Nanoencapsulation of 3-Chloropropylaminobenzoate Derivatives with Potential Insecticidal Activity †
Abstract
:1. Introduction
2. Results and Discussion
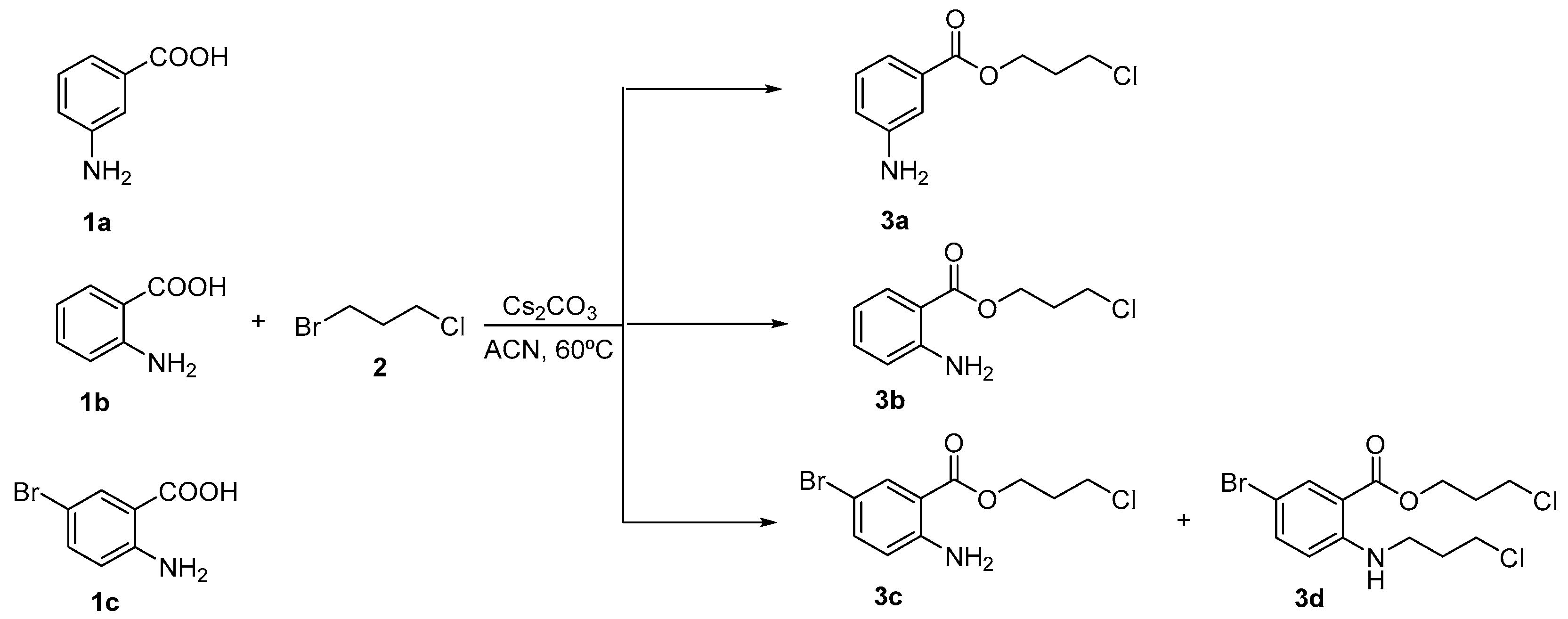
2.1. Synthesis of Aminobenzoic Acid Derivatives 3a–d
2.2. Toxicity of Aminobenzoic Acid Derivatives 3a–d
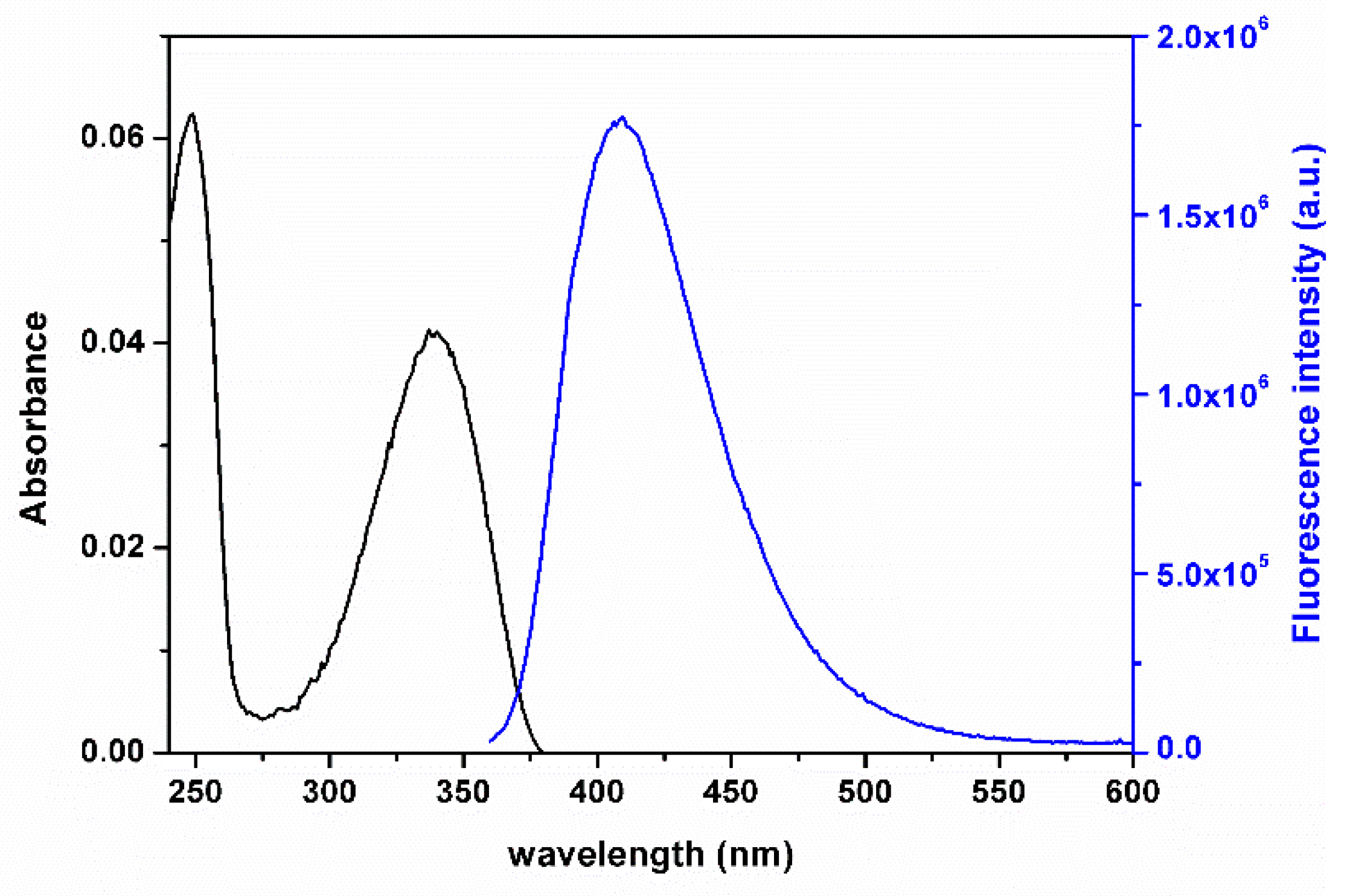
2.3. Nanoencapsulation Studies
3. Material and Methods
3.1. Typical Procedure for the Preparation of Compounds 3a–d (Illustrated for 3b)
3.2. Biological Assays of Aminobenzoic Acid Derivatives 3a–d
3.3. Nanoencapsulation and Release Studies of Compound 3b
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. How to Feed the World in 2050; FAO: Rome, Italy, 2009; Volume 2050, pp. 1–35.
- Bailey-Serres, J.; Parker, J.E.; Ainsworth, E.A.; Oldroyd, G.E.D.; Schroeder, J.I. Genetic strategies for improving crop yields. Nature 2019, 575, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Sparks, T.C.; Lorsbach, B.A. Perspectives on the agrochemical industry and agrochemical discovery. Pest Manag. Sci. 2017, 73, 672–677. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Sparks, T.C.; Storer, N.; Porter, A.; Slater, R.; Nauen, R. Insecticide resistance management and industry: The origins and evolution of the Insecticide Resistance Action Committee (IRAC) and the mode of action classification scheme. Pest Manag. Sci. 2021, 77, 2609–2619. [Google Scholar] [CrossRef]
- Prasher, P.; Sharma, M. Medicinal chemistry of anthranilic acid derivatives: A mini review. Drug Dev. Res. 2021, 82, 945–958. [Google Scholar] [CrossRef]
- Zoromba, M.S.; Ismail, M.I.M.; Bassyouni, M.I.; Abdel-Aziz, M.H.; Salah, N.; Alshahrie, A.; Memic, A. Fabrication and characterization of poly (aniline-co-o-anthranilic acid)/magnetite nanocomposites and their application in wastewater treatment. Colloids Surf. A Physicochem. Eng. Asp. 2017, 520, 121–130. [Google Scholar] [CrossRef]
- Shirazi, Z.; Golikand, A.N.; Keshavarz, M.H. A new nanocomposite based on poly (o-anthranilic acid), graphene oxide and functionalized carbon nanotube as an efficient corrosion inhibitor for stainless steel in severe environmental corrosion. Compos. Commun. 2020, 22, 100467. [Google Scholar] [CrossRef]
- Benelli, G.; Pavoni, L.; Zeni, V.; Ricciardi, R.; Cosci, F.; Cacopardo, G.; Gendusa, S.; Spinossi, E.; Petrelli, R.; Cappellacci, L.; et al. Developing a highly stable carlina acaulis essential oil nanoemulsion for managing Lobesia Botrana. Nanomaterials 2020, 10, 1867. [Google Scholar] [CrossRef]
- Lee, J.C.; Oh, Y.S.; Cho, S.H.; Lee, J.D. Efficient in situ esterification of carboxylic acids using cesium carbonate. Org. Prep. Proced. Int. 1996, 28, 480–483. [Google Scholar] [CrossRef]
- Fernandes, M.J.G.; Pereira, R.B.; Rodrigues, A.R.O.; Vieira, T.F.; Fortes, A.G.; Pereira, D.M.; Sousa, S.F.; Gonçalves, M.S.T.; Castanheira, E.M.S. Liposomal formulations loaded with a eugenol derivative for application as insecticides: Encapsulation studies and in silico identification of protein targets. Nanomaterials 2022, 12, 3583. [Google Scholar] [CrossRef]
- Jaafar-Maalej, C.; Diab, R.; Andrieu, V.; Elaissari, A.; Fessi, H. Ethanol injection method for hydrophilic and lipophilic drug-loaded liposome preparation. J. Liposome Res. 2010, 20, 228–243. [Google Scholar] [CrossRef]
- Papadopoulou, V.; Kosmidis, K.; Vlachou, M.; Macheras, P. On the use of the Weibull function for the discernment of drug release mechanisms. Int. J. Pharm. 2006, 309, 44–50. [Google Scholar] [CrossRef]
- Noyes, A.A.; Whitney, W.R. The rate of solution of solid substances in their own solutions. J. Am. Chem. Soc. 1987, 19, 930–934. [Google Scholar] [CrossRef]


| First-Order | Weibull | |||
|---|---|---|---|---|
| K (s−1) | R2 | b | a | R2 |
| 0.38 | 0.97 | 0.41 | 0.67 | 0.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coelho, R.G.G.; Pereira, R.B.; Rodrigues, A.R.O.; Pereira, D.M.; Castanheira, E.M.S.; Fortes, A.G.; Fernandes, M.J.G.; Gonçalves, M.S.T. Nanoencapsulation of 3-Chloropropylaminobenzoate Derivatives with Potential Insecticidal Activity. Chem. Proc. 2022, 12, 91. https://doi.org/10.3390/ecsoc-26-13719
Coelho RGG, Pereira RB, Rodrigues ARO, Pereira DM, Castanheira EMS, Fortes AG, Fernandes MJG, Gonçalves MST. Nanoencapsulation of 3-Chloropropylaminobenzoate Derivatives with Potential Insecticidal Activity. Chemistry Proceedings. 2022; 12(1):91. https://doi.org/10.3390/ecsoc-26-13719
Chicago/Turabian StyleCoelho, Ricardo G. G., Renato B. Pereira, Ana Rita O. Rodrigues, David M. Pereira, Elisabete M. S. Castanheira, A. Gil Fortes, Maria José G. Fernandes, and M. Sameiro T. Gonçalves. 2022. "Nanoencapsulation of 3-Chloropropylaminobenzoate Derivatives with Potential Insecticidal Activity" Chemistry Proceedings 12, no. 1: 91. https://doi.org/10.3390/ecsoc-26-13719
APA StyleCoelho, R. G. G., Pereira, R. B., Rodrigues, A. R. O., Pereira, D. M., Castanheira, E. M. S., Fortes, A. G., Fernandes, M. J. G., & Gonçalves, M. S. T. (2022). Nanoencapsulation of 3-Chloropropylaminobenzoate Derivatives with Potential Insecticidal Activity. Chemistry Proceedings, 12(1), 91. https://doi.org/10.3390/ecsoc-26-13719










