Abstract
This article presents the microwave-assisted extraction of biosilica from common reed (Phragmites australis) biomass and the utilization of the resulting aqueous extract to enrich porous ceramic granules based on diatomaceous earth and bentonite from white wine cleaning. The enriched porous ceramic granule generated a solution of soluble silicon that was +23.4 ± 2.2 more concentrated than the porous ceramic granules not enriched with reed extract. The water reactivity of Si-O-Si groups is higher in the polysilicic acid formed via the polycondensation of silicic acid extracted from reed, compared to the Si-O-Si group from diatomaceous earth or bentonite.
1. Introduction
The common reed (Phragmites australis) influences wetland biodiversity and wetland ecosystem resilience [1,2]. Harvesting reed biomass and its utilization in the construction, paper, and energy industries is one of the leading solutions for wetland ecosystem management that includes reeds control [3].
The main issue with the industrial processing of reed biomass is related to its high biosilica content, which increases the risk of silica scale [4]. The pretreatment of reed biomass for biosilica extraction reduces the silica scale risk and represents a solution to close the loop of the biosilica cycle [5].
One gap in reed biosilica loop closure is the appropriate soil application formulation of the extracted soluble silicon [5]. The solution investigated in this article is the embedment of the silicon species resulting from the concentrated aqueous reed extract in porous ceramic granules that include diatomaceous earth and bentonite from the winemaking industry.
Porous ceramic granules are inorganic soil conditioners that improve soil structure, increase soil resistance to compaction, and promote healthy root systems [6,7]. Our group previously developed ceramic granules, wherein diatomaceous earth was included as a source of soluble silicon species for an enhanced plant biostimulant effect [8]. This solution was further developed using bentonite from the wine clarification process [9] as a sacrificial pore-generating agent in ceramic granules intended for soil treatment. The utilization of spent bentonite recycles a byproduct of the winemaking industry. Spent bentonite gives superior properties to ceramic granules as a soil conditioner due to the addition of bentonite, with a proven ability to modulate the bioavailability of water and mineral nutrients in soil [10,11].
The embedment of the concentrated reed extract with polycondensate silicon species in ceramic porous granules is intended to enrich these granules with chemical structures that slow-release soluble silicon species. In a water environment, silicic acid at a concentration higher than 1 mM starts to polycondensate, generating polysilicic acid, SiO2×nH2O. This polycondensation reaction is reversible; the polysilicic acid structures hydrolyze in an aqueous solution wherein the silicic acid concentration is lower than 1 mM, releasing orthosilicic acid, H4SiO4. In a cultivated soil environment, the plant roots uptake silicic acid in concentrations between 0.1 and 0.6 mM [12], maintaining a constant slow release of soluble species into the soil solution.
This paper presents the microwave extraction of silicon species from reeds and the process of enriching the ceramic granules with the concentrated aqueous reeds extract.
2. Materials and Methods
2.1. Materials
Two reed (Phragmites australis) samples (S1 and S2) were taken from the Danube Delta, from a location near Mila 23 village, at 45°13′23′23″ N, 29°14′44′44″ E, 2 m altitude, in February 2023. For manufacturing ceramic granules, clay, quartz sand, silica sol, diatomaceous earth, and bentonite from white wine clarification were used. The clay was extracted from the Bodoc site and contains 69.97% SiO2, 15.41% Al2O3, 4.88% Fe2O3 [13]. The quartz sand contains more than 97% SiO2. The used diatomaceous earth (DE) was from Sibiciu de Sus quarry (Industriile de Diatomit, Pătârlagele, Buzău, Romania), and was formed by frustules of freshwater diatoms from the Aulacoseira (66%) and Actinocyclus genera [14]. The bentonite was from clarification of Muscat Ottonel white wine (Miniș, Romania). The montmorillonite content of the used bentonite was 72%.
The reagents used to determine the elemental composition of aqueous reed extract quantitatively were of analytical purity. Standard solutions, Certipur, with a concentration of 1000 mg/L, from Merck (Merck Group, Darmstadt, Germany) were used. Ultrapure water produced by Milli-Q, Integral Sistem (Millipore, Merck Group, Darmstadt) with a resistivity of 18.2 MΩ/cm was used to prepare the working solutions and samples. The purge gas for ICP-OES (inductively coupled plasma–optical emission spectrometry) was Argon 5.0 of 99.999% purity (Siad, Călărași, Romania).
2.2. Microwave-Assisted Extraction of Silicon Species from Reeds
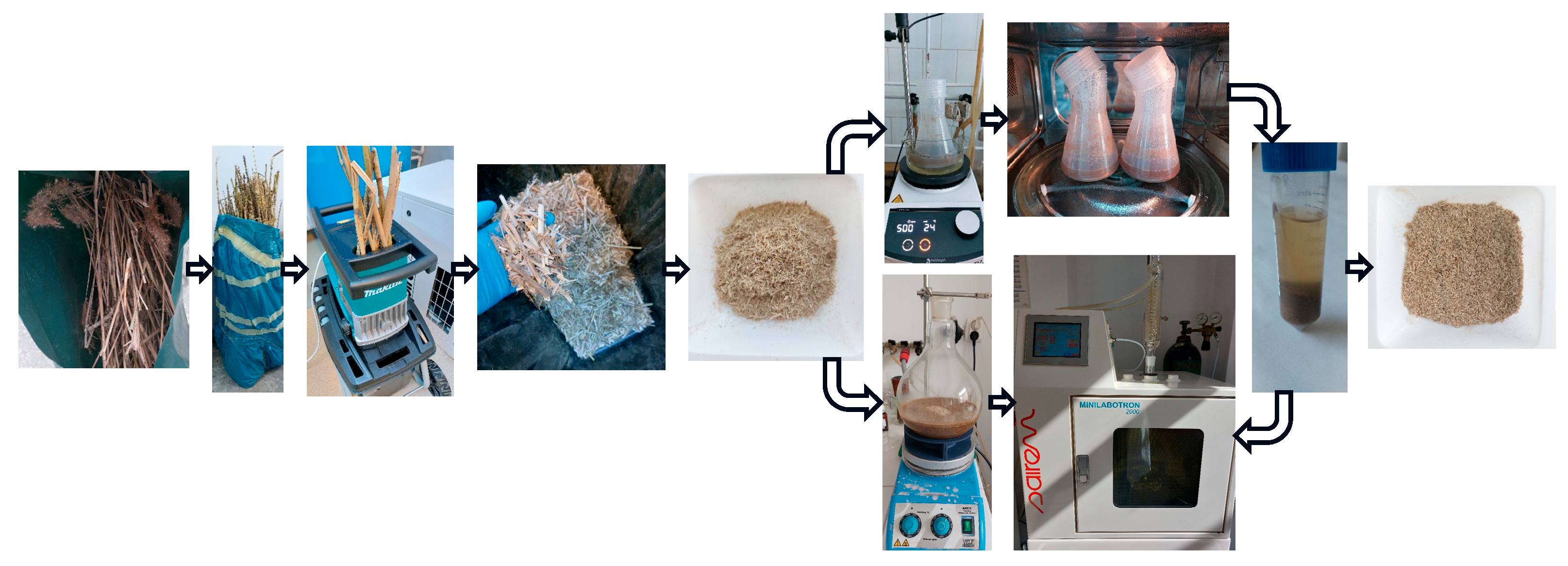
The microwave-assisted extraction of reed biomass is illustrated in Figure 1.
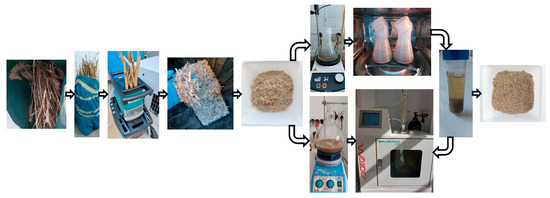
Figure 1.
The process for microwave-assisted extraction of silicon species from reed biomass.
The dried reed biomass was shredded with a UD2500 electric shredder (Makita, Anjo, Japan), followed by grinding with a laboratory mill with two knives (M20, IKA, Staufen, Germany), to a maximum size of 0.5 cm [15].
The microwave-assisted extraction was carried out in two main variants: heating on a microwave oven, after heating and mixing reed biomass with water in a hot stirring plate, and in a microwave extraction system.
In the first variant, two approaches were used. In the first approach, a solid/liquid (S/L) mixture was produced in a ratio of 1:30, respectively, 5 g processed reed to 150 mL ultrapure water Milli-Q, in 0.5 L Erlenmeyer flasks with a wide neck and screw cap, made from polypropylene (Corning®, Corning Inc., Corning, NY, USA). Extractions were performed in triplicate. The reed used had a moisture content of 4.36%. The mixture was heated on a magnetic hot plate, MR Hei-Tec (Heidolph, Schwabach, Germany), with a water bath at 50 °C for 2 h, and then the mixture was placed in a microwave oven, AT325 (Whirlpool, Benton Harbor, MI, USA), for 5 min. The microwave extraction was performed in stages. After every 30 s of heating, extraction was stopped for 1 min.
After microwave-assisted extraction, the mixture was centrifuged in a Universal 320R centrifuge (Hettich, Tuttlingen, Germany) for 15 min, 7200× g, temperature 25 °C, and the supernatant was filtered through filter paper, Filtrak no. 390. The volume was measured, and the total Si content of samples S1.1 and S2.1 was analyzed via ICP-OES. The sediment was recovered, dried in a laboratory oven, UN 200 (Memmert, Schwabach, Germany), at 105 °C for 4 h, and its dried weight was measured using a precision balance (XPR204S, Metler-Toledo, Columbus, OH, USA).
In the second approach, microwave-assisted extraction was performed with fresh pure water for 10 min. After 2 h on the magnetic plate, the samples were centrifuged, and the supernatant was filtered and analyzed for the total silicon content (leading to S1.2 and S2.2). Over the wet sediment, 100 mL of ultrapure water Milli-Q was added, and the wet reed biomass was further extracted for 10 min in the microwave oven. The final mixture was centrifuged, the supernatant was filtered and analyzed for total Si content (resulting S1.3 and S2.3), and the sediment was recovered, dried, and weighed.
The same S/L ratio was used in the second variant, but with larger quantities. A 20 g ground reed biomass sample was mixed with 600 mL ultrapure water Milli-Q. The extraction was conducted directly in a microwave system (Minilabotron 2000, model M20K230, Sairem, Décines-Charpieu, France) equipped with a round-bottomed glass flask, magnetic stirring, and ascending refrigerant connected to water. In this variant, a blank sample was performed only with ultrapure water Milli-Q, under the same conditions, to quantify and further deduct the silicon extracted from the glass flask. Before the microwave extraction, the reed sample was stirred for wetting at room temperature, at 900 rpm, on a magnetic stirrer (Arex 6, Velp Scientifica, Usmate Velate, Italy) for 5 min. Then, the mixture was placed in the microwave system and heated until it reached the boiling temperature of water (~95 °C). The sample was extracted at boiling temperature for 5 min, under reflux at an applied power (PI) of 200 W. After the microwave-assisted extraction, the sample was processed as in the first variant: centrifugation, supernatant filtration, sediment recovery, drying, and weighing. In the supernatant S2.4, besides the content of Si, other elements were determined: Ca, Mg, K, Na, P, Cu, Cd, Pb, Cr, and Ni.
2.3. Element Analysis Using ICP-OES
The content of Si Ca, Mg, K, Na, P, Cu, Cd, Pb, Cr, and Ni from the aqueous extract was determined using the Optima 2100 DV ICP-OES System (Perkin Elmer, Waltham, MA, USA), according to SR EN ISO 11885:2009 standard [16], with dual-view optical system—axial and radial plasma view in a single work sequence, which works with an independent transistorized radio frequency generator with a frequency of 40 MHz. The nebulization system used has a PEEK Mira Mist® nebulizer coupled with the Baffled Cyclonic spray chamber. The spectrometer comprises an optical module with an Echelle monochromator with a two-dimensional CCD (charged coupled device) detector, the spectral range being 165–800 nm [17]. The samples were mineralized according to the already-described protocol [18], and the sample concentration was calculated based on calibration curves.
2.4. Enrichment of Ceramic Granules with Silicon
Wet ceramic granules with diatomite and bentonite were fabricated at room temperature via pan granulation up to the desired size of approximately 10 mm, according to the already described method [13]. The granules were sintered at 960 °C in a laboratory furnace (CWF, Carbolite-Gero, Verder Scientific, Haan, Germany). The porous granules have a specific surface of 2.145 m2/g. The S2.4 extract was concentrated 10 times in a rotary evaporator (Rotavapor® R-300, Büchi, Flawil, Switzerland). Fifty grams of porous granules were mixed with 100 mL of concentrated extracts, dried at room temperature for 24 h, and then at 104 ± 2 °C for 4 h in a vacuum oven (VD, Binder, Tuttlingen, Germany). To demonstrate the enrichment with slow-releasing silicon species, 10 g of ceramic granules, enriched and non-enriched with reed extract, were mixed with 500 mL pure water MilliQ for 12 h in a magnetic stirrer (Arex 6, Velp Scientifica, Usmate Velate). The soluble silicon species were determined with ammonium molybdate reagent [19].
2.5. FT-IR Spectroscopy
The diatomaceous earth, bentonite, and freeze-dried reed extract (that contains amorphous silica) were subjected to attenuated total reflection (ATR)—Fourier transform infrared (FTIR) spectroscopy. The equipment used comprised an IRTracer 100 spectrophotometer (Shimadzu, Kyoto, Japan). The vibrational spectra were analyzed in the wavenumber range between 4000 and 400 cm−1, with a resolution of 4 cm−1, and 45 scans per sample.
3. Results and Discussion
The total silicon content in different samples of microwave-assisted extracted reed biomass is presented in Table 1.

Table 1.
Total silicon content in different samples of microwave-assisted extracted reed biomass.
The energy transferred to the system is essential for the solubilization of the biosilica from reed biomass. The application of 700 W power for 10 min determined an increase in the concentration of the extracted total silicon compared to the application for 5 min of the 700 W power.
The most efficient microwave-assisted extraction is with the Minilabotron 2000 microwave system. This system, designed for the targeted application of microwaves, extracted significantly more silicon from reed biomass when 200 W was applied for 5 min than the microwave oven at a power of 750 W for 5 or 10 min.
In the reed biomass, biosilica is present in two forms, phytoliths, i.e., opal silica bodies, SiO2×nH2O, formed via the polycondensation of the silicic acid accumulated between cytoplasmatic membranes and cell walls [20,21] and silicic acid complexed in the lignocellulosic matrix [22]. The energy is needed to extract the silicic acid from its hemicellulose complex and accelerate silicic acid solubilization from opal silica. The Minilabotron 2000 microwave system targets more efficient microwave energy and produces more efficient vibration at the molecular level—and further, this molecular vibration is transformed into heat. In a microwave oven, the energy is not focused and dissipates faster as heat.
The concentrations of the elements for the reed sample S2.4 analyzed are presented in Table 2, at the corresponding wavelengths (λ) of determination.

Table 2.
The concentrations of the elements for the reed sample.
Table 2 shows that the reed sample is rich in Si and K, followed by Na, Ca, Mg, P, and a small amount of the heavy metals Pb, Cu, Cd, Ni, and Cr, under the threshold limit for fertilizing products recovered from biomass [23].
The porous ceramic granules enriched with reed extract generated a solution of soluble silicon that was +23.4 ± 2.2 more concentrated than those not enriched with reed extract. The water reactivity of Si-O-Si groups is higher on the polysilicic acid formed via the polycondensation of silicic acid extracted from reed, compared to the Si-O-Si groups from diatomaceous earth or bentonite.
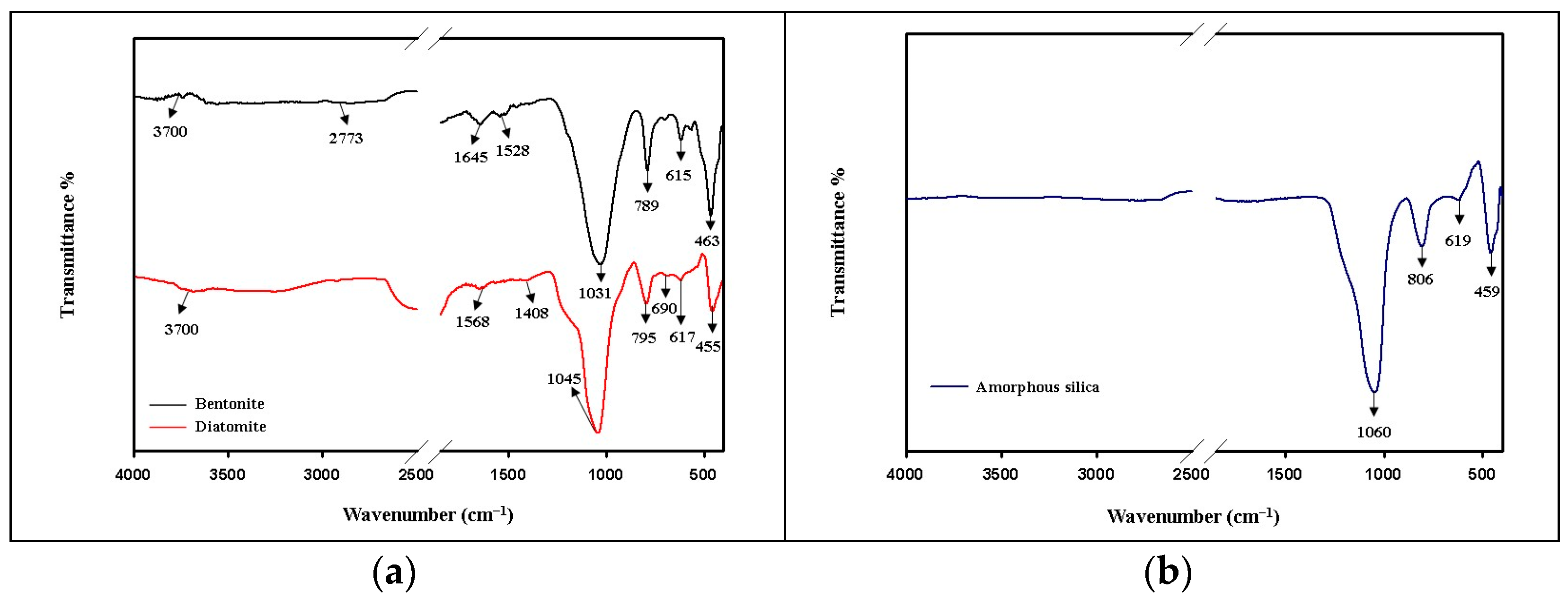
The FTIR analysis is relevant regarding Si-O-Si groups from diatomaceous earth, bentonite, and amorphous silica resulting from extracted biomass reed. Figure 2 shows the characteristic vibrational spectra of these materials.
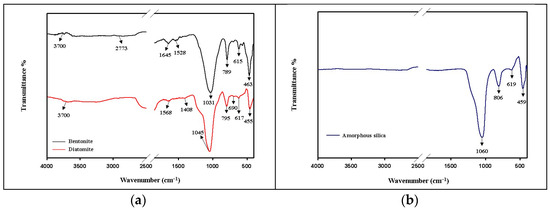
Figure 2.
ATR-FT-IR spectra for: (a) diatomaceous earth (diatomite) and bentonite, and (b) amorphous silica resulting from extracted reed biomass.
The stretching vibration spectra corresponding to diatomaceous earth are represented by a high silica content (over 80%). The silanol Si-OH groups are found at 3700 cm−1 (they were formed as a result of water adsorption in the diatomite structure), the peak at 1568 cm−1 corresponds to some carbonate groups (in small amounts, in the form of traces [24], and the peak at 1408 cm−1 belongs to some OH groups, suggesting the presence of residual water in the system. The peak at 1045 cm−1 describes the asymmetric stretching vibrations of the siloxane (Si-O-Si), and in the area 795-455 cm−1 are the symmetric bands of the silicates represented by the groups Si-O-Si, Si-O-, and O-Si-O. According to Figure 2a, in comparison to amorphous silica Figure 2b, it is found that in the structure of the diatomite, there is an amorphous structure (given by the presence of the peaks at 1045 cm−1; the other peaks at 795 cm−1, 617 cm−1, and 455 cm−1 show some delocalization and transformations), but also the presence of crystalline structures represented by calcite (the peak at 1568 cm−1), and quartz with a low content of cristobalite (through the symmetrical bands at 795 cm−1 and 455 cm−1, where the peaks become sharper compared to the amorphous form) [25].
Bentonite exhibits a dioctahedral structure of smectite (either calcium or sodium montmorillonite or both) [26] with 2:1 stacked layers (TOT) with two tetrahedral (T) starts of electrostatically cross-linked silica (SiO4) with a central layer of aluminum oxide (Al2O3) or iron (Fe2O3) arranged octahedrally (O) [27]. The layers are held together by van der Waals forces that allow the structure to rearrange. The tetrahedral structure given by the SiO4 groups is highlighted by the peaks at 1031 cm−1, 789 cm−1, and 463 cm−1 [28]. The peak at 1645 cm−1 indicates the presence of deformation vibrations of adsorbed water in the system [29], and the peak at 1528 cm−1 shows the stretching vibrations of the hydroxyl groups. The symmetrical stretching bands of the peak at 789 cm−1 attest to the presence of the Fe-OH-Mg bond, describing the Mg2+-enriched structure with low Fe3+ content [30]. In the area below 1000 cm−1, symmetrical stretching bands that correspond to Si-O-, Si-O-Si bonds can also be found.
4. Conclusions
The silicon is efficiently extracted with a microwave applied in the Minilabotron 2000 system, due to the targeted application of the energy. The extracted liquid with high silicon and potassium content was used to enrich porous ceramic granules. The enriched ceramic granules, with a higher releasing rate of soluble silicon species, are good candidates for a multifunctional product, acting as both a soil conditioner and plant biostimulant.
Author Contributions
Conceptualization, F.O. and D.C.-A.; methodology, M.D.-A. and O.C.R.; software, F.O.; validation, C.L., L.C. and D.C.-A.; formal analysis, F.O.; investigation, M.D.-A., I.T., O.C.R. and M.P.; resources, F.O.; data curation, D.C.-A.; writing—original draft preparation, M.D.-A.; writing—review and editing, F.O. and D.C.-A.; visualization, I.T. and D.C.-A.; supervision, F.O.; project administration, C.L. and M.D.-A.; funding acquisition, F.O. All authors have read and agreed to the published version of the manuscript.
Funding
The research leading to these results has received funding from European Regional Development Fund (ERDF), the Competitiveness Operational Program (POC), Axis 1, project POC-A1-A1.2.3-G-2015-P_40_352, My_SMIS 105684, “Sequential processes of closing the side streams from bioeconomy and innovative (bio)products resulting from it—SECVENT”, subsidiary projects 1818/2020—AgriCem and 617/2022 DiaCer.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.
Conflicts of Interest
O.C.R. was employed by Chemi Ceramic F and M.P. is employed by Primosal. The other authors declare no conflicts of interest. The above mentioned companies and the funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Wails, C.N.; Baker, K.; Blackburn, R.; Del Vallé, A.; Heise, J.; Herakovich, H.; Holthuijzen, W.A.; Nissenbaum, M.P.; Rankin, L.; Savage, K.; et al. Assessing changes to ecosystem structure and function following invasion by Spartina alterniflora and Phragmites australis: A meta-analysis. Biol. Invasions 2021, 23, 2695–2709. [Google Scholar] [CrossRef]
- Oteman, B.; Scrieciu, A.; Bouma, T.J.; Stanica, A.; van der Wal, D. Indicators of Expansion and Retreat of Phragmites Based on Optical and Radar Satellite Remote Sensing: A Case Study on the Danube Delta. Wetlands 2021, 41, 72. [Google Scholar] [CrossRef]
- Dragoni, F.; Giannini, V.; Ragaglini, G.; Bonari, E.; Silvestri, N. Effect of harvest time and frequency on biomass quality and biomethane potential of common reed (Phragmites australis) under paludiculture conditions. Bioenergy Res. 2017, 10, 1066–1078. [Google Scholar] [CrossRef]
- Ehrlich, H.; Demadis, K.D.; Pokrovsky, O.S.; Koutsoukos, P.G. Modern views on desilicification: Biosilica and abiotic silica dissolution in natural and artificial environments. Chem. Rev. 2010, 110, 4656–4689. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu-Aruxandei, D.; Oancea, F. Closing the Nutrient Loop—The New Approaches to Recovering Biomass Minerals during the Biorefinery Processes. Int. J. Environ. Res. Public Health 2023, 20, 2096. [Google Scholar] [PubMed]
- Li, D.; Joo, Y.K.; Christians, N.E.; Minner, D.D. Inorganic Soil Amendment Effects on Sand-Based Sports Turf Media. Crop Sci. 2000, 40, 1121–1125. [Google Scholar] [CrossRef]
- Raduly, O.C.; Fazakas, J.; Constantinescu-Aruxandei, D.; Benedek, C.; Avram-Deșliu, M.; Fazakas, E.; Oancea, F. Porous ceramic granules as inorganic soil conditioner. Sci. Bull. Ser. F. Biotechnol. 2021, 25, 32–41. [Google Scholar]
- Roşu, C.; Piştea, I.C.; Oancea, F.; Jozsef, F.; Roba, C.A. The study of biostimulating effect of porous ceramics functionalized with polyoxometalates. Int. Multidiscip. Sci. GeoConference: SGEM 2018, 18, 745–751. [Google Scholar]
- Pargoletti, E.; Sanarica, L.; Ceruti, M.; Elli, F.; Pisarra, C.; Cappelletti, G. A comprehensive study on the effect of bentonite fining on wine charged model molecules. Food Chem. 2021, 338, 127840. [Google Scholar] [CrossRef]
- Das, D.; Dakshinamurti, C. Bentonite as a soil conditioner. Soil Cond. 1975, 7, 65–76. [Google Scholar]
- Zhang, H.; Chen, W.; Zhao, B.; Phillips, L.A.; Zhou, Y.; Lapen, D.R.; Liu, J. Sandy soils amended with bentonite induced changes in soil microbiota and fungistasis in maize fields. Appl. Soil Ecol. 2020, 146, 103378. [Google Scholar] [CrossRef]
- Epstein, E. Silicon. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 641–664. [Google Scholar] [CrossRef] [PubMed]
- Fazakas, E.; Muntean, M.; Fazakas, J.; Muntean, O. Porous nutritive ceramic granules. Rev. Româna De Mater. 2007, 37, 205–210. [Google Scholar]
- Tulan, E.; Reinhard, F.S.; Tari, G.; Witkowski, J.; Tămaș, D.M.; Horvat, A.; Tămaș, A. Hydrocarbon source rock potential and paleoenvironment of lower Miocene diatomites in the Eastern Carpathians Bend Zone (Sibiciu de Sus, Romania). Geol. Carpathica. 2020, 71, 424–443. [Google Scholar] [CrossRef]
- Braune, C.; Lieberei, R.; Steinmacher, D.; Kaiser, T.M. A simple microwave extraction method for the isolation and identification of plant opal phytoliths. Biologia 2012, 67, 927–930. [Google Scholar] [CrossRef]
- EN ISO 11885:2009; Water Quality—Determination of Selected Elements by Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES). ISO: Geneva, Switzerland, 2009.
- Capra, L.; Manolache, M.; Ion, I.; Stoica, R.; Ion, A.C. Validation and Optimization of a Method for Sb Determination from Bottled Natural Mineral Waters by ICP-OES. Rev. Chim. 2018, 69, 2102–2106. [Google Scholar] [CrossRef]
- Capră, L.; Stoica, R.; Ivan, G.-R.; Șuică-Bunghez, I.-R.; Oancea, F. The Optimization and Validation of the Method for the Determination of Micronutrients in Organic Fertilizers by Inductively Coupled Plasma Optical Emission Spectrometry. Chem. Proc. 2023, 13, 10. [Google Scholar]
- Coradin, T.; Durupthy, O.; Livage, J. Interactions of amino-containing peptides with sodium silicate and colloidal silica: A biomimetic approach of silicification. Langmuir 2002, 18, 2331–2336. [Google Scholar] [CrossRef]
- Ollendorf, A.L.; Mulholland, S.C.; Rapp, G. Phytolith Analysis as a Means of Plant Identification: Arundo donax and Phragmites communis. Ann. Bot. 1988, 61, 209–214. [Google Scholar] [CrossRef]
- Hongyan, L.; Dongmei, J.; Lidan, L.; Zhuo, G.; Guizai, G.; Lianxuan, S.; Jixun, G.; Zhihe, Q. The research on phytoliths size variation characteristics in Phragmites communis under warming conditions. Silicon 2018, 10, 445–454. [Google Scholar] [CrossRef]
- Zexer, N.; Kumar, S.; Elbaum, R. Silica deposition in plants: Scaffolding the mineralization. Ann. Bot. 2023, 131, 897–908. [Google Scholar] [CrossRef] [PubMed]
- Huygens, D.; Saveyn, H.G.M.; Tonini, D.; Eder, P.D.S.L. Technical Proposals for Selected New Fertilising Materials under the Fertilising Products Regulation (Regulation (EU) 2019/1009); JRC117856; JRC: Luxembourg, 2019. [Google Scholar]
- Benkacem, T.; Hamdi, B.; Chamayou, A.; Balard, H.; Calvet, R. Physicochemical characterization of a diatomaceous upon an acid treatment: A focus on surface properties by inverse gas chromatography. Powder Technol. 2016, 294, 498–507. [Google Scholar] [CrossRef]
- Sun, N.; Zhang, P.C.; Qiu, K.H.; Li, J.F.; Liu, X.F.; Qiu, Y.C.; Yang, J.C.; Wang, X.; Xian, L.J. Preparation of high purity spherical silica powder from silica fume. In Materials Science Forum; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2015; pp. 298–302. [Google Scholar]
- Islam, K.N.; Bakar, M.Z.B.A.; Noordin, M.M.; Hussein, M.Z.B.; Abd Rahman, N.S.B.; Ali, M.E. Characterisation of calcium carbonate and its polymorphs from cockle shells (Anadara granosa). Powder Technol. 2011, 213, 188–191. [Google Scholar] [CrossRef]
- Aroke, U.; Abdulkarim, A.; Ogubunka, R. Fourier-transform infrared characterization of kaolin, granite, bentonite and barite. ATBU J. Environ. Technol. 2013, 6, 42–53. [Google Scholar]
- Hernández-Ortiz, M.; Hernández-Padrón, G.; Bernal, R.; Cruz-Vázquez, C.; Castaño, V. Nanocrystalline mimetic opals: Synthesis and comparative characterization vs. natural stones. Int. J. Basic Appl. Sci 2015, 4, 238–243. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Q.; Wu, Z.; Zhang, Y. Thermal behavior analysis of two bentonite samples selected from China. J. Therm. Anal. Calorim. 2015, 121, 1287–1295. [Google Scholar] [CrossRef]
- Alabarse, F.G.; Conceição, R.V.; Balzaretti, N.M.; Schenato, F.; Xavier, A.M. In-situ FTIR analyses of bentonite under high-pressure. Appl. Clay Sci. 2011, 51, 202–208. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).