DFT and Multinuclear NMR Spectroscopy in the Study of Five-Membered Saturated Metallocarbocycles of Main III Group Metals †
Abstract
:1. Introduction
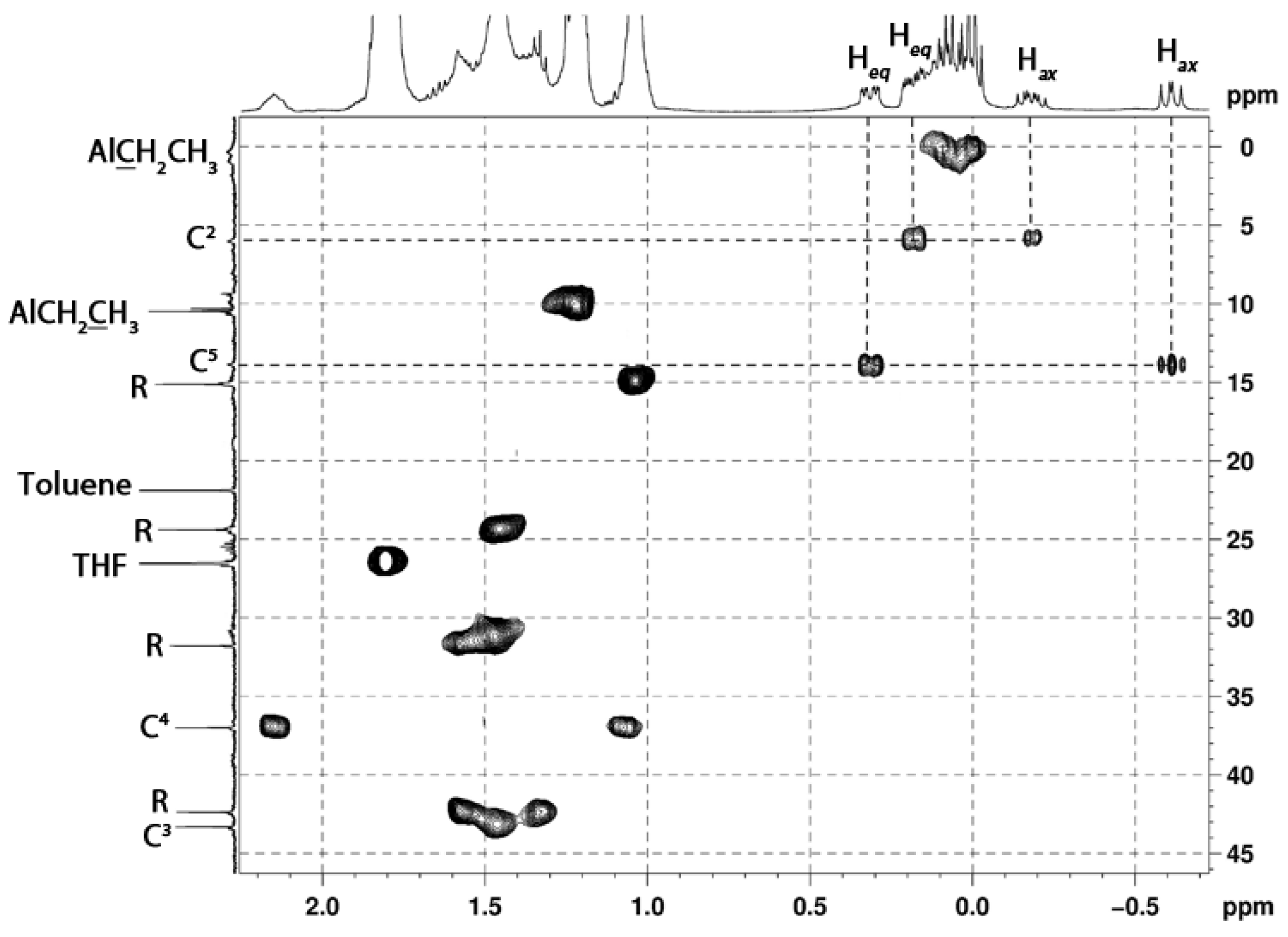
2. Results and Discussion
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dzhemilev, U.M.; Ibragimov, A.G.; Ermilova, O.E.; Sultanov, R.M.; Khalilov, L.M.; Kunakova, R.V.; Sharipova, A.Z. Method of Preparing 1-Chloro-trans-3,4-dialkyl Substituted Indium. Cyclopentanes. Patent RU2164227C2, 20 January 1999. [Google Scholar]
- Schumann, H.; Just, O.; Seuß, T.D.; Görlitz, F.H.; Weimann, R. Intramolekular stabilisierte galla- und Indacyclohexane, -cyclopentane und -cyclopentene; Röntgenstrukturanalyse von (CH2)5Ga(CH2)3NM. J. Organomet. Chem. 1994, 466, 5–14. [Google Scholar] [CrossRef]
- Cowley, A.H.; Corbelin, S.; Jones, R.A.; Lagow, R.J.; Nail, J.W. Synthesis and structure of [(CH2)4Ga-μ-AstBu2]2. The first example of a gallacyclopentane. J. Organomet. Chem. 1994, 464, C1–C3. [Google Scholar] [CrossRef]
- Ibragimov, A.G.; Khafizova, L.O.; Satenov, K.G.; Khalilov, L.M.; Yakovleva, L.G.; Rusakov, S.V.; Dzhemilev, U.M. Synthesis and transformations of metallacycles. Russ. Chem. Bull. 1999, 48, 1574–1580. [Google Scholar] [CrossRef]
- Dzhemilev, U.M.; D’yakonov, V.A. Hydro-, carbo-, and cycloalumination of unsaturated compounds. In Modern Organoaluminum Reagents: Preparation, Structure, Reactivity and Use; Woodward, S., Dagorne, S., Eds.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2013; pp. 215–244. [Google Scholar]
- D’yakonov, V.A. Dzhemilev Reaction in Organic and Organometallic Synthesis; Nova Science Publishers, Inc.: New York, NY, USA, 2010. [Google Scholar]
- D’yakonov, V.A.; Makhamatkhanova, A.L.; Dilmukhametova, L.K.; Agliullina, R.A.; Tyumkina, T.V.; Dzhemilev, U.M. Catalytic cycloalumination for the synthesis of norbornane-annulated phospholanes. Organometallics 2015, 34, 221–228. [Google Scholar] [CrossRef]
- D’yakonov, V.A.; Makhamatkhanova, A.L.; Agliullina, R.A.; Dilmukhametova, L.K.; Tyumkina, T.V.; Dzhemilev, U.M. Aluminacyclopentanes in the synthesis of 3-substituted phospholanes and α,ω-bisphospholanes. Beilstein J. Org. Chem. 2016, 12, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Khafizova, L.O.; Khusainova, L.I.; Tyumkina, T.V.; Dzhemilev, U.M. One-pot synthesis of borolanes by reaction of aluminacyclopentanes with BF3·Et2O. Russ. J. Org. Chem. 2012, 48, 755–760. [Google Scholar] [CrossRef]
- Smith, M.B. The monomer−dimer equilibria of liquid aliminum alkyls: III. Trimethylaluminum: The monomer−dimer equilibria of liquid and gaseous trimethylaluminum and triethylaluminum. J. Organomet. Chem. 1972, 46, 31–49. [Google Scholar] [CrossRef]
- Yamamoto, O. The Low Temperature NMR Spectrum of Triethylaluminum. Bull. Chem. Soc. Jpn. 1964, 37, 1125–1128. [Google Scholar] [CrossRef]
- Yamamoto, O.; Hayamizu, K.; Yanagisawa, M. Bridge-terminal exchange of aluminum trialkyl dimers. J. Organomet. Chem. 1974, 73, 17–25. [Google Scholar] [CrossRef]
- Ramey, K.C.; O’Brien, J.F.; Hasegawa, I.; Borchert, A.E. Nuclear Magnetic Resonance Study of Aluminum Alkyls. J. Phys. Chem. 1965, 69, 3418–3423. [Google Scholar] [CrossRef]
- Dzhemilev, U.M.; Ibragimov, A.G.; Zolotarev, A.P.; Muslukhov, R.R.; Tolstikov, G.A. First preparative synthesis of alumocyclopentanes involving zirconium complexes. Russ. Chem. Bull. 1989, 38, 194–195. [Google Scholar] [CrossRef]
- Muslukhov, R.R.; Khalilov, L.M.; Zolotarev, A.P.; Morozov, A.B.; Ibragimov, A.G.; Dzhemilev, U.M.; Tolstikov, G.A. Synthesis and conversions of metallocycles. 8.13C NMR spectra of aluminocyclopentanes. Russ. Chem. Bull. 1992, 41, 1646–1651. [Google Scholar] [CrossRef]
- Dzhemilev, U.M.; Ibragimov, A.G. Regio- and stereoselective synthesis for a novel class of organoaluminium compounds—Substituted aluminacyclopentanes and aluminacyclopentenes assis. J. Organomet. Chem. 1994, 466, 1–4. [Google Scholar] [CrossRef]
- Tyumkina, T.V.; Islamov, D.N.; Parfenova, L.V.; Khalilov, L.M.; Dzhemilev, U.M. Structure and conformations of 2-substituted and 3-substituted alumolanes in polar solvents: A direct NMR observation. Magn. Reson. Chem. 2016, 54, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Benn, R.; Rufińska, A. High-Resolution Metal NMR Spectroscopy of Organometallic Compounds. Angew. Chem. Int. Ed. 1986, 25, 861–881. [Google Scholar] [CrossRef]
- Wasano, T.; Agou, T.; Sasamori, T.; Tokitoh, N. Synthesis, structure and reactivity of a 1-bromoalumole. Chem. Commun. 2014, 50, 8148–8150. [Google Scholar] [CrossRef] [PubMed]
- Stammler, H.-G.; Blomeyer, S.; Berger, R.J.F.; Mitzel, N.W. Trimethylaluminum: Bonding by Charge and Current Topology. Angew. Chem. Int. Ed. 2015, 54, 13816–13820. [Google Scholar] [CrossRef] [PubMed]
- Tyumkina, T.V.; Idrisova, S.M.; Nurislamova, R.R.; Tullyabaeva, L.I. Multinuclear 1H, 13C, 27Al and 31P NMR spectroscopy in the study of the structure of the 3-spiro-substituted polycyclic borolanes, alumolanes and phospholanes. In Proceedings of the International Conferences “Modern Development of Magnetic Resonance” and “Spin Physics, Spin Chemistry, and Spin Technology”, Kazan, Russia, 25–30 September 2023. [Google Scholar]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tyumkina, T.V.; Islamov, D.N. DFT and Multinuclear NMR Spectroscopy in the Study of Five-Membered Saturated Metallocarbocycles of Main III Group Metals. Chem. Proc. 2023, 14, 36. https://doi.org/10.3390/ecsoc-27-16048
Tyumkina TV, Islamov DN. DFT and Multinuclear NMR Spectroscopy in the Study of Five-Membered Saturated Metallocarbocycles of Main III Group Metals. Chemistry Proceedings. 2023; 14(1):36. https://doi.org/10.3390/ecsoc-27-16048
Chicago/Turabian StyleTyumkina, Tatyana V., and Denis N. Islamov. 2023. "DFT and Multinuclear NMR Spectroscopy in the Study of Five-Membered Saturated Metallocarbocycles of Main III Group Metals" Chemistry Proceedings 14, no. 1: 36. https://doi.org/10.3390/ecsoc-27-16048
APA StyleTyumkina, T. V., & Islamov, D. N. (2023). DFT and Multinuclear NMR Spectroscopy in the Study of Five-Membered Saturated Metallocarbocycles of Main III Group Metals. Chemistry Proceedings, 14(1), 36. https://doi.org/10.3390/ecsoc-27-16048





