Abstract
Alzheimer’s disease (AD) is a neurodegenerative disease that affects the majority of people worldwide. To date, there is no cure for the disease, so new therapeutic targets need to be identified and studied. Acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) are promising therapeutic targets for AD treatment. In order to identify new inhibitors, a newly synthesized series containing thirty seven 2-hydroxy-N-phenylbenzamidederivatives were tested to study the inhibition of enzymes associated with this disease. Our work focuses on the use of molecular modeling methods based on molecular docking, QSAR and ADME property prediction. Our molecular docking results discussion is based on a number of parameters. Analysis of these obtained results showed that the ligands L18, L17 and L6 have a high inhibitory effect in the case of the enzyme AChE, while the ligands L6′, L30′ and L4′ have a high inhibitory effect in the case of the enzyme BuChE. In addition, the ADME-T properties calculation proved that these ligands respect the Lipinski, Veber and Egan rules, allowing us to select them as being probably the best inhibitors of Alzheimer’s disease (AD). Then, a QSAR model was developed to explain and predict the inhibitory activity of a series of compounds using different descriptors. This model has been validated by two methods: internal and external.
1. Introduction
Alzheimer’s disease is characterized by progressive cognitive decline due to multiple pathological changes in the brain, primarily in cholinergic neurons of the basal fore-brain [1]. This disease is the most common form of dementia and is associated with progressive and irreversible intellectual decline, resulting in impairment of mental performance and behavior, resulting in loss of autonomy [2]. Acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) are two different types of cholinesterases that hydrolyze acetylcholine (Ach) into acetate and choline, respectively [3]. AchE hydrolyzes Ach in the normal brain. Therefore, it is a major drug target [4]. To this end, we selected a series of recently synthesized derivatives of 2-hydroxy-N-phenylbenzamide as inhibitors to study their effects on the two targets, AChE and BuChE, which are responsible for these diseases. It is this approach of the treatment by inhibition of AChE and BuChE that we are interested in this work in order to contribute to the development of new inhibitors using different molecular modelling techniques, such as molecular docking, QSAR and ADME properties.
Molecular docking was performed to analyze the complex score and different types of interactions present between certain amino acids of the protein studied and that of ligands. On the other hand, the QSAR has become, at present, an indispensable tool in the field of drug design, especially in the absence of information on the active site of the enzyme. Technically, this approach is mainly based on the choice of descriptors and the learning algorithm [5].
Finally, to reduce the failure rate of drug candidates, the implementation of ADME (Absorption Distribution Metabolism and Elimination)-Tox (Toxicity) filters for chemo-therapies in any screening process gave good pharmacokinetic performance and bioavailability, as well as excellent results.
2. Materials and Methods
Thirty-seven compounds belonging to 2-hydroxy-N-phenylbenzamide derivatives were studied by molecular docking, QSAR and ADME, and their AChE and BuChE inhibitory activities were tested with, MOE [6] and; HyperChem software (Version 7.0, Hypercube, USA, http://www.hyper.com, accessed on 30 September 2023), and other software programs were used to find optimal high-affinity compounds.
3. Results and Discussion
The molecular docking results obtained in this work are based on three parameters: energy score (S-score, kcal/mol), interaction (type and distance) and RMSD value.
3.1. Interaction between Compounds and Targets (AChE/BuChE)
The results of the molecular docking of the three best compounds of thirty seven of 2-hydroxy-N-phenylbenzamide derivatives with active site residues of the AChE and BuChE targets are regrouped in Table 1.

Table 1.
Docking score energy, RMSD values and interactions of studied compounds and clinical test with active site residues of AChE/BuChE.
According to the energy value of the binding evaluation, compared with compounds L6 (−7.368 kcal/mol) and L17 (−7.461 kcal), we can see that compound L18 (−7.799 kcal/mol) is the most effective inhibitor of the AChE (target/mol); these results were confirmed by detecting two interactions of each compound.
It can be seen that the complex formed by compound L18 has a lower energy value (−7.799 kcal/mol), which is very close to the natural ligand donepezil (−11.247 kcal/mol). On the other hand, the RMSD value of the AChE-L18 complex is: 1.014, which is less than 2 A [7,8], which means that this compound fits well into the pocket of AChE (Table 1).
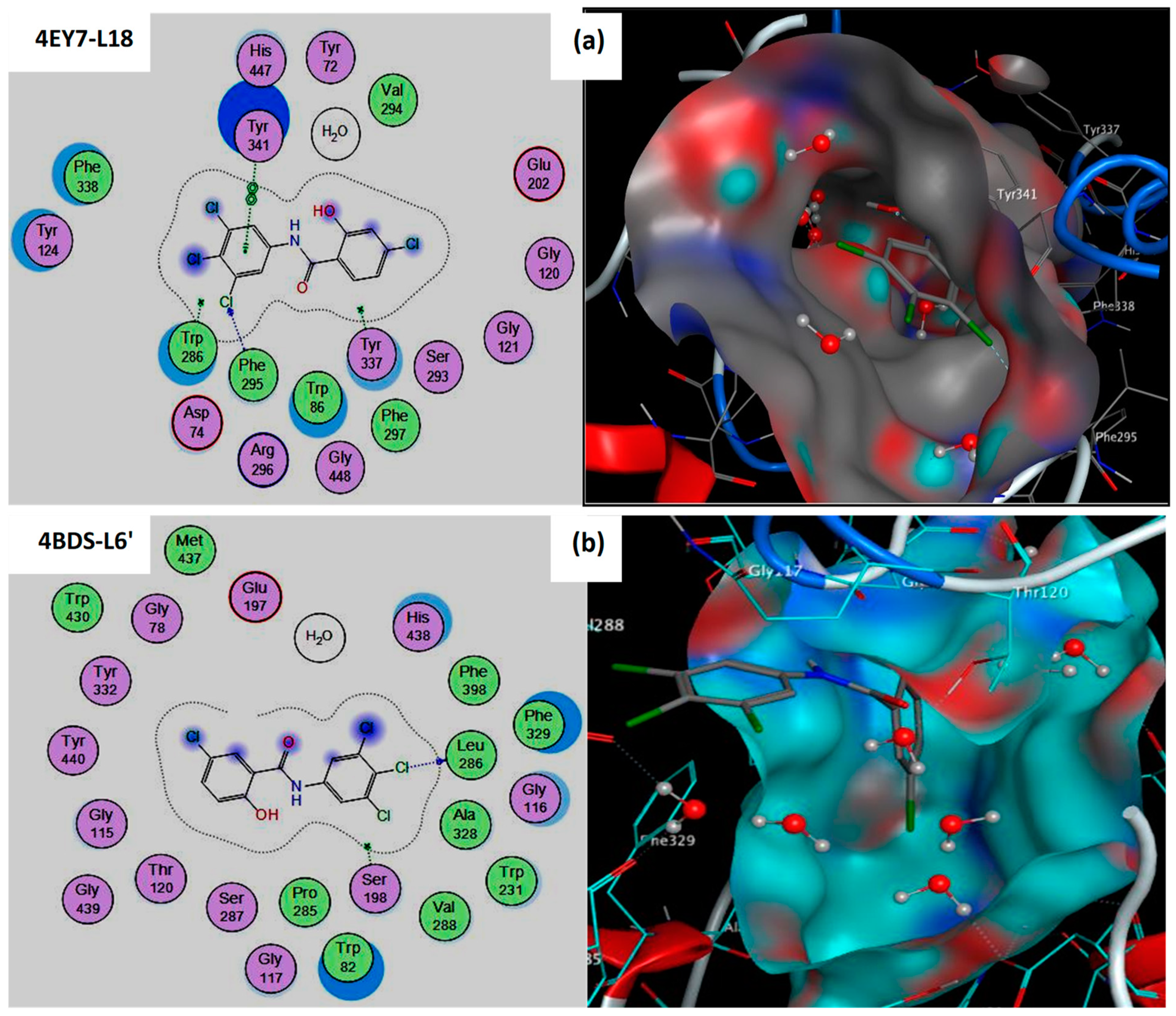
It is also evident that compound L18 forms strong hydrogen bonds with active site residues of the AChE target [9]. PHE295 forms a hydrogen bond (bond distance = 3.40 Å) with the Cl18 atom of compound L18.
Furthermore, the same compound establishes hydrophobic interactions with binding site residues of AChE: TYR341 interacts pi-pi with the 6-ring of compound L18 at a distance of 3.83 Å (Table 1 and Figure 1). This means that we have discovered an enzyme active site suitable for this study, which has been mentioned in many recent papers [10,11].
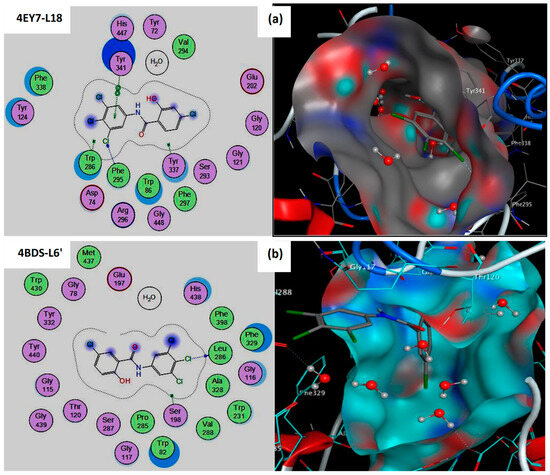
Figure 1.
2D and 3D representation of the best pose interactions of complexes: (a) 4EY7-L18, (b) 4BDS-L6′ using molecular docking simulation.
For BuChE, we found that compound L6′ (−6.603 kcal/mol) had the highest affinity for the BuChE target compared to compounds L4′ (−5.250 kcal/mol) and L30′ (−5.590 kcal/mol).
It is also evident that compound L6′ forms two strong hydrogen bonds with active site residues of the BuChE target [9]. HOH2153 forms a hydrogen bond (bond distance = 2.97 Å) with the O16 atom of compound L6′. In the second step, SER198 establishes another hydrogen bond (bond distance = 3.08 Å) with Cl22 of compound L6′ (Table 1 and Figure 1). In this context, many recent studies [12,13] confirmed that SER198 and molecular water play a central role in inhibiting BuChE targets.
On the other hand, the RMSD value of the BuChE-L6′ complex is: 0.979, which is less than 2 A [7,8], implying that this compound fit well into the BuChE binding site (Table 1).
3.2. QSAR Modeling
The correlation between BuChE inhibitory activity and calculated descriptors is given by the following relation:
Log (1/IC50)= 5.098 − 0.165 LogP + 0.005 MW − 0.037 MR − 0.94 qC3′ − 0.566 qC4′
n = 25; R = 0.869; R2 = 0.756; S = 0.0516; F = 11.749; Q = 6.470; p < 0.001
Our results suggest that the best QSAR model obtained is the one using the following descriptors: log p, MR, MW, qC3′, and qC4′.We know that the reliability and predictive power of this QSAR model has been validated by the right values of R2adj, q2 and, SPRESS.
3.3. Evaluation of ADME Properties
The molecular structures of the best compounds L18 and L6′ were analyzed using the SwissADME server (http://www.swissadme.ch/, accessed on 30 September 2014) to ensure compliance with the Lipinski, Veber and Egan rules, which describe various physicochemical properties of the calculated ligand molecules. All these molecules follow the rules of Lipinski, Ghose, Veber and Egan. Finally, toxicity prediction results indicated that none of the compounds were toxic. We can confirm that these compounds do not cause oral bioavailability issues, have good properties compared to drugs for both targets (natural ligands), and have the potential to be selected as oral drugs against this disease.
4. Conclusions
The molecular docking study revealed that ligands: L18; L17; and L6 are the best inhibitors in the case of AChE, and ligands L6′; L30′ and L4′ are best in the case of BuChE, this is justified by the presence of different types of interactions (mainly hydrogen bonds with low energy score values).
We also note that the increase in interactions between inhibitors and residues of the active site improves affinity (energy score); this means that these complexes have the lowest score energies compared to others, This is confirmed by the value of RMSD (root-mean-square deviation), which does not exceed 2 Å in most complexes formed by these inhibitors and the two enzymes AChE and BuChE.
In addition, a strong correlation was observed between the experimental and predicted values of BuChE inhibitory biological activity, indicating the reliability and validity of the QSAR model obtained.
The combination of several molecular modelling methods may be useful in the interest of discovering new anti-Alzheimer’s drugs, and these methods which allowed us to identify new inhibitors have raised potential new drugs to use against this disease.
Author Contributions
Data collection, software, formal analysis, and first draft of the manuscript were prepared by F.H. All authors commented on previous versions of the manuscript. I.D. contributed to the conceptualization and supervision of the study. N.M. and I.D. contributed to the interpretation of docking and ADME-T studies. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Spaio, T.B.; Savall, A.S.; Gutierrez, M.E.; Pinton, S. Neurotrophic factors in Alzheimer’s and Parkinson’s diseases: Implications for pathogenesis and therapy. Neural Regen. Res. 2017, 12, 549. [Google Scholar]
- Mohamed, T.; Yeung, J.C.; Vasefi, M.S.; Beazely, M.A.; Rao, P.P. Development and evaluation of multifunctional agents for potential treatment of Alzheimer’s disease: Application to a pyrimidine-2,4-diamine template. Bioorganic Med. Chem. Lett. 2012, 22, 4707–4712. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Li, Y.P.; He, Y.; Huang, S.L.; Tan, J.H.; Ou, T.M.; Li, D.; Gu, L.Q.; Huang, Z.S. Design, synthesis and evaluation of novel tacrine-multialkoxybenzene hybrids as dual inhibitors for cholinesterases and amyloid beta aggregation. Bioorganic Med. Chem. 2011, 19, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Guevara-Salazar, J.A.; Espinoza-Fonseca, M.; Beltrán, H.I.; Correa-Basurto, J.; Quintana Zavala, D.; Trujillo-Ferrara, J.G. The electronic influence on the active site-directed inhibition of acetylcholinesterase by N-aryl-substituted succinimides. J. Mex. Chem. Soc. 2007, 51, 222–227. [Google Scholar]
- Kola, I.; Landis, J. Can the pharmaceutical industry reduce attrition rates? Nat. Rev. Drug Discov. 2004, 3, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Molecular Operating Environment (MOE); Version 2014.09; Chemical Computing Group Inc.: Montreal, QC, Canada, 2014.
- Ibrahim, M.T.; Uzairu, A.; Shallangwa, G.A.; Ibrahim, A. In-silico studies of someoxadiazoles derivatives as anti-diabetic compounds. J. King Saud. Univ. Sci. 2020, 32, 423–432. [Google Scholar] [CrossRef]
- Brooijmans, N. Chapter: Docking methods, ligand design, and validating data sets in the structural genomics era. In Structural Bioinformatics; Gu, J., Bourne, P.E., Eds.; Wiley: New York, NY, USA, 2009; pp. 635–663. [Google Scholar]
- Imberty, A.; Hardman, K.D.; Carver, J.P.; Perez, S. Molecular modeling of protein-carbohydrate interactions. Docking of monosaccharides in the binding site of concanavalin A. Glycobiology 1991, 1, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Daoud, I.; Melkemi, N.; Salah, T.; Ghalem, S. Combined QSAR, molecular docking and molecular dynamics study on new Acetylcholinesterase and Butyrylcholinesterase inhibitors. Comput. Biol. Chem. 2018, 74, 304–326. [Google Scholar] [CrossRef] [PubMed]
- Kherachi, R.; Daoud, I.; Melkemi, N.; Kenouche, S.; Mettai, M.; Mesli, F. Investigation of spirooxindole-pyrrolidine derivatives as acetylcholinesterase inhibitors using molecular docking/dynamics simulations, bioisosteric replacement, MEP, and ADME/Tox properties. In Biologia; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1–21. [Google Scholar]
- Nikseresht, A.; Ghasemi, S.; Parak, S. [Cu3(BTC)2]: A metal–organic framework as an environment-friendly and economically catalyst for the synthesis of tacrine analogues by Friedlander reaction under conventional and ultrasound irradiation. Polyhedron 2018, 151, 112–117. [Google Scholar] [CrossRef]
- Baba-Ahmed, I.; Kibou, Z.; Daoud, I.; Belhadj, F.; Lahcen, B.; Daich, A.; Choukchou-Braham, N. Synthesis, molecular docking and ADME-TOX studies of new tacrine analogs as promising for alzheimer’s disease therapy. Curr. Org. Chem. 2022, 26, 1218–1233. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).