Structural Analogues of Thyronamines: Some Aspects of the Structure and Bioactivity of 4-[4-(2-Aminoetoxy)benzyl]aniline †
Abstract
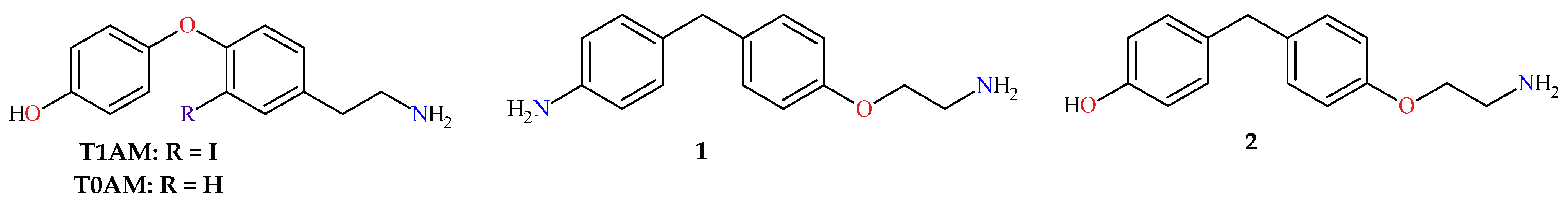
1. Introduction
2. Experimental and Computational Details
2.1. Synthesis of 4-[4-(2-Aminoethoxy)benzyl]aniline
2.2. NMR 1H and 13C Spectroscopy
2.3. Bioactivity of 4-[4-(2-Aminoethoxy)benzyl]aniline
2.4. Semiempirical and DFT Calculations
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Filimonov, D.A.; Evtushenko, S.K.; Fedorova, A.A. Molecular mechanisms of neuroprotective effects of thyroid hormones and their metabolites in acute brain ischemia. Ann. Clin. Exp. Neurol. 2023, 17, 43–54. [Google Scholar] [CrossRef]
- Buchan, A.M.; Pelz, D.M. Neuroprotection in Acute Ischemic Stroke: A Brief Review. Can. J. Neurol. Sci. 2022, 49, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Filimonov, D.A.; Trubnikova, N.N.; Belotserkovskaya, M.A.; Fedorova, A.A.; Eresko, A.B.; Marusichenko, V.V. Thermoregulatory effects of triiodothyronine derivatives: In vivo study and review of potential neuroprotective effects. Int. Neurol. J. 2020, 16, 65–71. [Google Scholar] [CrossRef]
- Chiellini, G.; Nesi, G.; Sestito, S.; Chiarugi, S.; Runfola, M.; Espinoza, S.; Sabatini, M.; Bellusci, L.; Laurino, A.; Cichero, E.; et al. Hit-to-Lead Optimization of Mouse Trace Amine Associated Receptor 1 (mTAAR1) Agonists with a Diphenylmethane-Scaffold: Design, Synthesis, and Biological Study. J. Med. Chem. 2016, 59, 9825–9836. [Google Scholar] [CrossRef]
- Chiellini, G.; Nesi, G.; Digiacomo, M.; Malvasi, R.; Espinoza, S.; Sabatini, M.; Frascarelli, S.; Laurino, A.; Cichero, E.; Macchia, M.; et al. Design, Synthesis, and Evaluation of Thyronamine Analogues as Novel Potent Mouse Trace Amine Associated Receptor 1 (mTAAR1) Agonists. J. Med. Chem. 2015, 58, 5096–5107. [Google Scholar] [CrossRef]
- Runfola, M.; Perni, M.; Yang, X.; Marchese, M.; Bacci, A.; Mero, S.; Santorelli, F.M.; Polini, B.; Chiellini, G.; Giuliani, D.; et al. Identification of a Thyroid Hormone Derivative as a Pleiotropic Agent for the Treatment of Alzheimer’s Disease. Pharmaceuticals 2021, 14, 1330. [Google Scholar] [CrossRef]
- Cody, V. Structure of thyroxine: Role of thyroxine hydroxyl in protein binding. Acta Crystallogr. Sect. B 1981, 37, 1685–1689. [Google Scholar] [CrossRef]
- Mondal, S.; Mugesh, G. Structure Elucidation and Characterization of Different Thyroxine Polymorphs. Angew. Chem. Int. Ed. 2015, 54, 10833–10837. [Google Scholar] [CrossRef]
- Schweizer, U.; Steegborn, C. Thyroid hormones—From Crystal Packing to Activity to Reactivity. Angew. Chem. Int. Ed. 2015, 54, 12856–12858. [Google Scholar] [CrossRef]
- Mondal, S.; Mugesh, G. Conformational Flexibility and Halogen Bonding in Thyroid Hormones and Their Metabolites. Cryst. Growth Des. 2016, 16, 5896–5906. [Google Scholar] [CrossRef]
- Duggan, B.M.; Craik, D.J. 1H and 13C NMR relaxation studies of molecular dynamics of the thyroid hormones thyroxine, 3,5,3′-triiodothyronine, and 3,5-diiodothyronine. J. Medic. Chem. 1996, 39, 4007–4016. [Google Scholar] [CrossRef] [PubMed]
- Duggan, B.M.; Craik, D.J. Conformational dynamics of thyroid hormones by variable temperature nuclear magnetic resonance: The role of side chain rotations and cisoid/transoid interconversions. J. Med. Chem. 1997, 40, 2259–2265. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Ko, Y.H.; Si, J.; Na, J.; Ortore, G.; Chiellini, G.; Kim, J.H. Thyroxine metabolite-derived 3-iodothyronamine (T1AM) and synthetic analogs as efficient suppressors of transthyretin amyloidosis. Comput. Struct. Biotechn. J. 2023, 21, 4717–4728. [Google Scholar] [CrossRef] [PubMed]
- Romańczyk, P.P.; Kurek, S.S. Noncatalytic Reductive Deiodination of Thyroid Hormones. Electrochemistry and Quantum Chemical Calculations. ChemElectroChem 2024, 11, e202300527. [Google Scholar] [CrossRef]
- Spils, J.; Caspers, L.D.; Puylaert, P.; Nachtsheim, B.J. Oxidative cyclization and enzyme-free deiodination of thyroid hormones. Org. Chem. Front. 2024, 11, 2800–2806. [Google Scholar] [CrossRef]
- Semenov, V.A.; Krivdin, L.B. Computational NMR of natural products. Russ. Chem. Rev. 2022, 91, RCR5027. [Google Scholar] [CrossRef]
- Filimonov, D.A.; Eresko, A.B.; Raksha, E.V.; Trubnikova, N.N.; Ischenko, R.V.; Tereschenko, D.A.; Kisilenko, I.A.; Nosova, I.N. Antioxidant effects of the synthetic thyronamine analogue in experimental cerebral ischemia. Extrem. Med. 2024, 57–63. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of parameters for semiempirical methods V: Modification of NDDO approximations and application to 70 elements. J. Mol. Model. 2007, 13, 1173–1213. [Google Scholar] [CrossRef]
- Korth, M.; Pitoňák, M.; Řezáč, J.; Hobza, P. A Transferable H-bonding Correction for Semiempirical Quantum-Chemical Methods. J. Chem. Theory Comput. 2010, 6, 344–352. [Google Scholar] [CrossRef]
- Řezáč, J.; Fanfrlík, J.; Salahub, D.; Hobza, P. Semiempirical Quantum Chemical PM6 Method Augmented by Dispersion and H-Bonding Correction Terms Reliably Describes Various Types of Noncovalent Complexes. J. Chem. Theory Comput. 2010, 5, 1749–1760. [Google Scholar] [CrossRef]
- MOPAC2016, J.J.P. Stewart, Stewart Computational Chemistry, Colorado Springs, CO, USA. Available online: http://openmopac.net (accessed on 14 September 2024).
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA quantum chemistry program package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.J.; Taylor, P.R. A diagnostic for determining the quality of single-reference electron correlation methods. Int. J. Quantum Chem. 1989, 36, 199–207. [Google Scholar] [CrossRef]
- Wolinski, K.; Hilton, J.F.; Pulay, P. Efficient implementation of the gauge-independent atomic orbital method for NMR chemical shift calculations. J. Am. Chem. Soc. 1990, 112, 8251–8260. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eresko, A.B.; Raksha, E.V.; Filimonov, D.A.; Trubnikova, N.N.; Kisilenko, I.A.; Chudoba, D.M. Structural Analogues of Thyronamines: Some Aspects of the Structure and Bioactivity of 4-[4-(2-Aminoetoxy)benzyl]aniline. Chem. Proc. 2024, 16, 22. https://doi.org/10.3390/ecsoc-28-20165
Eresko AB, Raksha EV, Filimonov DA, Trubnikova NN, Kisilenko IA, Chudoba DM. Structural Analogues of Thyronamines: Some Aspects of the Structure and Bioactivity of 4-[4-(2-Aminoetoxy)benzyl]aniline. Chemistry Proceedings. 2024; 16(1):22. https://doi.org/10.3390/ecsoc-28-20165
Chicago/Turabian StyleEresko, Alexander B., Elena V. Raksha, Dmitry A. Filimonov, Nadezhda N. Trubnikova, Irina A. Kisilenko, and Dorota M. Chudoba. 2024. "Structural Analogues of Thyronamines: Some Aspects of the Structure and Bioactivity of 4-[4-(2-Aminoetoxy)benzyl]aniline" Chemistry Proceedings 16, no. 1: 22. https://doi.org/10.3390/ecsoc-28-20165
APA StyleEresko, A. B., Raksha, E. V., Filimonov, D. A., Trubnikova, N. N., Kisilenko, I. A., & Chudoba, D. M. (2024). Structural Analogues of Thyronamines: Some Aspects of the Structure and Bioactivity of 4-[4-(2-Aminoetoxy)benzyl]aniline. Chemistry Proceedings, 16(1), 22. https://doi.org/10.3390/ecsoc-28-20165






