Synthesis and Characterisation of Menthol-Based Hydrophobic Deep Eutectic Solvents †
Abstract
:1. Introduction
2. Materials and Methods
Hydrophobic Deep Eutectic Solvent Synthesis
3. Results and Discussion
3.1. Fourier Transform Infrared of HDESs
3.2. Physicochemical Properties
3.2.1. Density
3.2.2. Viscosity
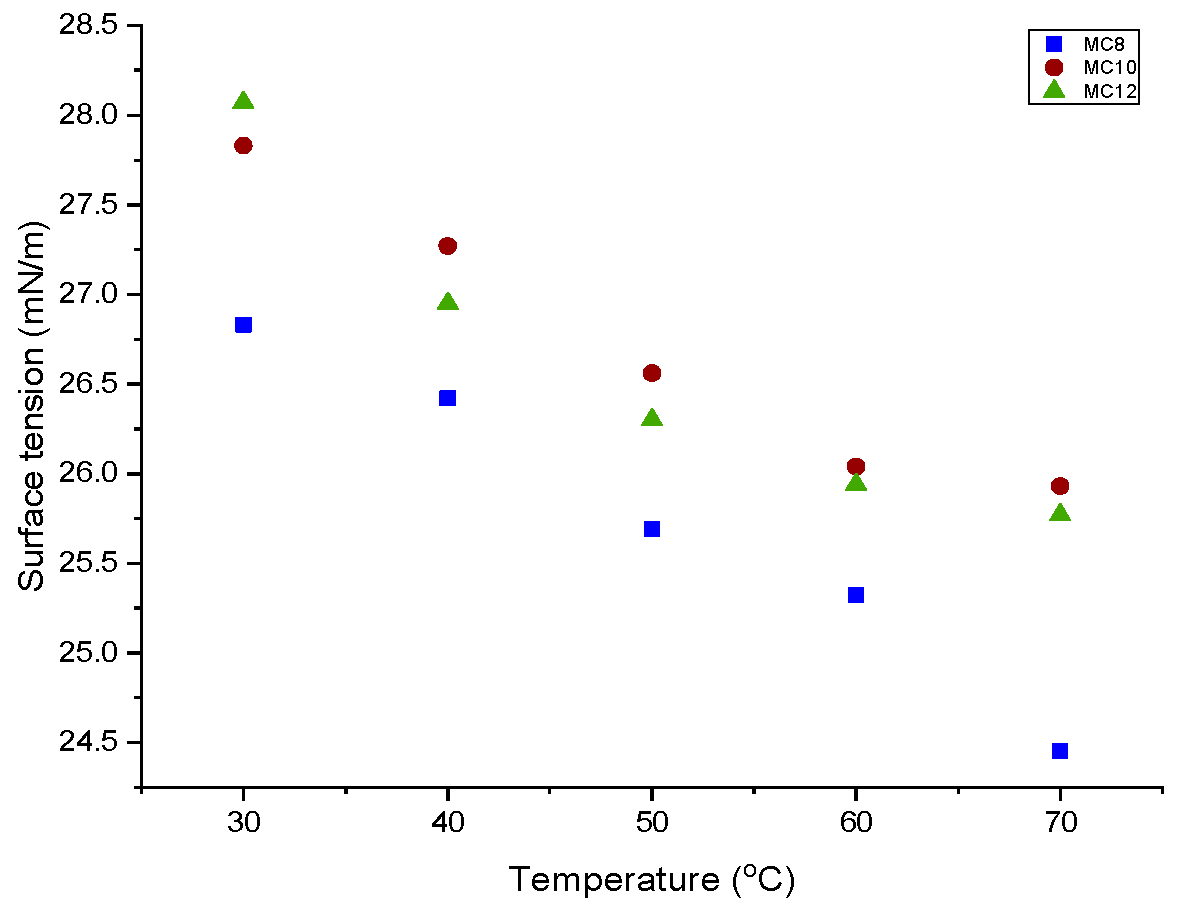
3.2.3. Surface Tension
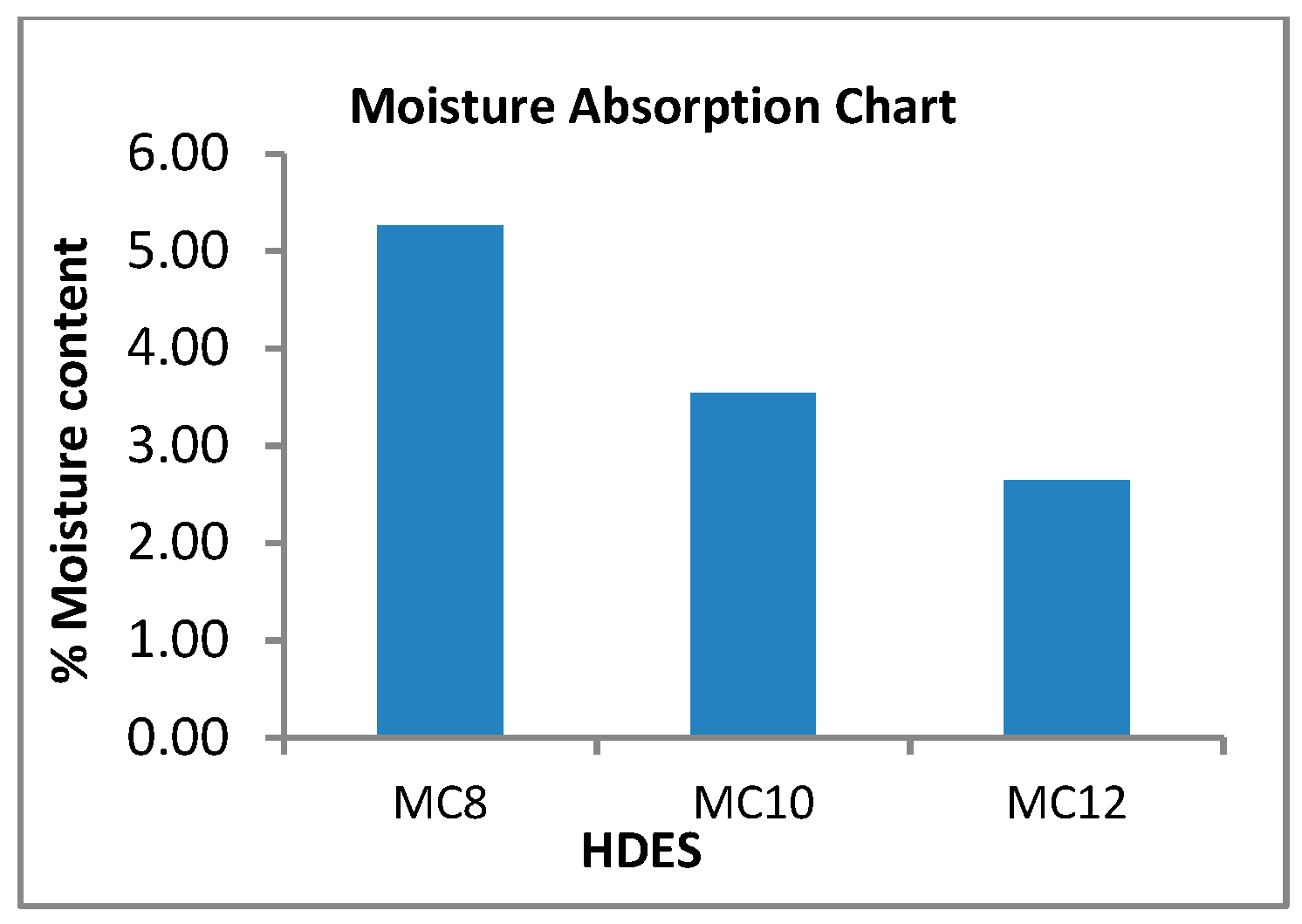
3.3. Hydrophobicity Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Francisco, M.; Van Den Bruinhorst, A.; Kroon, M.C. Low-transition-temperature mixtures (LTTMs): A new generation of designer solvents. Angew. Chem.-Int. Ed. 2013, 52, 3074–3085. [Google Scholar] [CrossRef] [PubMed]
- Paiva, A.; Craveiro, R.; Aroso, I.; Martins, M.; Reis, R.L.; Duarte, A.R.C. Natural deep eutectic solvents-Solvents for the 21st century. ACS Sustain. Chem. Eng. 2014, 2, 1063–1071. [Google Scholar] [CrossRef]
- Abbott, A.P.; Alaysuy, O.; Antunes, A.P.M.; Douglas, A.C.; Guthrie-Strachan, J.; Wise, W.R. Processing of leather using deep eutectic solvents. ACS Sustain. Chem. Eng. 2015, 3, 1241–1247. [Google Scholar] [CrossRef]
- Passos, H.; Tavares, D.J.P.; Ferreira, A.M.; Freire, M.G.; Coutinho, J.A.P. Are Aqueous Biphasic Systems Composed of Deep Eutectic Solvents Ternary or Quaternary Systems? ACS Sustain. Chem. Eng. 2016, 4, 2881–2886. [Google Scholar] [CrossRef]
- Silva, N.H.C.S.; Pinto, R.J.B.; Freire, C.S.R.; Marrucho, I.M. Production of lysozyme nanofibers using deep eutectic solvent aqueous solutions. Colloids Surf. B Biointerfaces 2016, 147, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.H.; Ghareeb, M.M.; Al-Remawi, M.; TAl-Akayleh, F. New insight into sin7gle phase formation of capric acid/menthol eutectic mixtures by Fourier-transform infrared spectroscopy and differential scanning calorimetry. Trop. J. Pharm. Res. 2020, 19, 361–369. [Google Scholar] [CrossRef]
- Zhang, Q.; De Oliveira Vigier, K.; Royer, S.; Jérôme, F. Deep eutectic solvents: Syntheses, properties and applications. Chem. Soc. Rev. 2012, 41, 7108–7146. [Google Scholar] [CrossRef]
- Kareem, M.A.; Mjalli, F.S.; Hashim, M.A.; Alnashef, I.M. Phosphonium-based ionic liquids analogues and their physical properties. J. Chem. Eng. Data 2010, 55, 4632–4637. [Google Scholar] [CrossRef]
- Nunes, R.J.; Saramago, B.; Marrucho, I.M. Surface Tension of dl -Menthol: Octanoic Acid Eutectic Mixtures. J. Chem. Eng. Data 2019, 64, 4915–4923. [Google Scholar] [CrossRef]
- Shah, D.; Mjalli, F.S. Effect of water on the thermo-physical properties of Reline: An experimental and molecular simulation-based approach. Phys. Chem. Chem. Phys. 2014, 16, 23900–23907. [Google Scholar] [CrossRef]
- D’Agostino, C.; Harris, R.C.; Abbott, A.P.; Gladden, L.F.; Mantle, M.D. Molecular motion and ion diffusion in choline chloride-based deep eutectic solvents studied by 1H pulsed field gradient NMR spectroscopy. Phys. Chem. Chem. Phys. 2011, 13, 21383–21391. [Google Scholar] [CrossRef] [PubMed]
- Florindo, C.; Branco, L.C.; Marrucho, I.M. Quest for Green-Solvent Design: From Hydrophilic to Hydrophobic (Deep) Eutectic Solvents. ChemSusChem 2019, 12, 1549–1559. [Google Scholar] [CrossRef] [PubMed]
- Shekaari, H.; Zafarani-Moattar, M.T.; Mohammadi, B. Thermophysical characterization of aqueous deep eutectic solvent (choline chloride/urea) solutions in full ranges of concentration at T = (293.15–323.15) K. J. Mol. Liq. 2017, 243, 451–461. [Google Scholar] [CrossRef]
- Abbott Andrew, P.; Barron, J.C.; Ryder, K.S.; Wilson, D. Eutectic-based ionic liquids with metal-containing anions and cations. Chem. A Eur. J. 2007, 13, 6495–6501. [Google Scholar] [CrossRef] [PubMed]
- Abbott Andrew, P.; Harris, R.C.; Ryder, K.S. Application of hole theory to define ionic liquids by their transport properties. J. Phys. Chem. B 2007, 111, 4910–4913. [Google Scholar] [CrossRef]
- Abbott Andrew, P.; Harris, R.C.; Ryder, K.S.; Agostino, C.D.; Gladden, F.; Mantle, M.D. Green Chemistry Glycerol eutectics as sustainable solvent systems. Green Chem. 2011, 13, 82–90. [Google Scholar] [CrossRef]
- Cui, Y.; Li, C.; Yin, J.; Li, S.; Jia, Y.; Bao, M. Design, synthesis and properties of acidic deep eutectic solvents based on choline chloride. J. Mol. Liq. 2017, 236, 338–343. [Google Scholar] [CrossRef]
- Florindo, C.; Oliveira, F.S.; Rebelo, L.P.N.; Fernandes, A.M.; Marrucho, I.M. Insights into the synthesis and properties of deep eutectic solvents based on cholinium chloride and carboxylic acids. ACS Sustain. Chem. Eng. 2014, 2, 2416–2425. [Google Scholar] [CrossRef]
- García, G.; Aparicio, S.; Ullah, R.; Atilhan, M. Deep eutectic solvents: Physicochemical properties and gas separation applications. Energy Fuels 2015, 29, 2616–2644. [Google Scholar] [CrossRef]
- Abbott Andrew, P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 1, 70–71. [Google Scholar] [CrossRef]
- Abbott, A.P.; Boothby, D.; Capper, G.; Davies, D.L.; Rasheed, R. Deep Eutectic Solvents Formed Between Choline Chloride and Carboxylic Acids. J. Am. Chem. Soc. 2004, 126, 9142–9147. [Google Scholar] [CrossRef] [PubMed]
- Abbott Andrew, P.; Capper, G.; Davies, D.L.; Rasheed, R.K. Ionic Liquid Analogues Formed from Hydrated Metal Salts. Chem. A Eur. J. 2004, 10, 3769–3774. [Google Scholar] [CrossRef] [PubMed]
- Abbott Andrew, P.; Capper, G.; Gray, S. Design of improved deep eutectic solvents using hole theory. ChemPhysChem 2006, 7, 803–806. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Tailoring properties of natural deep eutectic solvents with water to facilitate their applications. Food Chem. 2015, 187, 14–19. [Google Scholar] [CrossRef]
- Du, C.; Zhao, B.; Chen, X.B.; Birbilis, N.; Yang, H. Effect of water presence on choline chloride-2urea ionic liquid and coating platings from the hydrated ionic liquid. Sci. Rep. 2016, 6, 29225. [Google Scholar] [CrossRef]
- Lapeña, D.; Lomba, L.; Artal, M.; Lafuente, C.; Giner, B. The NADES glyceline as a potential Green Solvent: A comprehensive study of its thermophysical properties and effect of water inclusion. J. Chem. Thermodyn. 2019, 128, 164–172. [Google Scholar] [CrossRef]







| HBA | HBD | Abbreviation | Mole Ratio | Physical Appearance |
|---|---|---|---|---|
| Menthol | Octanoic acid | MC8 | 1:1 | Pale-yellow liquid |
| Decanoic acid | MC10 | 1:1 | Colourless liquid | |
| Dodecanoic acid | MC12 | 1:1 | Golden-yellow liquid |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adeoye, D.O.; Gano, Z.S.; Ahmed, O.U.; Shuwa, S.M.; Atta, A.Y.; Iwarere, S.A.; Jubril, B.Y.; Daramola, M.O. Synthesis and Characterisation of Menthol-Based Hydrophobic Deep Eutectic Solvents. Chem. Proc. 2023, 14, 98. https://doi.org/10.3390/ecsoc-27-16334
Adeoye DO, Gano ZS, Ahmed OU, Shuwa SM, Atta AY, Iwarere SA, Jubril BY, Daramola MO. Synthesis and Characterisation of Menthol-Based Hydrophobic Deep Eutectic Solvents. Chemistry Proceedings. 2023; 14(1):98. https://doi.org/10.3390/ecsoc-27-16334
Chicago/Turabian StyleAdeoye, Deborah O., Zaharaddeen S. Gano, Omar U. Ahmed, Suleiman M. Shuwa, Abdulazeez Y. Atta, Samuel A. Iwarere, Baba Y. Jubril, and Michael O. Daramola. 2023. "Synthesis and Characterisation of Menthol-Based Hydrophobic Deep Eutectic Solvents" Chemistry Proceedings 14, no. 1: 98. https://doi.org/10.3390/ecsoc-27-16334
APA StyleAdeoye, D. O., Gano, Z. S., Ahmed, O. U., Shuwa, S. M., Atta, A. Y., Iwarere, S. A., Jubril, B. Y., & Daramola, M. O. (2023). Synthesis and Characterisation of Menthol-Based Hydrophobic Deep Eutectic Solvents. Chemistry Proceedings, 14(1), 98. https://doi.org/10.3390/ecsoc-27-16334








