Abstract
The homomeric α7 nicotinic acetylcholine receptor (α7 nAChR) is a cation-permeable pentameric ligand-gated channel present in the nervous system and non-neuronal cells. α7 nAChRs are highly expressed in brain regions critical for cognition and memory, such as the hippocampus and cerebral cortex. Therefore, enhancing its function with positive allosteric modulators (PAMs) is a promising therapeutic approach for treating cognitive deficits and neurodegenerative disorders. Continuing with our previous work in the search for novel PAMs of α7 nAChR, this study presents the synthesis and biological evaluation of novel isoxazole-vanillin derivatives exhibiting α7-PAM activity. Isoxazole derivatives with functional α7-PAM activity were identified by determining the effects of the synthetic compounds at different concentrations on the properties of the single channels elicited by ACh (100 μM). We found that only vanillin-derived isoxazoles (containing the 4-hydroxy-3-methoxy fragment) exhibited α7-enhancing activity in comparison to isoxazoles derived from dihydroxy- or dimethoxybenzaldehydes. The use of different substituted phenylacetylenes allowed us to create a small library of compounds with α7-PAM activity.
1. Introduction
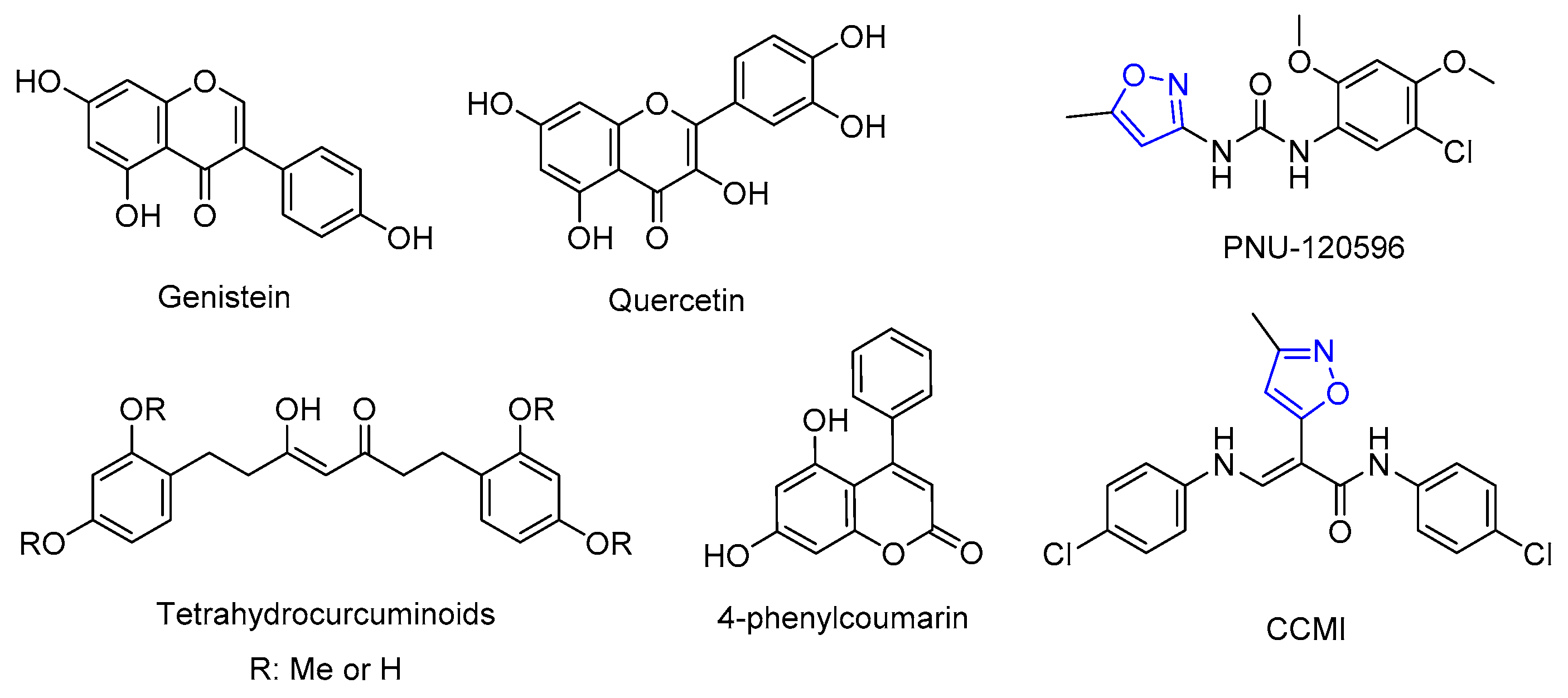
As one of the most abundant nicotinic acetylcholine receptor (nAChRs) subtypes present in the central nervous system, α7 nAChRs are highly expressed in the hippocampus, cortex, and subcortical limbic regions, where they play modulatory roles in neural circuits associated with cognition, learning, attention, memory, and sensory gating information. α7 nAChRs are also expressed astrocytes, microglia, and several non-neuronal cells, where they are involved in neuroprotection, immunity, and inflammation. Decreased α7 activity is associated with neurological, neurodegenerative, and inflammatory disorders, such as schizophrenia, Alzheimer’s disease, depression, and pain. Therefore, α7 nAChRs potentiation using positive allosteric modulators (PAMs) is a promising therapeutic strategy for the treatment of cognitive deficits and neurodegenerative disorders. The use of PAMs to enhance α7 activity offers certain advantages compared to the use of orthosteric agonists, like the conservation of the temporal and spatial pattern of endogenous activation, fewer side effects, higher selectivity, and a wide structural diversity. Among these, the isoxazole core and phenolic compounds (Figure 1) are privileged scaffolds exerting potent α7-PAM activity [1,2,3].
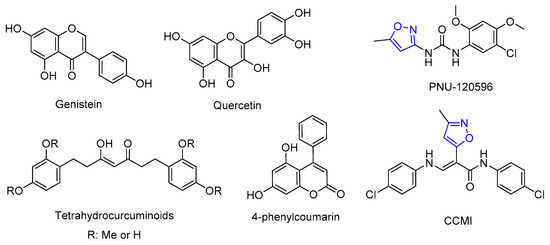
Figure 1.
α7-PAMs containing phenolic compounds or the 3,5-isoxazole ring.
Isoxazole derivatives, similar to 1,2,3-triazoles, can be obtained through a copper-catalyzed cycloaddition reaction between alkynes and the corresponding dipole [4]. In previous works, using supported copper nanoparticles (CuNPs) as the catalyst, we were able to access a small library of triazole derivatives with α7-PAM activity [5].
Continuing with our previous work, here we present the synthesis and biological evaluation of novel isoxazole-vanillin derivatives exhibiting α7-PAM activity. The one-pot synthesis of 3,5-disubstituted isoxazoles was carried out through the cycloaddition reaction between in situ-generated nitrile oxides and terminal alkynes catalyzed by supported CuNPs.
2. Experimental Procedures
2.1. General Methods—Chemistry
All starting materials were of the best available grade (Aldrich, Merck, Rahway, NJ, USA) and used without further purification. The catalyst consisting of copper nanoparticles supported on montmorillonite K10 was prepared according to the methods previously reported by our group. [6] Analytical thin-layer chromatography (TLC) was carried out on TLC aluminum sheets with silica gel 60 F254 (Merck) visualized under UV light and/or phosphomolybdic acid solution spray reagent (10% in ethanol), vanillin, or ferric trichloride solutions. Column chromatography was performed, using Merck silica gel 60 (0.040–0.063 μm, 240–400 mesh) and hexane/ethyl acetate (EtOAc) as the eluent. Microwave-assisted reactions were carried out using a CEM Discover BenchMate microwave operating at 60 W.
2.2. General Procedure for the Synthesis of Isoxazoles [6]
Hydroxylamine hydrochloride (57 mg, 0.825 mmol) and NaHCO3 (63 mg, 0.75 mmol) were added to aldehyde 1 (0.75 mmol) in DMF (2 mL). The reaction mixture was stirred at room temperature until the starting aldehyde was completely converted to the corresponding oxime, as indicated via TLC (30–60 min). N-Chlorosuccinimide (NCS, 120 mg, 0.9 mmol) was then gradually added. The mixture was stirred at room temperature until the oxime was fully transformed into the corresponding N-hydroxyimidoyl chloride, as confirmed via TLC using a FeCl3 solution in MeOH as a stain. Subsequently, CuNPs catalyst (20 mg, 1 mol% Cu), the alkyne (0.5 mmol), and NaHCO3 (63 mg, 0.75 mmol) were added, and the reaction mixture was heated under microwave irradiation (standard method, 60 W, 80 °C) for 30 min. Upon completion, the reaction mixture was diluted with water (10 mL) and extracted with EtOAc (3 × 10 mL). The collected organic phases were washed with water (10 mL) and brine (10 mL) and dried over Na2SO4. The solvent was evaporated under vacuum, and the resulting product was purified via flash column chromatography (hexane-EtOAc) to yield the corresponding isoxazole 5.
2.3. Expression of Human α7 wild Type Receptor
In order to achieve α7 expression, BOSC-23 cells were transfected via the calcium phosphate precipitation protocol with wild-type α7 subunit cDNA together with the α7 chaperone cDNAs, Ric-3 and NACHO, using a cDNA ratio of α7:Ric-3:NACHO 1:1:0.2. Green fluorescence protein cDNA was added during the transfection to allow the identification of transfected cells. All transfections were carried out for about 8–12 h in DMEM with 10% fetal bovine serum and terminated by exchanging the medium, as previously described [7]. Cells were used for experiments two-to-three days after transfection because, at that time, maximum functional expression levels were achieved.
2.4. Single-Channel Recordings
Single channels were recorded in the cell-attached patch configuration, as described previously [1,7]. The bath and pipette solutions contained 142 mM of KCl, 5.4 mM of NaCl, 1.8 mM of CaCl2, 1.7 mM of MgCl2, and 10 mM of HEPES (pH 7.4). Ach and the tested compounds were included in the pipette solution. DMSO was used to solubilize the compounds, and its final concentration was lower than 0.1% (v/v), which does not affect the α7 activation properties [7]. Single-channel currents were digitized at 5–10 μs intervals and low-pass filtered at a cutoff frequency of 10 kHz via an Axopatch 200B patch-clamp amplifier (Molecular Devices, San Jose, CA, USA). Analysis was performed with the program TAC (Bruxton Corporation, Seattle, WA, USA) using the Gaussian digital filter at 9 kHz (Final cut-off frequency 6.7 kHz). Events were detected via the half-amplitude threshold criterion [7]. Using the program TACFit (Bruxton Corporation, Seattle, WA, USA), open-time and closed-time histograms were constructed and fitted based on the sum of exponential functions via the maximum likelihood criterion. The mean open duration (τopen) was taken from the slowest component of the corresponding histogram. A critical duration (τcrit), which was taken as the point of intersection between closed components, was used to define a burst of channel openings, which is defined as a series of closely separated openings preceded and followed by closings longer than τcrit. Each series of opening events spaced by durations briefer than τcrit was considered to be a single burst. The mean burst duration (τburst) was determined from the longest duration component of the open time histogram constructed with the imposed τcrit. Critical durations were defined by the intersection between the first and second briefest components in the closed-time histogram for bursts of α7 activated by ACh (∼0.2−0.5 ms) and between the second and the third closed components in the presence of the compounds (∼1.5−5 ms).
2.5. Statistical Analysis
For each condition, n indicates the number of recordings from different cell patches (independent experiments) and N corresponds to the number of cell transfections, each from different days and cell batches. Data are presented as mean ± SD. For pairwise comparisons, data sets were analyzed using the two-tailed Student’s t-test or Mann–Whitney rank sum test via SigmaPlot 12.0 (Systat Software, Inc., San Jose, CA, USA). Statistically significant differences between two groups of data were established at p values < 0.05.
3. Results and Discussion
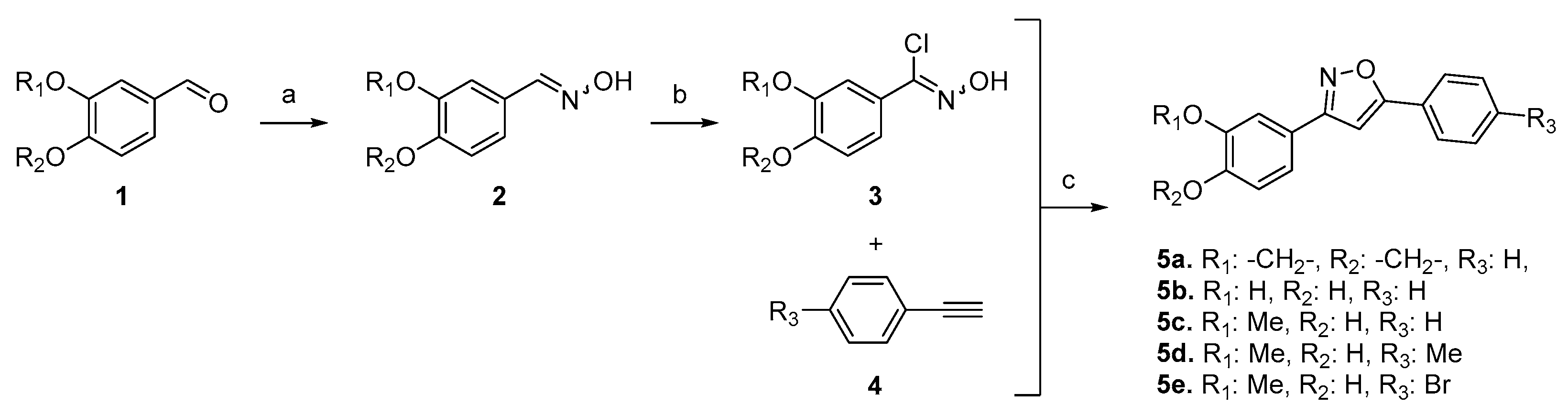
For the synthesis of 3,5-disubstituted isoxazoles, we employed a one-pot sequential approach (Scheme 1) [6]. Initially, hydroxylamine was added to aldehydes 1 to yield the corresponding aldoximes 2. These aldoximes were then treated with NCS to generate N-hydroxyimidoyl chlorides 3 as the nitrile oxide precursors. Subsequently, the addition of 1 equivalent of base (NaHCO3) generated these 1,3-dipoles, which were subjected to a cycloaddition reaction with terminal alkynes, resulting in the desired 3,5-isoxazoles with complete regioselectivity and very good yields (70–87%).
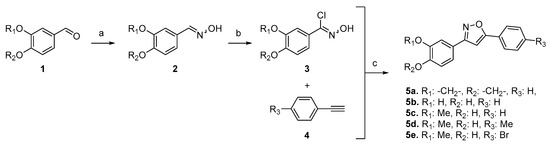
Scheme 1.
One-pot synthesis of 3,5-disubstituted isoxazoles from aldehydes. Reaction conditions: (a) NH2OH.HCl, NaHCO3, DMF, r.t; (b) NCS, r.t.; (c) alkyne, NaHCO3, CuNPs/MK-10, MW (80 °C, 30′).
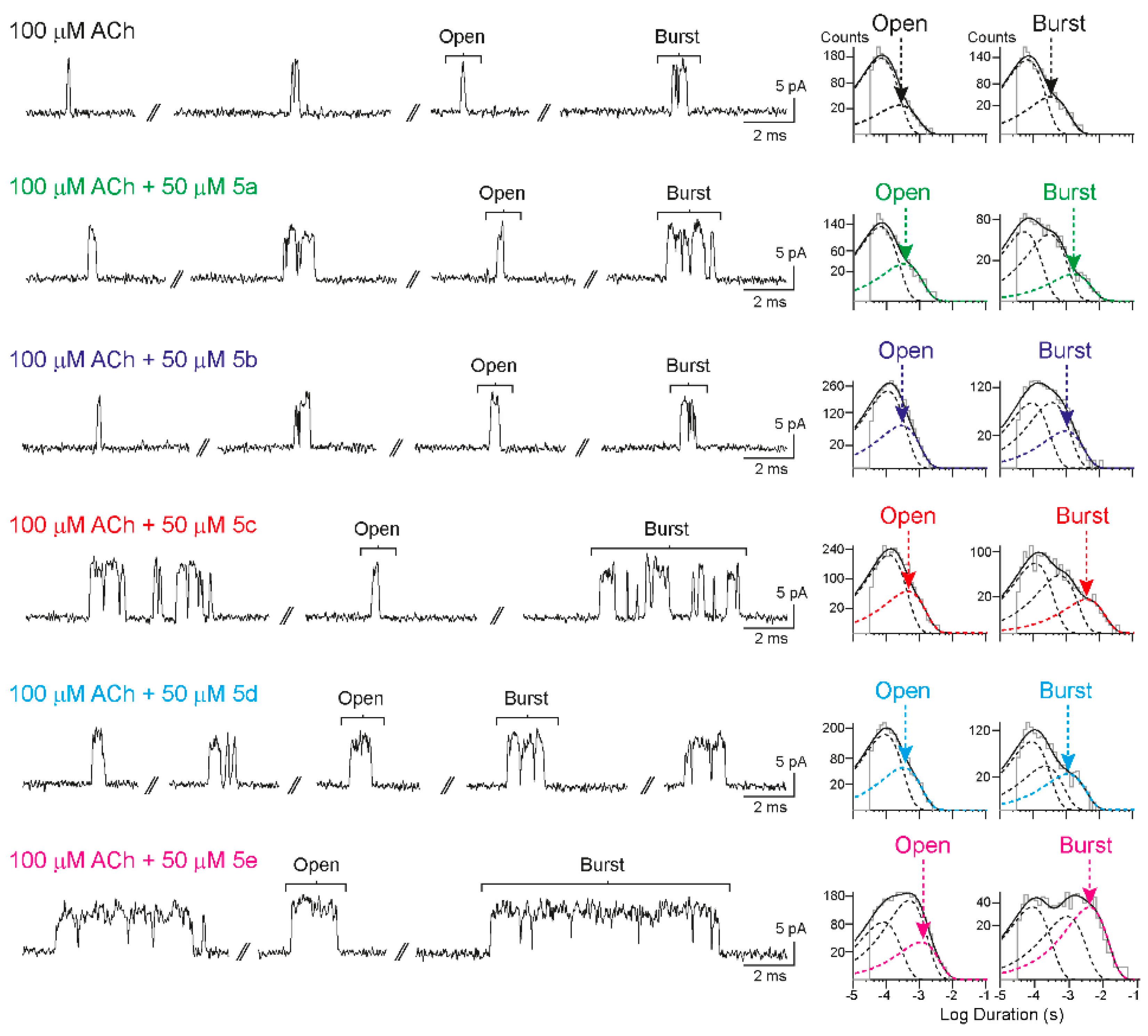
The α7 activity was evaluated through high-resolution single-channel recordings in the cell-attached patch configuration from BOSC23 cells expressing the receptor. In the control condition, α7 activity induced by 100 µM of ACh appeared as brief isolated openings flanked by long closed periods and with lower frequency as activation episodes (bursts). As described in experimental procedures, bursts consist of a few opening events in quick succession, which are evoked by a single receptor molecule (Figure 2). The mean open and burst durations were obtained from the corresponding histograms.
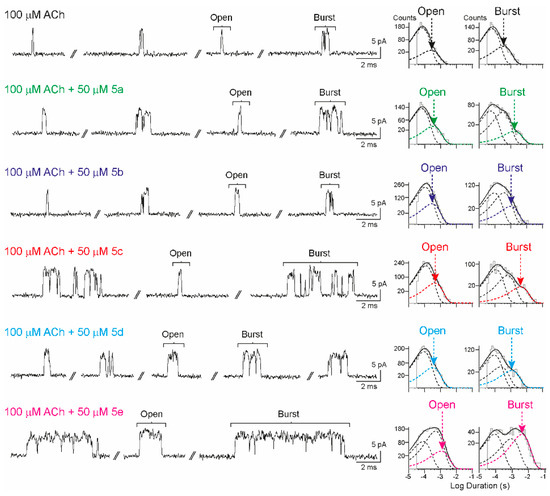
Figure 2.
Effects of isoxazoles 5a–e on α7 receptors at the single-channel level. (Left) Typical patterns of α7 channel currents in the presence of 100 μM of ACh alone or combined with each isoxazole (50 μM). Upward deflections indicate channel openings. (Right) Representative open and burst duration histograms for each condition. Membrane potential = −70 mV. Filter = 9 kHz.
The synthesized compounds were applied together with 100 μM of ACh, which is close to the EC50 concentration required for receptor activation, to evaluate their potential activity as PAMs of α7. 3,5-Isoxazoles were initially screened at 50 μM. Potentiation is typically detected as an increase in burst and open durations. The results are summarized in Table 1.

Table 1.
Single-channel parameters of α7 activated by 100 μM of ACh in the absence or presence of the synthetic compounds shown in Scheme 1. Values are mean ± SD.
It is noteworthy that all isoxazoles evaluated showed α7-PAM activity. This can be seen from the significant increase in the mean burst duration in the presence of isoxazoles 5a–e compared to the control (ACh 100 µM). However, there were differences in the type of potentiation among isoxazoles, as only the vanillin derivatives (containing the 4-OH, 3-MeO fragment, compounds 5c–e) markedly enhanced the mean open duration. The choice of the starting aldehyde seems to be crucial for the α7-PAM activity. Piperonal (5a) and 2,3-dihydroxybenzaldehyde (5b) derivatives are the least active compounds. Therefore, we continued to work with vanillin derivatives and made variations in the alkyne employed in the cycloaddition reaction. Substitution at position C4 with a methyl group leads to isoxazole 5d, showing a decrease in the mean burst duration while maintaining the mean open duration, whereas substitution with bromine leads to 5e, i.e., the most active compound in the series. 5e presents robust α7-PAM activity with a ∼4-fold increase in the mean open duration and a ∼10-fold increase in the mean burst duration.
4. Conclusions
A novel series of 3,5-disubstituted isoxazole derivatives was synthesized in high yields via the microwaved-assisted [3+2] cycloaddition of in situ-generated nitrile oxides and terminal alkynes catalyzed by CuNPs/MK-10. The α7 activity of these compounds, evaluated through high-resolution single-channel recordings in the cell-attached patch configuration from BOSC23 cells expressing the receptor, showed that vanillin-isoxazole derivatives exhibited potent α7-PAM activity. The most active isoxazole, i.e., 5e, showed a ∼4-fold increase in the mean open duration (0.87 ± 0.18 ms) compared to the control condition (0.24 ± 0.03 ms) and a ∼10-fold increase in the mean burst duration (3.88 ± 0.59 ms). These preliminary studies suggest that isoxazole-vanillin derivatives are promising candidates for the development of new families of compounds with α 7-PAM activity. The generation of a small compound library and its biological evaluation are underway.
Author Contributions
Conceptualization, S.S. and J.F.C.; methodology, S.S. and J.F.C.; software, S.S. and J.F.C.; validation, S.S. and J.F.C.; formal analysis, J.F.C.; investigation, S.S. and J.F.C.; resources, G.R. and C.B.; data curation, S.S. and J.F.C.; writing—original draft preparation, S.S. and J.F.C.; writing—review and editing, G.R. and C.B.; visualization, S.S. and J.F.C.; supervision, G.R. and C.B.; project administration, G.R. and C.B.; funding acquisition, G.R. and C.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was generously supported by the Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, PIP-2021-1665, PIP-11220200102356), Agencia Nacional de Promoción Científica y Tecnológica (PICT-2018-2471; PICT-2020-00936), and Universidad Nacional del Sur (UNS, PGI 24/Q106; PGI 24/B298) in Argentina.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article.
Acknowledgments
S.S and J.F.C offer their thanks to CONICET for funding a postdoctoral fellowship.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Nielsen, B.E.; Bermudez, I.; Bouzat, C. Flavonoids as Positive Allosteric Modulators of α7 Nicotinic Receptors. Neuropharmacology 2019, 160, 107794. [Google Scholar] [CrossRef] [PubMed]
- Ximenis, M.; Mulet, J.; Sala, S.; Sala, F.; Criado, M.; González-Muñiz, R.; Pérez de Vega, M.J. Natural Polyhydroxy Flavonoids, Curcuminoids, and Synthetic Curcumin Analogs as α7 nAChRs Positive Allosteric Modulators. Int. J. Mol. Sci. 2021, 22, 973. [Google Scholar] [CrossRef] [PubMed]
- Hurst, R.S.; Hajós, M.; Raggenbass, M.; Wall, T.M.; Higdon, N.R.; Lawson, J.A.; Rutherford-Root, K.L.; Berkenpas, M.B.; Hoffmann, W.E.; Piotrowski, D.W.; et al. A Novel Positive Allosteric Modulator of the α7 Neuronal Nicotinic Acetylcholine Receptor: In Vitro and In Vivo Characterization. J. Neurosci. 2005, 25, 4396–4405. [Google Scholar] [CrossRef] [PubMed]
- Hansen, T.V.; Wu, P.; Fokin, V.V. One-Pot Copper(I)-Catalyzed Synthesis of 3,5-Disubstituted Isoxazoles. J. Org. Chem. 2005, 70, 7761–7764. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, B.E.; Stabile, S.; Vitale, C.; Bouzat, C. Design, Synthesis, and Functional Evaluation of a Novel Series of Phosphonate-Functionalized 1,2,3-Triazoles as Positive Allosteric Modulators of α7 Nicotinic Acetylcholine Receptors. ACS Chem. Neurosci. 2020, 11, 2688–2704. [Google Scholar] [CrossRef] [PubMed]
- Stabile, S.; Bjerg, E.E.; Radivoy, G.E. Copper Nanoparticles on Montmorillonite K-10: A Versatile Catalyst for the One-Pot Synthesis of 3,5-Disubstituted Isoxazoles Using Various Methodologies. Synthesis 2023, 55, A. [Google Scholar] [CrossRef]
- Chrestia, J.F.; Bruzzone, A.; del Esandi, M.C.; Bouzat, C. Tyrosine Phosphorylation Differentially Fine-Tunes Ionotropic and Metabotropic Responses of Human α7 Nicotinic Acetylcholine Receptor. Cell. Mol. Life Sci. 2021, 78, 5381–5395. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).