discussion
3.1. Synthesis and Characterization of Dabcyl 2
The dye was prepared by an azo coupling reaction between an aryl diazonium salt and an activated electron-rich aromatic component (Scheme 1). Firstly, the diazotization of 4-aminobenzoic acid using sodium nitrite as a nitrosating agent resulted in the diazonium cation 1, which was not isolated. Then, the SEAr-type reaction of the diazonium salt 1 with N,N-dimethylaniline gave Dabcyl 2 with an excellent yield (91%) without additional purification. The synthesized compound was characterized by 1H and 13C NMR spectroscopy, and the obtained data was in agreement with the expected structure.
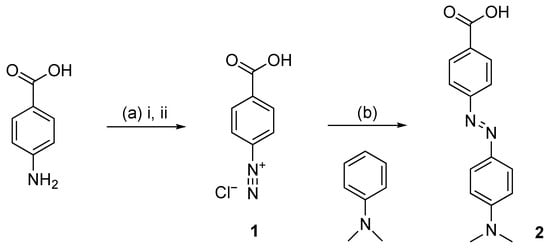
Scheme 1.
Synthesis of Dabcyl 2: (a) i: HCl 1 M, HCl 6 M, r.t.; ii: NaNO2, H2O, 0 °C, 30 min (b) AcOH, H2O, r.t.
The photophysical properties of Dabcyl 2 were analyzed in acetonitrile solution (1 × 10−5 M). The compound exhibited a large UV–Vis absorbance band with a high molar absorption coefficient (log ε = 4.49) at 439 nm. This is a typical feature of azobenzene derivatives related to their significant number of vibronic states in each energy level, Sn [19]. Furthermore, because of the isomerization of their azo bridge, either through a rotation mechanism around the -N=N- double bond or an inversion mechanism upon UV–Vis irradiation, these dyes are usually weakly or non-fluorescent compounds. Hence, the fluorescence properties of Dabcyl have not been studied in this work [19,20].
3.2. Preliminary Chemosensing Studies of Dabcyl 2
A preliminary evaluation of Dabcyl 2 as a colorimetric chemosensor was performed in acetonitrile solution in the presence of various cations. The study was performed by addition of 20 equivalents of each cation to a solution of the compound (Figure 2). The chromogenic response of compound 2 was remarkably visible to the naked eye, with a color change from light yellow to pink in the presence of Hg2+, Fe2+, Pd2+, Sn2+ and Al3+.

Figure 2.
Color changes observed for Dabcyl 2 in acetonitrile solution after addition of 20 equivalents of each cation.
3.3. Spectrophotometric Titrations of Dabcyl 2
Considering the preliminary results and the above-mentioned effects of Hg2+, Sn2+ and Al3+ on human health and the environment, the sensing properties of Dabcyl 2 towards these cations were examined in more detail using UV–Vis spectroscopy in acetonitrile solution. The increase in the ratio of these cations led to the gradual disappearance of the absorption band at 439 nm and the appearance of a new peak at 510 nm, with an isosbestic point at 465 nm (Figure 3). The significant red shift in the UV–Vis spectra (Δλ = 71 nm) resulted in a color change from light yellow to pink, which can be easily detected by the naked eye. Furthermore, the sensitivity of Dabcyl 2 towards these cations was evident, since the addition of only 1.5 equivalents of Al3+ was necessary to reach the absorbance plateau, while the interaction with Hg2+ and Sn2+ required a higher amount to reach the plateau, with six and seven equivalents required, respectively. Therefore, Dabcyl 2 can be used to detect these cations with remarkable sensitivity.
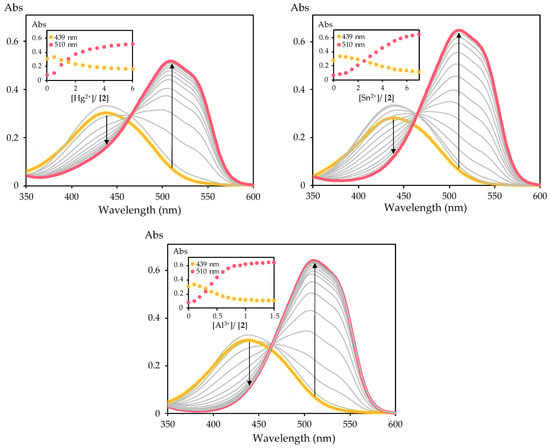
Figure 3.
Spectrophotometric titration of Dabcyl 2 with addition of increasing amounts of Hg2+, Sn2+ and Al3+ in acetonitrile. The inset represents the normalized absorption ([2] = 1 × 10−5 M).
4. Conclusions
This work reports the synthesis and evaluation of Dabcyl acid 2 as a colorimetric chemosensor for metal cations. This compound had a colorimetric response for different cations in acetonitrile with a marked color change from light yellow to pink in the presence of Hg2+, Fe2+, Pd2+, Sn2+ and Al3+. Moreover, the sensing properties of Dabcyl 2 with selected cations were analyzed by UV–Vis spectroscopy in acetonitrile solution, confirming the sensitivity of this dye towards Hg2+, Sn2+ and Al3+. Therefore, Dabcyl 2 could be a potential sensitive colorimetric probe for the detection of these analytes. In the near future, further studies will be performed in water solutions to determine the potential application of Dabcyl as a chromogenic probe for cations in environmental and biological samples.
Author Contributions
Conceptualization, S.P.G.C.; methodology, C.D.F.M. and S.P.G.C.; validation, S.P.G.C. and M.M.M.R.; formal analysis, C.D.F.M., M.M.M.R. and S.P.G.C.; investigation, C.D.F.M.; resources, S.P.G.C. and M.M.M.R.; writing—original draft preparation, C.D.F.M.; writing—review and editing, C.D.F.M., M.M.M.R. and S.P.G.C.; supervision, S.P.G.C. and M.M.M.R.; project administration, S.P.G.C. and M.M.M.R.; funding acquisition, M.M.M.R. and S.P.G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Foundation for Science and Technology (FCT) for financial support to CQ/UM (UID/QUI/00686/2020), project PTDC/QUI-COL/28052/2017 and a PhD grant to C. D. F. Martins (SFRH/BD/05277/2020). The NMR spectrometer Bruker Avance III 400 is part of the National NMR Network and was purchased within the framework of the National Pro-gram for Scientific Re-equipment, contract REDE/1517/RMN/2005 with funds from POCI 2010 (FEDER) and FCT.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- You, L.; Zha, D.; Anslyn, E.V. Recent advances in supramolecular analytical chemistry using optical sensing. Chem. Rev. 2015, 115, 7840–7892. [Google Scholar] [CrossRef] [PubMed]
- Kaur, B.; Kaur, N.; Kumar, S. Colorimetric metal ion sensors—A comprehensive review of the years 2011–2016. Coord. Chem. Rev. 2018, 358, 13–69. [Google Scholar] [CrossRef]
- Wu, D.; Sedgwick, A.C.; Gunnlaugsson, T.; Akkaya, E.U.; Yoon, J.; James, T.D. Fluorescent chemosensors: The past, present and future. Chem. Soc. Rev. 2017, 46, 7105–7123. [Google Scholar] [CrossRef] [PubMed]
- Heo, G.; Manivannan, R.; Kim, H.; Kim, M.J.; Min, K.S.; Son, Y.-A. Developing an RGB—Arduino device for the multi-color recognition, detection and determination of Fe(III), Co(II), Hg(II) and Sn(II) in aqueous media by a terpyridine moiety. Sens. Actuators B Chem. 2019, 297, 126723. [Google Scholar] [CrossRef]
- Ando, S.; Koide, K. Development and applications of fluorogenic probes for mercury(II) based on vinyl ether oxymercuration. J. Am. Chem. Soc. 2011, 133, 2556–2566. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Tan, S.; Hu, H.; Chen, Z.; Pu, S. Novel colorimetric and fluorescent chemosensor for Hg2+/Sn2+ based on a photochromic diarylethene with a styrene-linked pyrido[2,3-b] pyrazine unit. J. Photochem. Photobiol. A Chem. 2021, 418, 113439. [Google Scholar] [CrossRef]
- Manna, S.K.; Mondal, S.; Jana, B.; Samanta, K. Recent advances in tin ion detection using fluorometric and colorimetric chemosensors. New J. Chem. 2022, 46, 7309–7328. [Google Scholar] [CrossRef]
- Gupta, V.K.; Shoora, S.K.; Kumawat, L.K.; Jain, A.K. A highly selective colorimetric and turn-on fluorescent chemosensor based on 1-(2-pyridylazo)-2-naphthol for the detection of aluminium(III) ions. Sens. Actuators B Chem. 2015, 209, 15–24. [Google Scholar] [CrossRef]
- Singh, V.P.; Tiwari, K.; Mishra, M.; Srivastava, N.; Saha, S. 5-[{(2-Hydroxynaphthalen-1-yl)methyl}amino]pyridine-2,4(1H,3H)-dione as Al3+ selective colorimetric and fluorescent chemosensor. Sens. Actuators B Chem. 2013, 182, 546–554. [Google Scholar] [CrossRef]
- Sareen, D.; Paramjit Kaur, P.; Singh, K. Strategies in detection of metal ions using dyes. Coord. Chem. Rev. 2014, 265, 125–154. [Google Scholar] [CrossRef]
- Kaur, P.; Kaur, S.; Mahajan, A.; Singh, K. Highly selective colorimetric sensor for Zn2+ based on hetarylazo derivative. Inorg. Chem. Commun. 2008, 11, 626–629. [Google Scholar] [CrossRef]
- Kempf, O.; Kempf, K.; Schobert, R.; Bombarda, E. Hydrodabcyl: A superior hydrophilic alternative to the dark fluorescence quencher Dabcyl. Anal. Chem. 2017, 89, 11893–11897. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Huang, C.; Emery, B.P.; Sedgwick, A.C.; Bull, S.D.; He, X.-P.; Tian, H.; Yoon, J.; Sessler, J.L.; James, T.D. Förster resonance energy transfer (FRET)-based small-molecule sensors and imaging agents. Chem. Soc. Rev. 2020, 49, 5110–5139. [Google Scholar] [CrossRef] [PubMed]
- Saremi, M.; Kakanejadifard, A.; Adeli, M. A ratiometric fluorescent sensor based azo compound of 4-(4-Dimethylamino-phenylazo)-N-pyridin-2-ylmethyl-benzamide for rapid and selective detection of Fe3+ ion. J. Mol. Liq. 2022, 358, 119168. [Google Scholar] [CrossRef]
- Presti, M.L.; Martínez-Máñez, R.; Ros-Lis, J.V.; Batista, R.M.F.; Costa, S.P.G.; Raposo, M.M.M.; Sancenón, F. A dual channel sulphur-containing macrocycle functionalised BODIPY probe for the detection of Hg(II) in mixed aqueous solution. New J. Chem. 2018, 42, 7863–7868. [Google Scholar] [CrossRef]
- Martins, C.D.F.; Batista, P.M.R.; Raposo, M.M.M.; Costa, S.P.G. Crown ether benzoxazolyl-alanines as fluorimetric chemosensors for the detection of palladium in aqueous environment. Chem. Proc. 2021, 3, 5. [Google Scholar]
- Esteves, C.I.C.; Ferreira, R.C.M.; Raposo, M.M.M.; Costa, S.P.G. New fluoroionophores for metal cations based on benzo[d]oxazol-5-yl-alanine bearing pyrrole and imidazole. Dyes Pigm. 2018, 151, 211–218. [Google Scholar] [CrossRef]
- Okda, H.E.; El Sayed, S.; Ferreira, R.C.M.; Gonçalves, R.C.R.; Costa, S.P.G.; Raposo, M.M.M.; Martínez-Máñez, R.; Sancenón, F. N,N-diphenylanilino-heterocyclic aldehyde-based chemosensors for UV-vis/NIR and fluorescence Cu(II) detection. New J. Chem. 2019, 43, 7393–7402. [Google Scholar] [CrossRef]
- Chevalier, A.; Renard, P.-Y.; Romieu, A. Azo-based fluorogenic probes for biosensing and bioimaging: Recent advances and upcoming challenges. Chem. Asian J. 2017, 16, 2008–2028. [Google Scholar] [CrossRef] [PubMed]
- Bandara, H.M.D.; Burdette, S.C. Photoisomerization in different classes of azobenzene. Chem. Soc. Rev. 2012, 41, 1809–1825. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).