1. Introduction
Biosurfactants are natural surface-active biomolecules produced by a wide range of microorganisms belonging to the genera
Pseudomonas,
Bacillus,
Candida,
Rhodococcus, or
Corynebacterium. Among them,
Pseudomonas species are mainly used to produce this important group of bioactive compounds [
1,
2].
Biosurfactants exhibit superior properties over synthetic ones in terms of biodegradability, surface activity, stability in a wide range of pH and temperature, low toxicity, and extraordinary emulsifying and demulsifying activity [
3]. Due to these unique physicochemical properties, biosurfactants present potential applications in various fields, such as medicine, food, cosmetic and pharmaceutical industries, bioremediation, and agriculture sectors.
According to their chemical compositions, biosurfactants can be glycolipids, lipopeptides, lipoproteins, phospholipids, and polymeric surfactants. Apart from these, fatty acids, neutral lipids, and particulate compounds can also be considered as biosurfactants [
4].
The actual production of biosurfactants is limited, due to the high costs of the media components used in the fermentation processes.
The most common carbon sources used for production of biosurfactants are glucose and sucrose, while yeast extract, urea, or NaNO3 are used as nitrogen sources [
5].
Also, due to the environmental concerns about chemical surfactants, the interest in obtaining environmentally friendly bio-compounds has increased in recent years [
6].
Pseudomonas strains are capable of using different waste substrates, such as carbon and nitrogen sources, for the economical production of biosurfactants [
7,
8].
Therefore, in the present study, whey and corn extract, by-products obtained from industrial processes, were evaluated as potential cheap substrates for the production of biosurfactants by two Pseudomonas strains.
2. Materials and Methods
2.1. Biologic Material
The Pseudomonas putida ICCF 391 and Pseudomonas fluorescens ICCF 392 strains used in this study belong to the Collection of Microorganisms of Industrial Importance-CMII–ICCF-WFCC 232.
2.2. Culture Medium and Cultivation Conditions
The strains were maintained on M44 agar slant (pre-inoculum) until further use, with the following composition % (g/v): glycerol 5.0, yeast extract 1.0, bacto-peptone 1.0, and agar 2.0. The culture medium was prepared with distilled water, adjusted to a pH of 6.5–7.0, and sterilized at 115 °C for 30 min. The pre-inoculum consisted of bacterial strains incubated at 30 °C for 48–72 h, on M44 agarized medium.
The liquid medium used for biosurfactant production contained % (v/v): whey 3.0, as a carbon source, and corn extract 2.0, as a nitrogen source. Also in this case, the culture media were prepared with distilled water, adjusted to a pH of 6.5–7.0, and sterilized at 121 °C for 20 min. For the submerged fermentation, Erlenmeyer flasks of 500 mL capacity, with 100 mL medium, were used. Bioprocess conditions were performed at 30 °C, for 72 h, with an inoculation volume of 10%.
2.3. Emulsification Test
In order to evaluate the production of biosurfactants by the Pseudomonas putida ICCF 391 and Pseudomonas fluorescens ICCF 392 strains, submerged bioprocesses in 500 mL flasks with 100 mL medium on a rotary shaker at 220 rpm were performed. The strains were grown at 30 °C, for 72 h, in liquid media containing whey, as a carbon source, and corn extract 2.0, as a nitrogen source.
After centrifugation of the culture broths (20 min, at 4 °C, and 9000 rpm), the emulsification index (E
24) of the biosurfactant produced by both
Pseudomonas strains was determined. In total, 4 mL of supernatant was mixed with 6 mL of heptane, octane, and sunflower oil, in separate tubes. Then, tubes were mixed vigorously for 5 min and kept for 24 h [
9]. The emulsification index was determined by using the following formula:
The experiments were performed in triplicate.
3. Results and Discussion
The cell growth of both strains, tested for biosurfactant production, was monitored by pH and OD (λ = 550 nm) measurements. At the end of the fermentation process, both strains registered good values for O.D., being 15.7 for Pseudomonas putida ICCF 391, and 15.2, in the case of Pseudomonas fluorescens ICCF 392. Regarding the pH value, it was almost similar, being in the range of 8-8.5 for both strains.
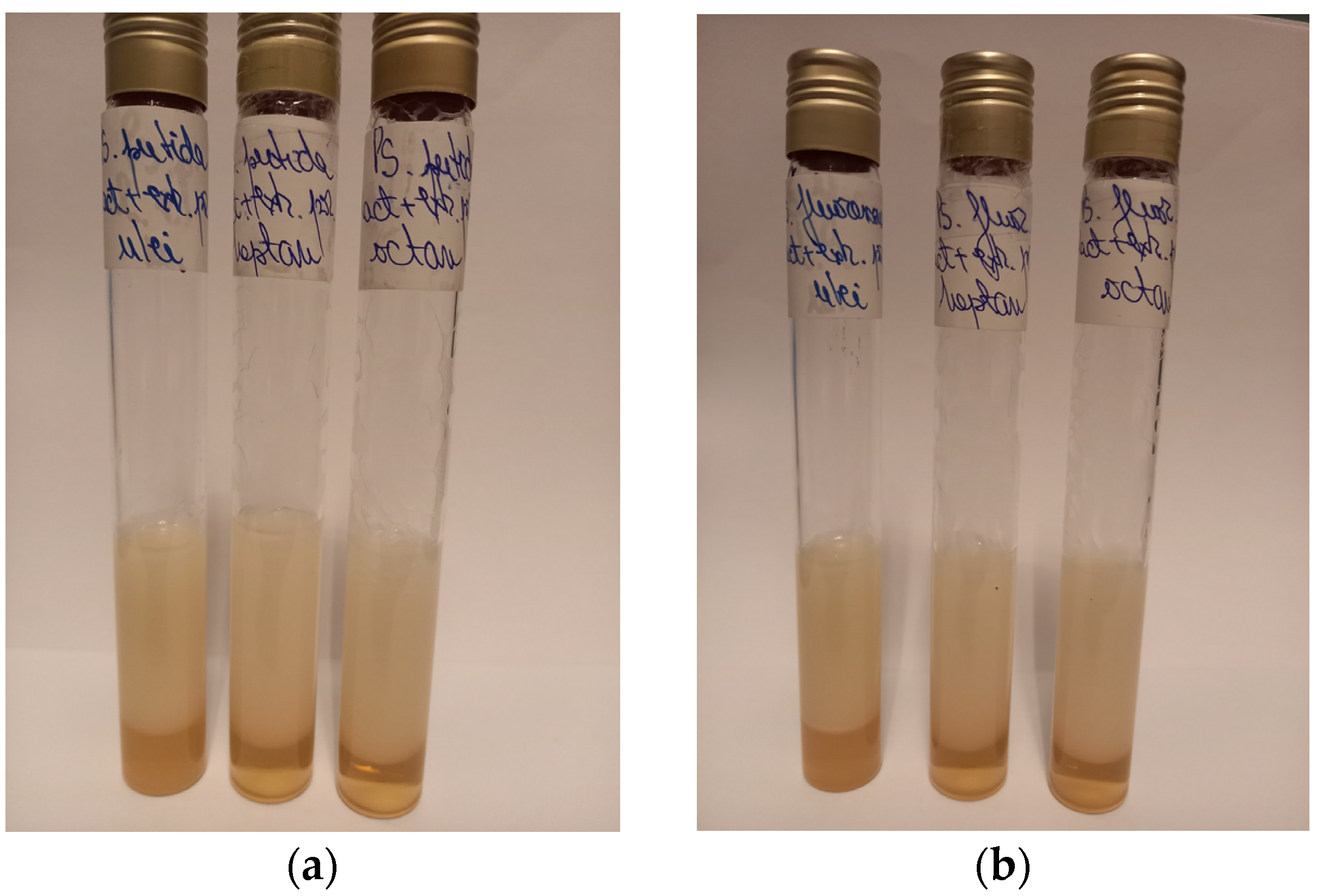
Also, after 72 h of fermentation, the media were centrifuged, and the supernatants were evaluated for their capacity to emulsify heptane, octane, and sunflower oil (
Figure 1).
The supernatants of
Pseudomonas putida ICCF 391 and
Pseudomonas fluorescens ICCF 392 produced stable emulsions with heptane, octane, and sunflower oil. The figure below presents the values of the emulsification index (E
24%) obtained with both
Pseudomonas strains (
Figure 2).
In the case of Pseudomonas putida ICCF 391, the values of the emulsification index (E24) were higher in the case of heptane (75.43%) and octane (72.72%), compared to the sunflower oil (70.37%). Regarding the supernatants obtained with Pseudomonas fluorescens ICCF 392 strain, the results were almost similar, with the values of the emulsification index of 74.07% for heptane, 71.42% for octane, and 69.09% for sunflower oil, respectively.
Our results showed that both the Pseudomonas strains were good producers of biosurfactants on substrates containing whey, as a carbon source, and corn extract, as a nitrogen source. The emulsions obtained were stable for more than one month, and according to the literature, it is considered that emulsion stability is one of the most important properties of a biosurfactant.
The values of the emulsifying indices obtained are in accordance with the observations made by Joice and Parthasarathi [
10], who obtained an emulsifying index almost of 70.0% for heptane, and also, with the study of Matatkova et al. [
11], who reported an emulsification index of 70% for sunflower oil.
4. Conclusions
The supernatants of both the Pseudomonas strains formed emulsions with heptane, octane, and sunflower oil. The emulsions formed by the supernatants of Pseudomonas putida ICCF 391 and Pseudomonas fluorescens ICCF 392, grown on liquid media containing whey and corn extract as single sources of nutrients, presented good results, with the biggest values of the emulsification index being obtained with the Pseudomonas putida ICCF 391 strain, followed by the Pseudomonas fluorescens ICCF 392 strain.
Therefore, the use of inexpensive by-products from the agro-food industry as substrates represents a promising source for the production of biosurfactants with the Pseudomonas strains.
Author Contributions
Concept, R.M.S.; writing–original draft preparation, R.M.S.; writing and editing, R.M.S., M.M., S.N., C.B., E.S.L. and L.I.C.; performed the experiments, R.M.S., M.M., S.N. and C.B.; analyzed the data, R.M.S. and M.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant from the Ministry of Research, Innovation and Digitization, CCCDI-UEFISCDI, project number PN-III-P2-2.1-PED-2021-3528/731PED-2022 within PNCDI III.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Deshmukh, N.; Kathwate, G. Biosurfactant production by Pseudomonas aeruginosa strain LTR1 and its application. Biointerface Res. Appl. Chem. 2023, 13, 10. [Google Scholar] [CrossRef]
- Mohanty, S.S.; Koul, Y.; Varjani, S.; Pandey, A.; Ngo, H.H.; Chang, J.S.; Wong, J.W.C.; Bui, X.T. A critical review on various feedstocks as sustainable substrates for biosurfactants production: A way towards cleaner production. Microb. Cell Fact. 2021, 20, 120. [Google Scholar] [CrossRef] [PubMed]
- Ciurko, D.; Chebbi, A.; Kruszelnicki, M.; Czapor-Irzabek, H.; Urbanek, A.K.; Polowczyk, I.; Franzetti, A.; Jane, T. Production and characterization of lipopeptide biosurfactant from a new strain of Pseudomonas antarctica 28E using crude glycerol as a carbon source. RSC Adv. 2023, 13, 24129–24139. [Google Scholar] [CrossRef] [PubMed]
- Gurkok, S.; Ozdal, M. Microbial biosurfactants: Properties, types, and production. Anatol. J. Biol. 2021, 2, 7–12. [Google Scholar]
- Eras-Munoz, E.; Farre, A.; Sanchez, A.; Font, X.; Gea, T. Microbial biosurfactants: A review of recent environmental applications. Bioengineered 2022, 13, 12365–12391. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Patil, Y.; Rale, V. Biosurfactant production: Emerging trends and promising strategies. J. Appl. Microbiol. 2018, 126, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Praveesh, B.V.; Soniyamby, A.R.; Mariappan, C.; Kavithakumari, P.; Palaniswamy, M.; Lalitha, S. Biosurfactant production by Pseudomonas sp. from soil using whey as carbon source. N. Y. Sci. J. 2011, 4, 99–103. [Google Scholar]
- Rita de Cássia, F.; Almeida, D.G.; Meira, H.M.; Silva, E.J.; Farias, C.B.B.; Rufino, R.D.; Luna, J.M.; Sarrubbo, L.A. Production and characterization of a new biosurfactant from Pseudomonas cepacia grown in low-cost fermentative medium and its application in the oil industry. Biocatal. Agric. Biotechnol. 2017, 12, 206–215. [Google Scholar] [CrossRef]
- Pathak, K.V.; Keharia, H. Application of extracellular lipopeptide biosurfactant produced by endophytic Bacillus subtilis K1 isolated from aerial roots of banyan (Ficus benghalensis) in microbially enhanced oil recovery (MEOR). 3Biotech 2014, 4, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Joice, P.A.; Parthasarathi, R. Optimization of biosurfactant production from Pseudomonas aeruginosa PBSC1. Int. J. Curr. Microbiol. App. Sci. 2014, 3, 140–151. [Google Scholar]
- Matátková, O.; Michailidu, J.; Ježdík, R.; Kolouchová, I.J.; Rezanka, T.; Jirku, V.; Masák, J. Production and characterization of rhamnolipids produced by Pseudomonas aeruginosa DBM 3774: Response surface methodology approach. Microorganisms 2022, 10, 1272. [Google Scholar] [CrossRef] [PubMed]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).