Nematicidal Activity of Oxygen-Containing Aliphatic Compounds on Bursaphelenchus xylophilus, B. mucronatus and B. fraudulentus †
Abstract
1. Introduction
2. Materials and Methods
2.1. Nematicidal Chemicals
2.2. Bursaphelenchus spp. In Vitro Culture
2.3. Toxicological Characterization of Nematicidal Compounds
2.4. Data Treatment and Statistical Analysis
3. Results
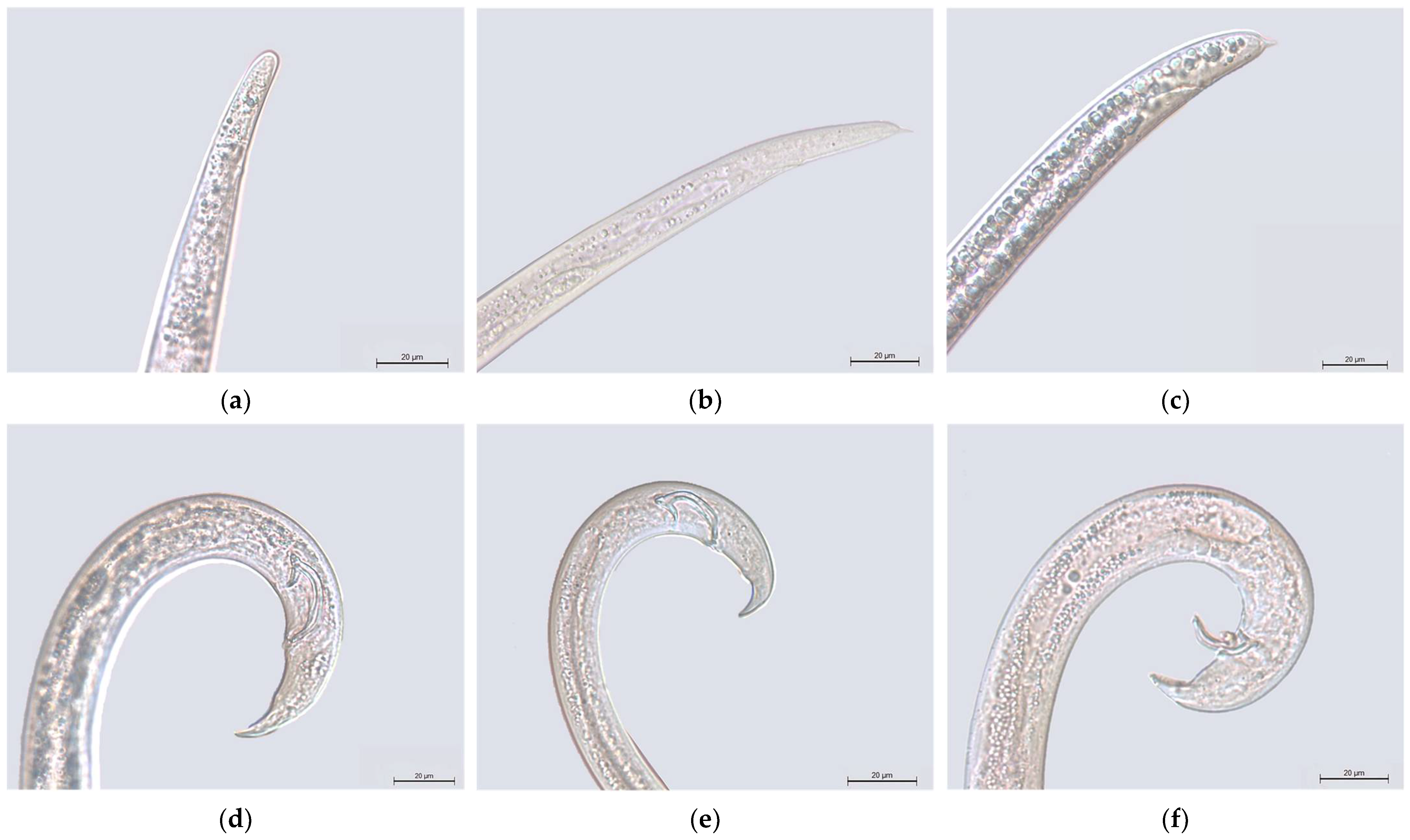
3.1. Bursaphelenchus spp. Morphology
3.2. Toxicity of Oxygen-Containing Aliphatic Compounds
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, B.G. Pine Wilt Disease in China. In Pine Wilt Disease; Zhao, B.G., Futai, K., Sutherland, J.R., Takeuchi, Y., Eds.; Springer: Tokyo, Japan, 2008; pp. 18–25. ISBN 978-4-431-75655-2. [Google Scholar]
- Shin, S.-C. Pine Wilt Disease in Korea. In Pine Wilt Disease; Zhao, B.G., Futai, K., Sutherland, J.R., Takeuchi, Y., Eds.; Springer: Tokyo, Japan, 2008; pp. 26–32. ISBN 978-4-431-75655-2. [Google Scholar]
- Futai, K. Pine Wilt in Japan: From First Incidence to the Present. In Pine Wilt Disease; Zhao, B.G., Futai, K., Sutherland, J.R., Takeuchi, Y., Eds.; Springer: Tokyo, Japan, 2008; pp. 5–12. ISBN 978-4-431-75655-2. [Google Scholar]
- Mota, M.; Braasch, H.; Bravo, M.A.; Penas, A.C.; Burgermeister, W.; Metge, K.; Sousa, E. First Report of Bursaphelenchus xylophilus in Portugal and in Europe. Nematology 1999, 1, 727–734. [Google Scholar] [CrossRef]
- Fonseca, L.; Cardoso, J.M.S.; Lopes, A.; Pestana, M.; Abreu, F.; Nunes, N.; Mota, M.; Abrantes, I. The Pinewood Nematode, Bursaphelenchus xylophilus, in Madeira Island. Helminthologia 2012, 49, 96–103. [Google Scholar] [CrossRef]
- Abelleira, A.; Picoaga, A.; Mansilla, J.P.; Aguin, O. Detection of Bursaphelenchus xylophilus, Causal Agent of Pine Wilt Disease on Pinus Pinaster in Northwestern Spain. Plant Disease 2011, 95, 776. [Google Scholar] [CrossRef] [PubMed]
- Futai, K. Pine Wood Nematode, Bursaphelenchus xylophilus. Annu. Rev. Phytopathol. 2013, 51, 61–83. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.M.; Faria, J.M.S. Profiling the Variability of Eucalyptus Essential Oils with Activity against the Phylum Nematoda. Biol. Life Sci. Forum 2021, 2, 26. [Google Scholar] [CrossRef]
- Hirata, A.; Nakamura, K.; Nakao, K.; Kominami, Y.; Tanaka, N.; Ohashi, H.; Takano, K.T.; Takeuchi, W.; Matsui, T. Potential Distribution of Pine Wilt Disease under Future Climate Change Scenarios. PLoS ONE 2017, 12, e0182837. [Google Scholar] [CrossRef]
- Ryss, A.; Vieira, P.; Mota, M.; Kulinich, O. A Synopsis of the Genus Bursaphelenchus Fuchs, 1937 (Aphelenchida: Parasitaphelenchidae) with Keys to Species. Nematology 2005, 7, 393–458. [Google Scholar] [CrossRef]
- Cheng, X.-Y.; Xie, P.-Z.; Cheng, F.-X.; Xu, R.-M.; Xie, B.-Y. Competitive Displacement of the Native Species Bursaphelenchus mucronatus by an Alien Species Bursaphelenchus xylophilus (Nematoda: Aphelenchida: Aphelenchoididae): A Case of Successful Invasion. Biol. Invasions 2009, 11, 205–213. [Google Scholar] [CrossRef]
- Tomalak, M. Parasitic Association of the Mycetophagous Wood Nematode, Bursaphelenchus fraudulentus with the Honey Fungus Armillaria ostoyae. For. Pathol. 2017, 47, e12325. [Google Scholar] [CrossRef]
- Kamata, N. Integrated Pest Management of Pine Wilt Disease in Japan: Tactics and Strategies. In Pine Wilt Disease; Springer: Tokyo, Japan, 2008; pp. 304–322. ISBN 9784431756545. [Google Scholar]
- Woodcock, B.A.; Bullock, J.M.; Shore, R.F.; Heard, M.S.; Pereira, M.G.; Redhead, J.; Ridding, L.; Dean, H.; Sleep, D.; Henrys, P.; et al. Country-Specific Effects of Neonicotinoid Pesticides on Honey Bees and Wild Bees. Science 2017, 356, 1393–1395. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Barbosa, P.; Vieira, P.; Vicente, C.S.L.; Figueiredo, A.C.; Mota, M. Phytochemicals as Biopesticides against the Pinewood Nematode Bursaphelenchus xylophilus: A Review on Essential Oils and Their Volatiles. Plants 2021, 10, 2614. [Google Scholar] [CrossRef] [PubMed]
- Ihara, M.; Matsuda, K. Neonicotinoids: Molecular Mechanisms of Action, Insights into Resistance and Impact on Pollinators. Curr. Opin. Insect Sci. 2018, 30, 86–92. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-R.; Lee, S.-C.; Lee, J.-E.; Seo, S.-M.; Jeong, Y.-C.; Jung, C.-S.; Moloney, M.; Park, I.-K. Nematicidal Activity of 3-Acyltetramic Acid Analogues Against Pine Wood Nematode, Bursaphelenchus xylophilus. Molecules 2017, 22, 1568. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.; Vicente, C.S.L.; Inácio, M.L.; Mota, M. The Potential of Esteya spp. for the Biocontrol of the Pinewood Nematode, Bursaphelenchus xylophilus. Microorganisms 2022, 10, 168. [Google Scholar] [CrossRef]
- Guo, Y.; Weng, M.; Sun, Y.; Carballar-Lejarazú, R.; Wu, S.; Lian, C. Bacillus Thuringiensis Toxins with Nematocidal Activity against the Pinewood Nematode Bursaphelenchus xylophilus. J. Invertebr. Pathol. 2022, 189, 107726. [Google Scholar] [CrossRef]
- Faria, J.M.S.; Barbosa, P.; Bennett, R.N.; Mota, M.; Figueiredo, A.C. Bioactivity against Bursaphelenchus xylophilus: Nematotoxics from Essential Oils, Essential Oils Fractions and Decoction Waters. Phytochemistry 2013, 94, 220–228. [Google Scholar] [CrossRef]
- Kim, J.C.; Baek, S.; Park, S.E.; Kim, S.; Lee, M.R.; Jo, M.; Im, J.S.; Ha, P.; Kim, J.S.; Shin, T.Y. Colonization of Metarhizium anisopliae on the Surface of Pine Tree Logs: A Promising Biocontrol Strategy for the Japanese Pine Sawyer, Monochamus alternatus. Fungal Biol. 2020, 124, 125–134. [Google Scholar] [CrossRef]
- Lengai, G.M.W.; Muthomi, J.W.; Mbega, E.R. Phytochemical Activity and Role of Botanical Pesticides in Pest Management for Sustainable Agricultural Crop Production. Sci. Afr. 2020, 7, e00239. [Google Scholar] [CrossRef]
- Damalas, C.; Koutroubas, S. Current Status and Recent Developments in Biopesticide Use. Agriculture 2018, 8, 13. [Google Scholar] [CrossRef]
- Rani, L.; Thapa, K.; Kanojia, N.; Sharma, N.; Singh, S.; Grewal, A.S.; Srivastav, A.L.; Kaushal, J. An Extensive Review on the Consequences of Chemical Pesticides on Human Health and Environment. J. Clean. Prod. 2021, 283, 124657. [Google Scholar] [CrossRef]
- Dicke, M.; van Loon, J.J.A. Multitrophic Effects of Herbivore-Induced Plant Volatiles in an Evolutionary Context. Entomol. Exp. Appl. 2000, 97, 237–249. [Google Scholar] [CrossRef]
- Delory, B.M.; Delaplace, P.; Fauconnier, M.-L.; du Jardin, P. Root-Emitted Volatile Organic Compounds: Can They Mediate Belowground Plant-Plant Interactions? Plant Soil 2016, 402, 1–26. [Google Scholar] [CrossRef]
- Broekgaarden, C.; Snoeren, T.A.L.; Dicke, M.; Vosman, B. Exploiting Natural Variation to Identify Insect-Resistance Genes. Plant Biotechnol. J. 2011, 9, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Matsui, K. Green Leaf Volatiles: Hydroperoxide Lyase Pathway of Oxylipin Metabolism. Curr. Opin. Plant Biol. 2006, 9, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xu, S.; Lu, H.; Zhang, D.; Liu, F.; Lin, J.; Zhou, C.; Mu, W. Effects of the Plant Volatile trans-2-Hexenal on the Dispersal Ability, Nutrient Metabolism and Enzymatic Activities of Bursaphelenchus xylophilus. Pestic. Biochem. Physiol. 2017, 143, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Faria, J.M.S.; Sena, I.; Vieira da Silva, I.; Ribeiro, B.; Barbosa, P.; Ascensão, L.; Bennett, R.N.; Mota, M.; Figueiredo, A.C. In Vitro Co-Cultures of Pinus pinaster with Bursaphelenchus xylophilus: A Biotechnological Approach to Study Pine Wilt Disease. Planta 2015, 241, 1325–1336. [Google Scholar] [CrossRef]
- Whitehead, A.G.; Hemming, J.R. A Comparison of Some Quantitative Methods of Extracting Small Vermiform Nematodes from Soil. Ann. Appl. Biol. 1965, 55, 25–38. [Google Scholar] [CrossRef]
- Kong, J.O.; Lee, S.M.; Moon, Y.S.; Lee, S.G.; Ahn, Y.J. Nematicidal Activity of Plant Essential Oils against Bursaphelenchus xylophilus (Nematoda: Aphelenchoididae). J. Asia-Pac. Entomol. 2006, 9, 173–178. [Google Scholar] [CrossRef]
- Seefeldt, S.S.; Jensen, J.E.; Feurst, E.P. Log-Logistic Analysis of Herbicide Dose-Response Relationships. Weed Technol. 1995, 9, 218–227. [Google Scholar] [CrossRef]
- Seo, S.-M.; Kim, J.; Kim, E.; Park, H.-M.; Kim, Y.-J.; Park, I.-K. Structure—Activity Relationship of Aliphatic Compounds for Nematicidal Activity against Pine Wood Nematode (Bursaphelenchus xylophilus). J. Agric. Food Chem. 2010, 58, 1823–1827. [Google Scholar] [CrossRef]
- Slater, S.J.; Cox, K.J.A.; Lombardi, J.V.; Ho, C.; Kelly, M.B.; Rubin, E.; Stubbs, C.D. Inhibition of Protein Kinase C by Alcohols and Anaesthetics. Nature 1993, 364, 82–84. [Google Scholar] [CrossRef] [PubMed]
- Balgavy, P.; Iadv, F.D. Cut-off Effects in Biological Activities of Surfactants. Adv. Colloid Interface Sci. 1996, 66, 23–63. [Google Scholar] [CrossRef] [PubMed]
- Faria, J.M.S.; Sena, I.; Moiteiro, C.; Bennett, R.N.; Mota, M.; Cristina Figueiredo, A. Nematotoxic and Phytotoxic Activity of Satureja montana and Ruta graveolens Essential Oils on Pinus pinaster Shoot Cultures and P. pinaster with Bursaphelenchus xylophilus in Vitro Co-Cultures. Ind. Crops Prod. 2015, 77, 59–65. [Google Scholar] [CrossRef]
| Compounds | Bursaphelenchus spp. | EC50 24h (mg/mL) | R2 of Fit |
|---|---|---|---|
| C10H22O | B. xylophilus | 0.045 | 0.98 |
| B. mucronatus | 0.082 | 0.96 | |
| B. fraudulentus | 0.042 | 0.99 | |
| C11H24O | B. xylophilus | 0.014 | 0.99 |
| B. mucronatus | 0.022 | 0.98 | |
| B. fraudulentus | 0.020 | 0.99 | |
| C12H26O | B. xylophilus | 0.009 | 0.99 |
| B. mucronatus | 0.023 | 0.99 | |
| B. fraudulentus | 0.011 | 0.99 | |
| C13H28O | B. xylophilus | 0.012 | 0.99 |
| B. mucronatus | 0.019 | 0.98 | |
| B. fraudulentus | 0.012 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavaco, T.; Gonçalves, D.; Pombo, A.; Moiteiro, C.; Inácio, M.L.; Faria, J.M.S. Nematicidal Activity of Oxygen-Containing Aliphatic Compounds on Bursaphelenchus xylophilus, B. mucronatus and B. fraudulentus . Chem. Proc. 2022, 12, 55. https://doi.org/10.3390/ecsoc-26-13536
Cavaco T, Gonçalves D, Pombo A, Moiteiro C, Inácio ML, Faria JMS. Nematicidal Activity of Oxygen-Containing Aliphatic Compounds on Bursaphelenchus xylophilus, B. mucronatus and B. fraudulentus . Chemistry Proceedings. 2022; 12(1):55. https://doi.org/10.3390/ecsoc-26-13536
Chicago/Turabian StyleCavaco, Tomás, Diogo Gonçalves, Ana Pombo, Cristina Moiteiro, Maria L. Inácio, and Jorge M. S. Faria. 2022. "Nematicidal Activity of Oxygen-Containing Aliphatic Compounds on Bursaphelenchus xylophilus, B. mucronatus and B. fraudulentus " Chemistry Proceedings 12, no. 1: 55. https://doi.org/10.3390/ecsoc-26-13536
APA StyleCavaco, T., Gonçalves, D., Pombo, A., Moiteiro, C., Inácio, M. L., & Faria, J. M. S. (2022). Nematicidal Activity of Oxygen-Containing Aliphatic Compounds on Bursaphelenchus xylophilus, B. mucronatus and B. fraudulentus . Chemistry Proceedings, 12(1), 55. https://doi.org/10.3390/ecsoc-26-13536










