Abstract
Due to their ability to reversibly isomerize under the influence of external stimuli, spiropyrans represent the most interesting class of organic photochromic molecules. The photochromic properties of the isomeric forms of spiropyrans differ significantly from each other, which makes it possible to use these photochromes as sensors, optoelectronic and holographic devices, memory elements, etc. Also, an undoubted advantage of spiropyrans compared to other classes of organic photochromes is the relative ease of their preparation and chemical transformation. At the same time, modification of the structure of spiropyrans by introducing various functional groups opens up great synthetic possibilities for obtaining new photochromic molecules with various spectral-kinetic characteristics. In the development of research aimed at expanding the boundaries of the use of spirophotochromic compounds, in order to obtain new light-controlled materials with different characteristics, as well as to study the influence of functional groups in the spirophotochromic molecule on the spectral and photochromic properties, we have synthesized a new spiropyran. In this work, we synthesized a new salt of photochromic spiropyran containing various functional groups (–CHO, –NO2, –OCH3, –(CH2)5N(CH3)2*HBr), capable of reversibly responding to external influences. Photoinduced transformations and the spectral and kinetic characteristics of the synthesized compound were studied.
1. Introduction
The synthesis and design of functional molecules in information technology, medical applications, opto-and optobioelectronics, transport systems, photocontrolled nanostructured materials, catalysis, biomaterials, and molecular photodynamic sensors is an actively developing area of research [1,2,3]. These compounds include spiropyrans [4]. Due to the relative ease of their preparation and structural transformation and the possibility of directed changes in optical characteristics over a wide range when changing the structure, spiropyrans have attracted special attention as one of the most important and widely studied classes of photochromic organic molecules.
2. Results and Discussion
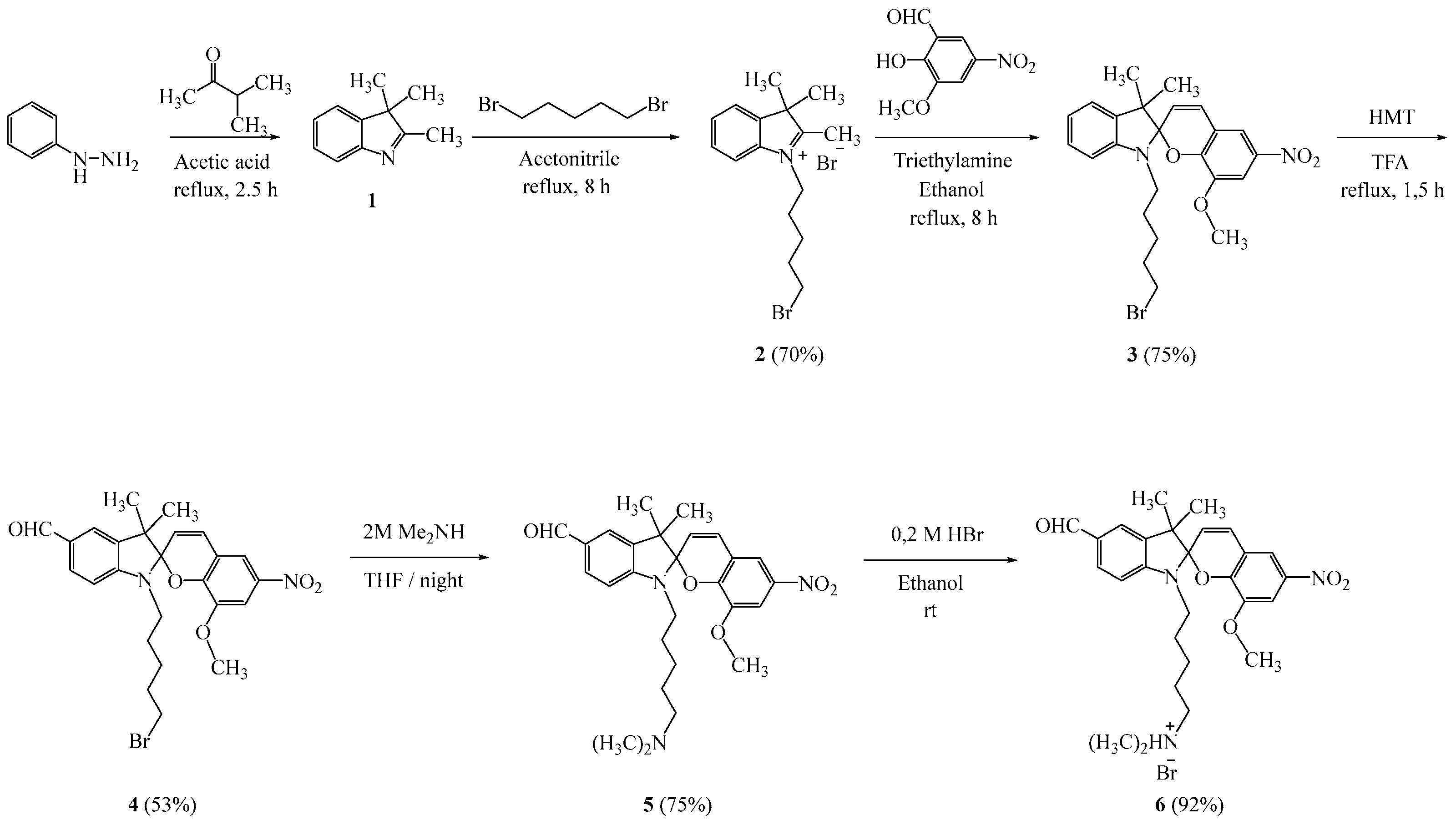
Taking into account the above and trying to expand the class of photochromic compounds and to study the effect of substituents on the electronic properties and stability of the new molecule, we synthesized a new photochromic salt of spiropyran containing various functional groups. The target spiropyran was synthesized using methods described in the literature [5,6] according to Scheme 1.
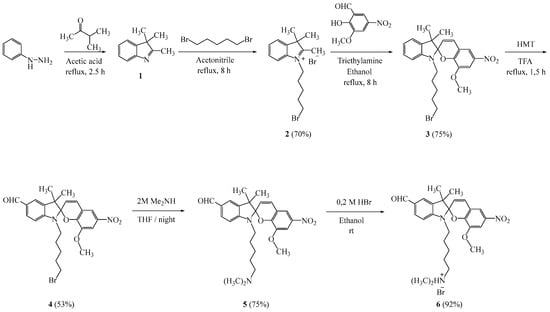
Scheme 1.
Synthesis of compounds 6.
The resulting reaction product 6 was purified via silica column chromatography using 10:1 CHCl3:EtOH as the eluant. The structures of compound 6 were determined using 1H and 13C NMR spectroscopy and high-resolution mass spectrometry.
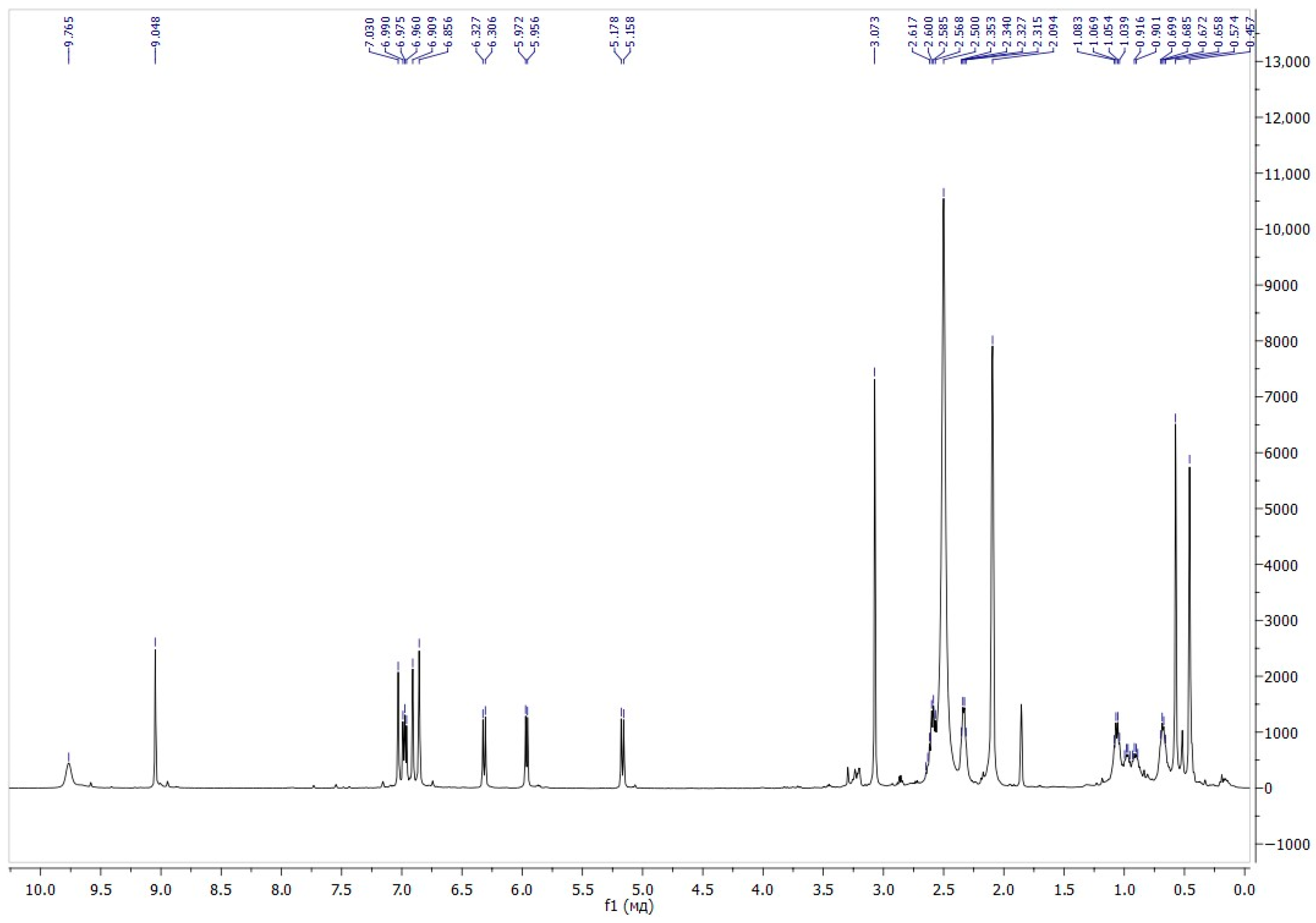
The position of the signals in the 1H NMR spectra of compound 6 in a mixture of chloroform and DMSO, along with the spin–spin interaction constants, is completely consistent with the presented structure (Figure 1). In the region of aliphatic protons, two singlets at 0.45 and 0.57 ppm correspond to the protons of the methyl groups at the nitrogen atom of the indole fragment of the molecule. The singlet signal at 2.09 ppm belongs to protons of two methyl groups at the ammonium nitrogen atom. The signal of O-CH3 was detected at 3.07 ppm. A doublet in the aromatic region at 5.17 ppm with a spin–spin interaction constant of 10.3 Hz corresponds to the CH group in the pyran moiety, which indicates the presence of a spirocyclic structure. The singlet signal at 9.04 ppm belongs to the proton of the formyl group.
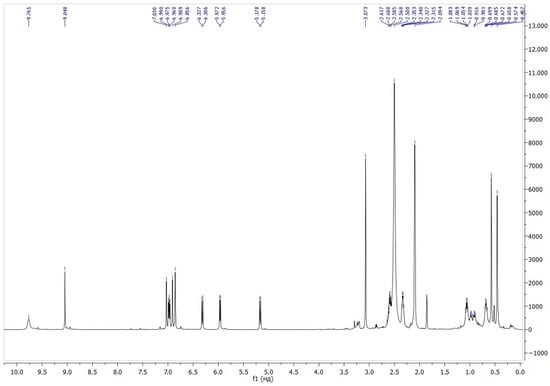
Figure 1.
1H NMR spectra of compound 6.
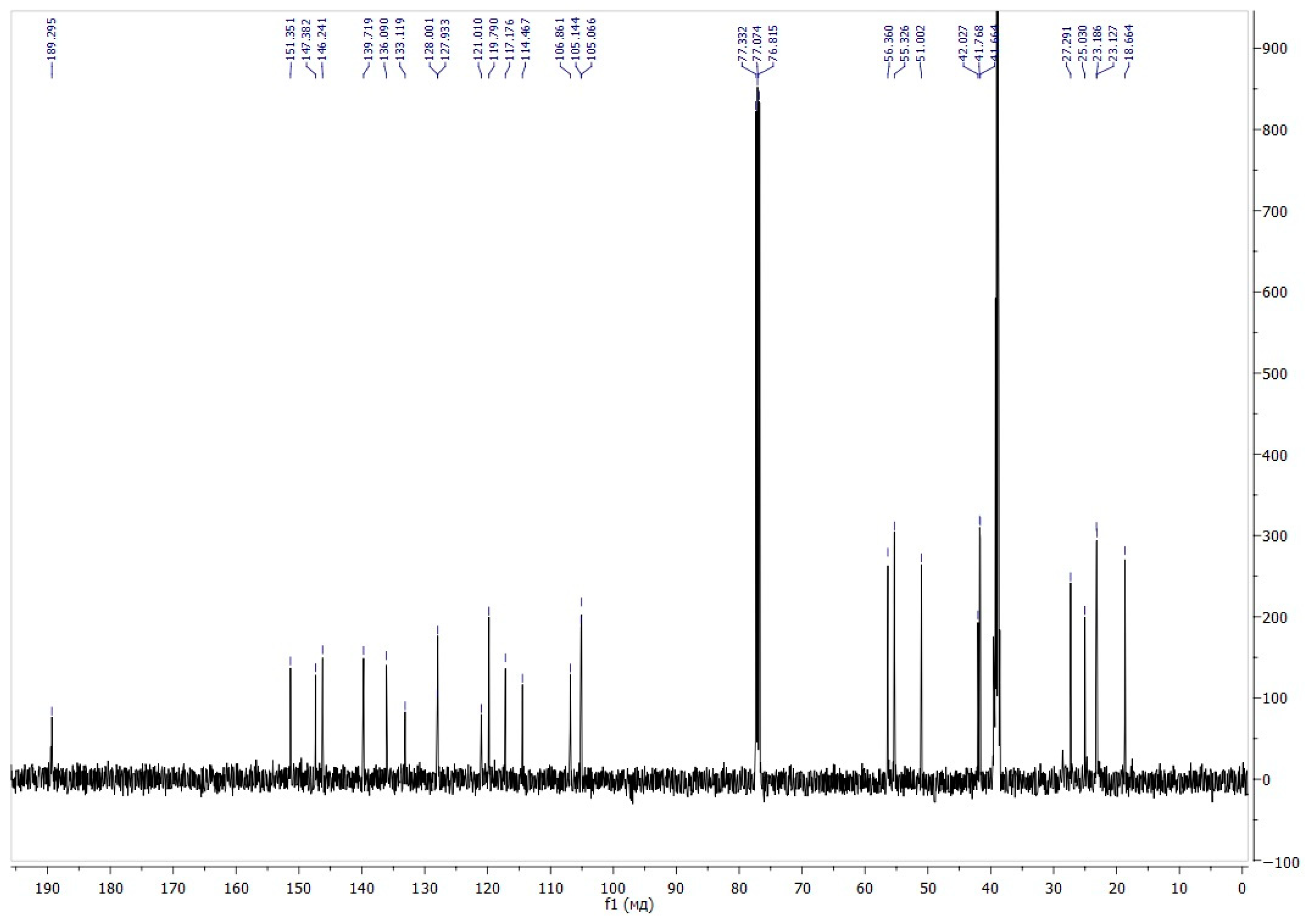
The signal at 105.06 ppm, which correlates in the 1H–13C HMBC spectrum with the signal of the protons of the methyl groups and the signals of the protons at the C3’ and C4’ carbon atoms, is characteristic and belongs to the spiro carbon atom (Figure 2). The signal at 189.29 ppm belongs to the proton of the formyl group.
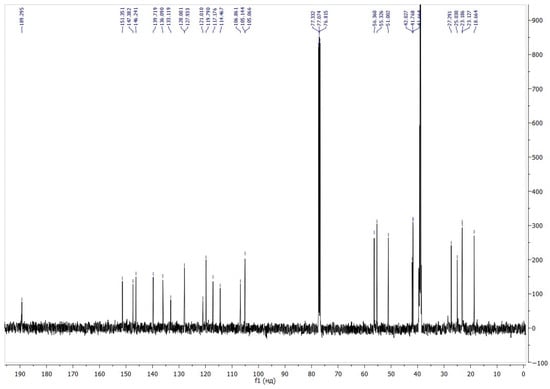
Figure 2.
13C NMR spectra of compound 6.
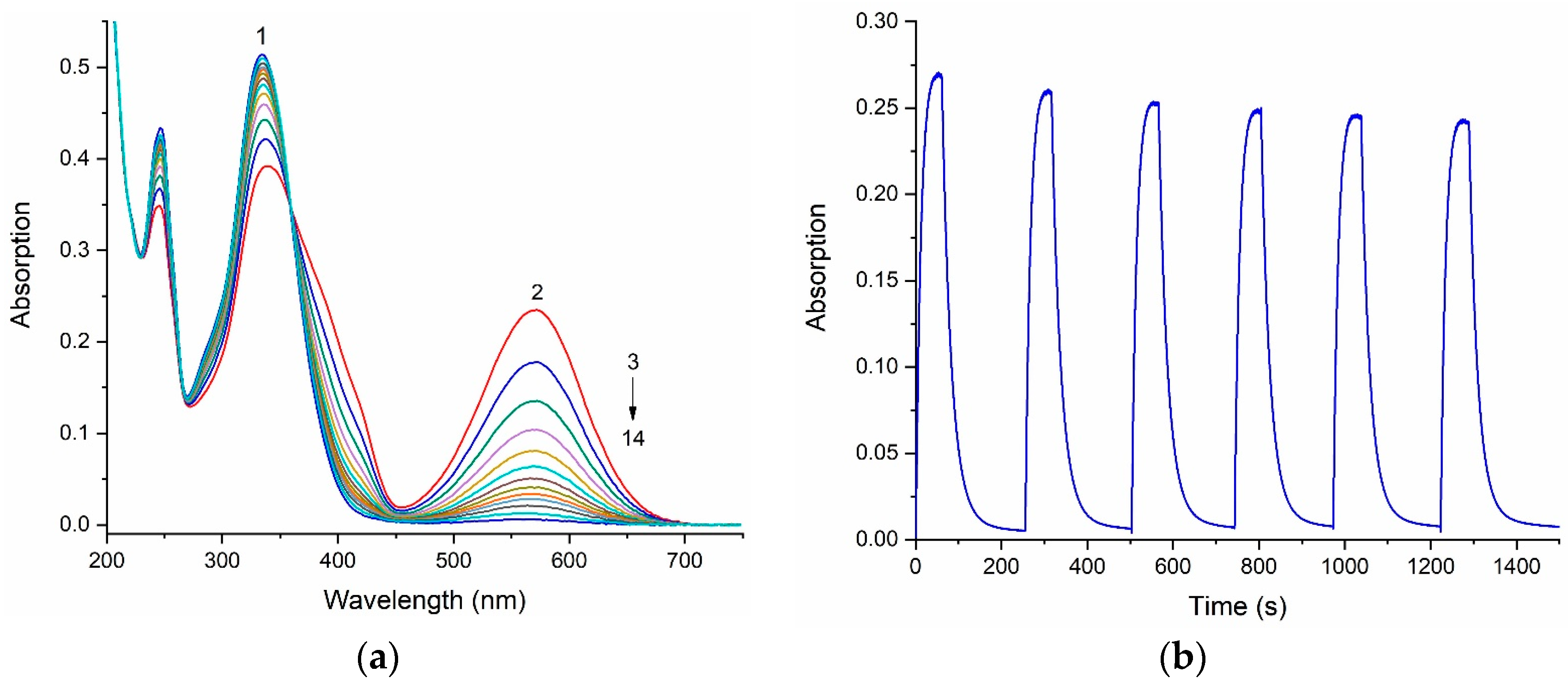
When a solution of spiropyran 6 in ethanol is exposed to UV radiation, a change in the absorption spectrum is observed (Figure 3a). In this case, a new absorption band appears in the visible range with a maximum in the visible region at 572 nm, indicating the formation of the merocyanine form. During the subsequent dark relaxation, a gradual decrease in the intensity of the photoinduced absorption band occurs, indicating the isomerization of the open form into the closed form.
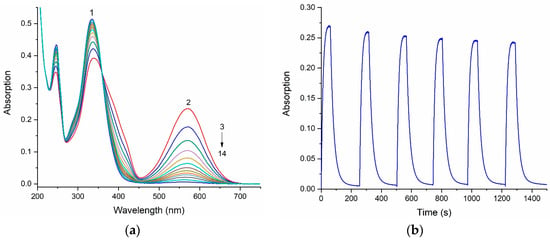
Figure 3.
(a) Change in the absorption spectra of spiropyran 6 before (1) and after irradiation with UV during 60 s through a UFS−1 optical filter in the photoequilibrium state (2), and after dark relaxation in ethanol (3−14). (b) Cyclic change in optical density at the maximum of the long-wavelength absorption band (572 nm) of spiropyran 6 upon irradiation with UV light and after dark relaxation in ethanol.
The change in the intensity of the optical density, namely, the decrease in the amplitude at the maximum of the long-wave absorption band of the photoinduced merocyanine form of compound 6, indicates that this spiropyran is subject to photochromic transformations, which is accompanied by the gradual photodegradation of the molecule (Figure 3b).
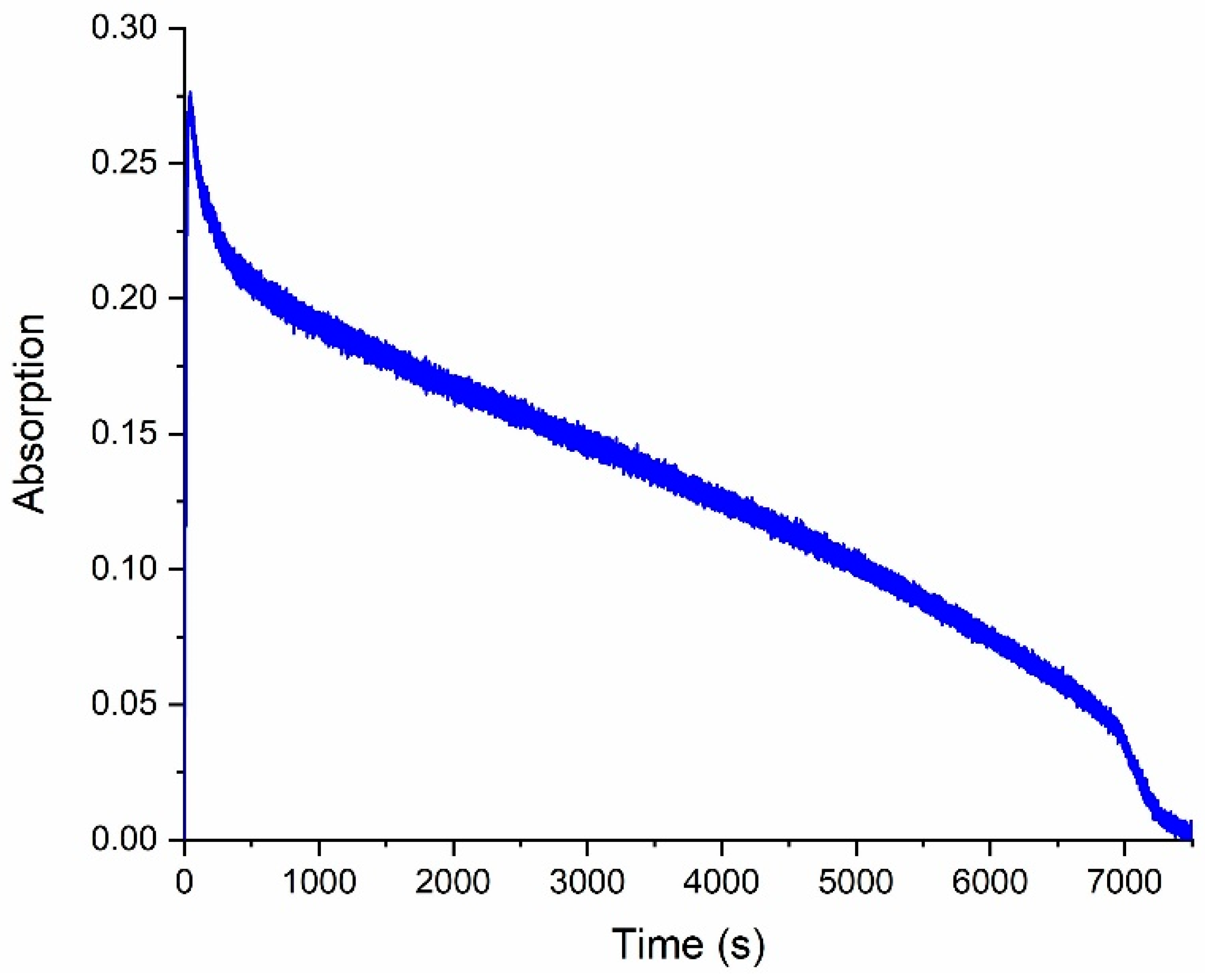
With the continuous exposure of a solution of compound 6 to UV light, a decrease in optical density in the open-form absorption spectrum is observed, which indicates the gradual destruction of spirophotochrome. Complete photodecomposition is observed after 2 h (Figure 4).
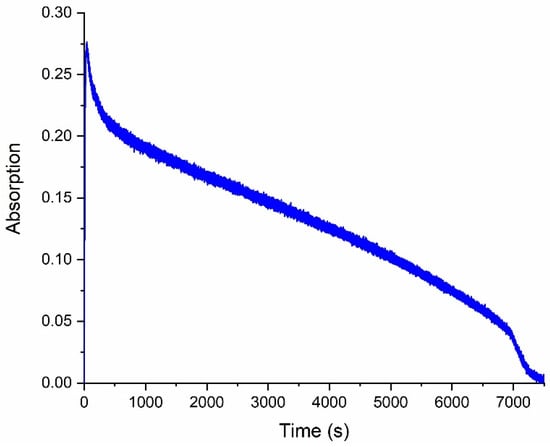
Figure 4.
Degradation kinetics of compound 6 by UV under continuous irradiation through a light filter (UFS−1) registered at a wavelength of 572 nm.
3. Conclusions
In summary, we synthesized a new salt photochromic spiropyran comprising various functional groups that can react reversibly to external influences and also studied photoinduced transformations and the spectral and kinetic characteristics of the synthesized compound. It was found that the resulting spiropyran exhibits positive photochromism and is quite resistant to irreversible photochemical transformations. This opens up prospects for the use of such molecules as molecular switches.
Author Contributions
Conceptualization, A.K.; methodology, A.K. and L.K.; validation, A.K. and L.K.; formal analysis, L.K.; investigation, A.K. and L.K.; writing—original draft preparation, A.K.; writing—review and editing, A.K.; data analysis and visualization, and L.K.; funding acquisition, A.K. and L.K. I, the corresponding author. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Russian Ministry of Science and Higher Education (Government themes FMRS-2022-0075).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available in this paper.
Acknowledgments
The structural studies of compounds were performed with the use of unique equipment in “Agidel” Collective Usage Centre at the Institute of Petrochemistry and Catalysis, Ufa Federal Research Center of the Russian Academy of Sciences.
Conflicts of Interest
All authors declare that they have no conflicts of interest.
References
- Minkin, V.I. Light-controlled molecular switches based on bistable spirocyclic organic and coordination compounds. Russ. Chem. Rev. 2013, 82, 1–26. [Google Scholar] [CrossRef]
- Klajn, R. Spiropyran-based dynamic materials. Chem. Soc. Rev. 2014, 43, 148–184. [Google Scholar] [CrossRef] [PubMed]
- Kortekaas, L.; Browne, W.R. The evolution of spiropyran: Fundamentals and progress of an extraordinarily versatile photochrome. Chem. Soc. Rev. 2019, 48, 3406–3424. [Google Scholar] [CrossRef] [PubMed]
- Guglielmetti, R.J. Photochromism: Molecules and Systems; Durr, H., Bouas-Laurent, H., Eds.; Elsevier: Amsterdam, The Netherlands, 1990; pp. 314–466. [Google Scholar]
- Hammarson, M.; Andersson, J.; Li, S.; Lincoln, P.; Andréasson, J. Molecular AND-logic for dually controlled activation of a DNA-binding spiropyran. Chem. Commun. 2010, 46, 7130–7132. [Google Scholar] [CrossRef] [PubMed]
- Khuzin, A.A.; Galimov, D.I.; Tulyabaev, A.R.; Khuzina, L.L. Synthesis, Photochromic and Luminescent Properties of Ammonium Salts of Spiropyrans. Molecules 2022, 27, 8492. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).