A Computational and Spectroscopic Approach to Elucidate the Coordination Structures in Iron–Catechol Polymers †
Abstract
1. Introduction
2. Methods
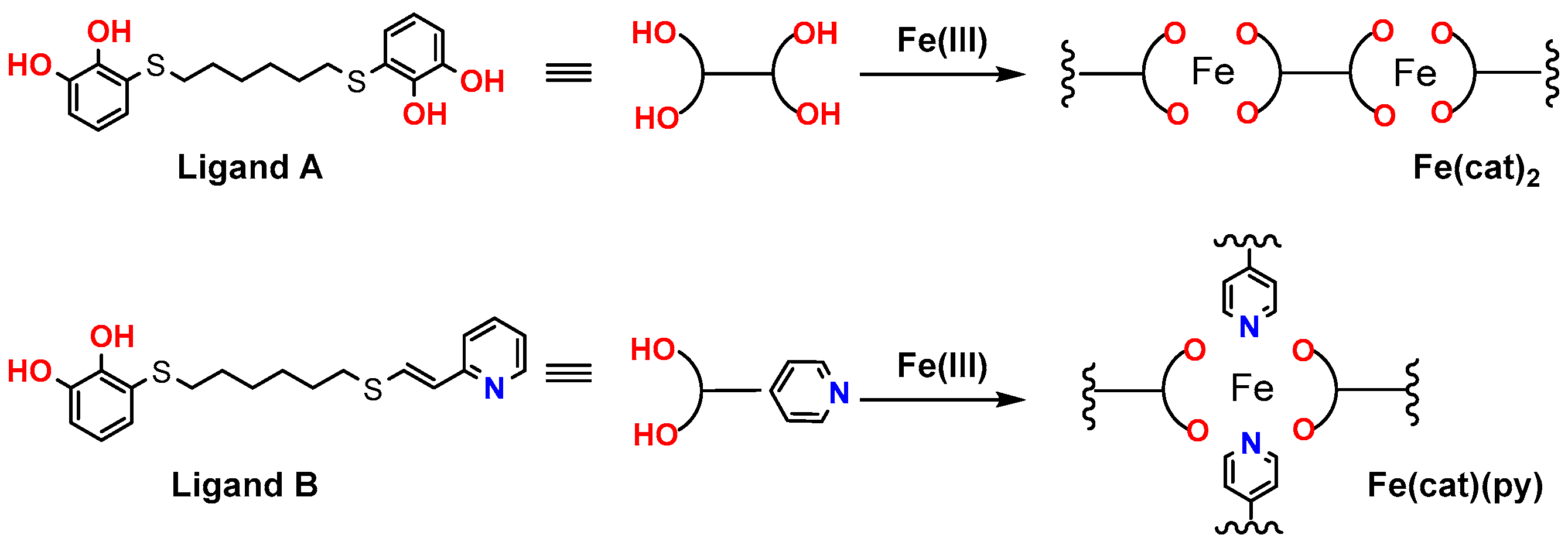
2.1. Polymer Preparation
2.2. Spectra
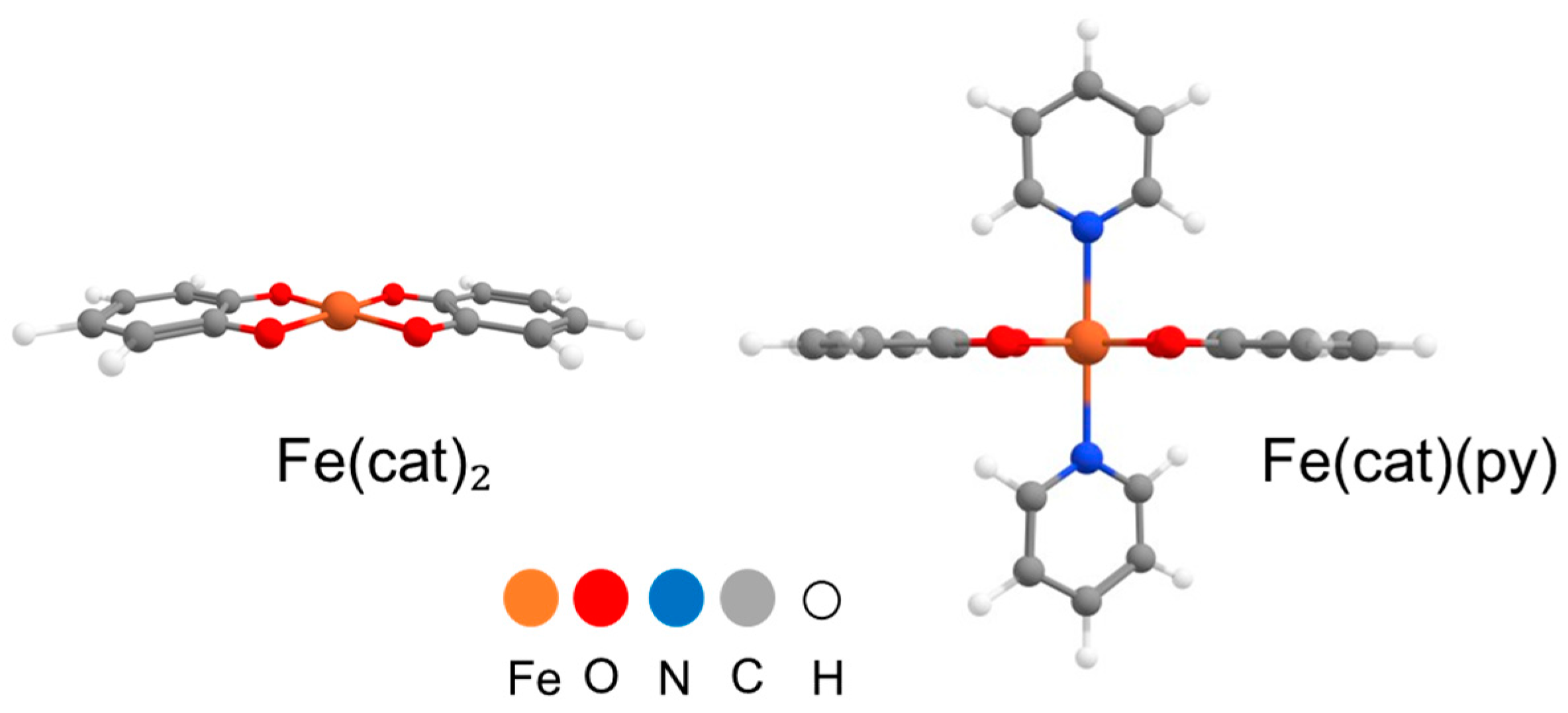
2.3. Calculations
3. Results and Discussion
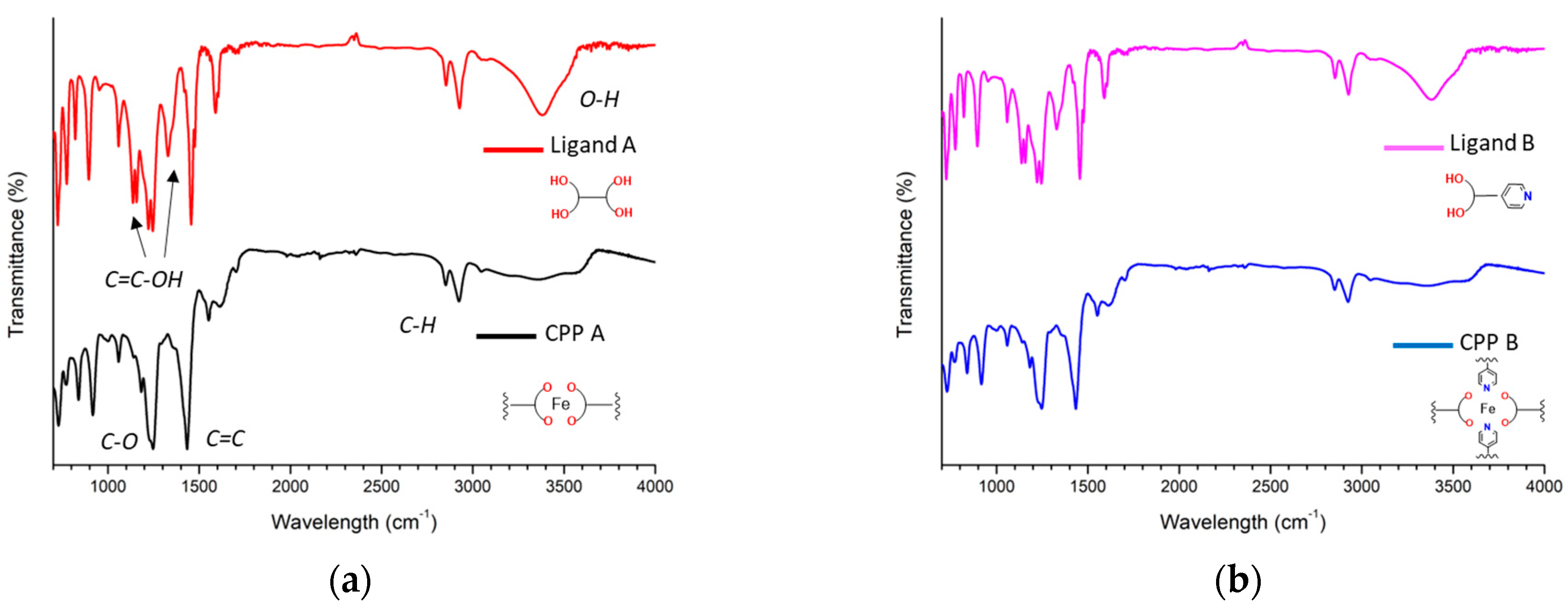
3.1. IR Spectral Assignment
- Region from 4000 to 2000 cm−1
- Region from 2000 to 1000 cm−1
- Region from 1000 to 700 cm−1
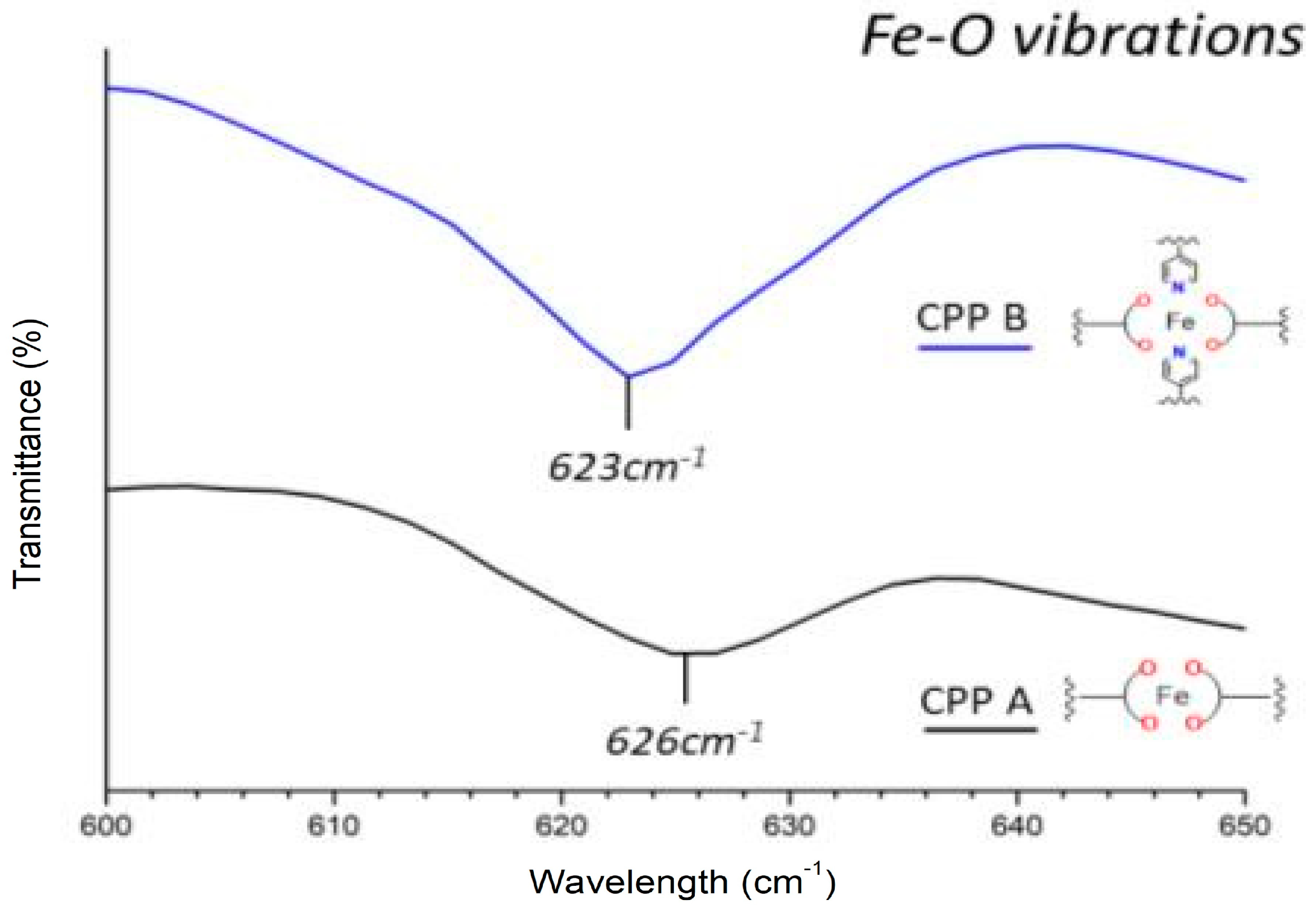
- Region from 700 to 400 cm−1
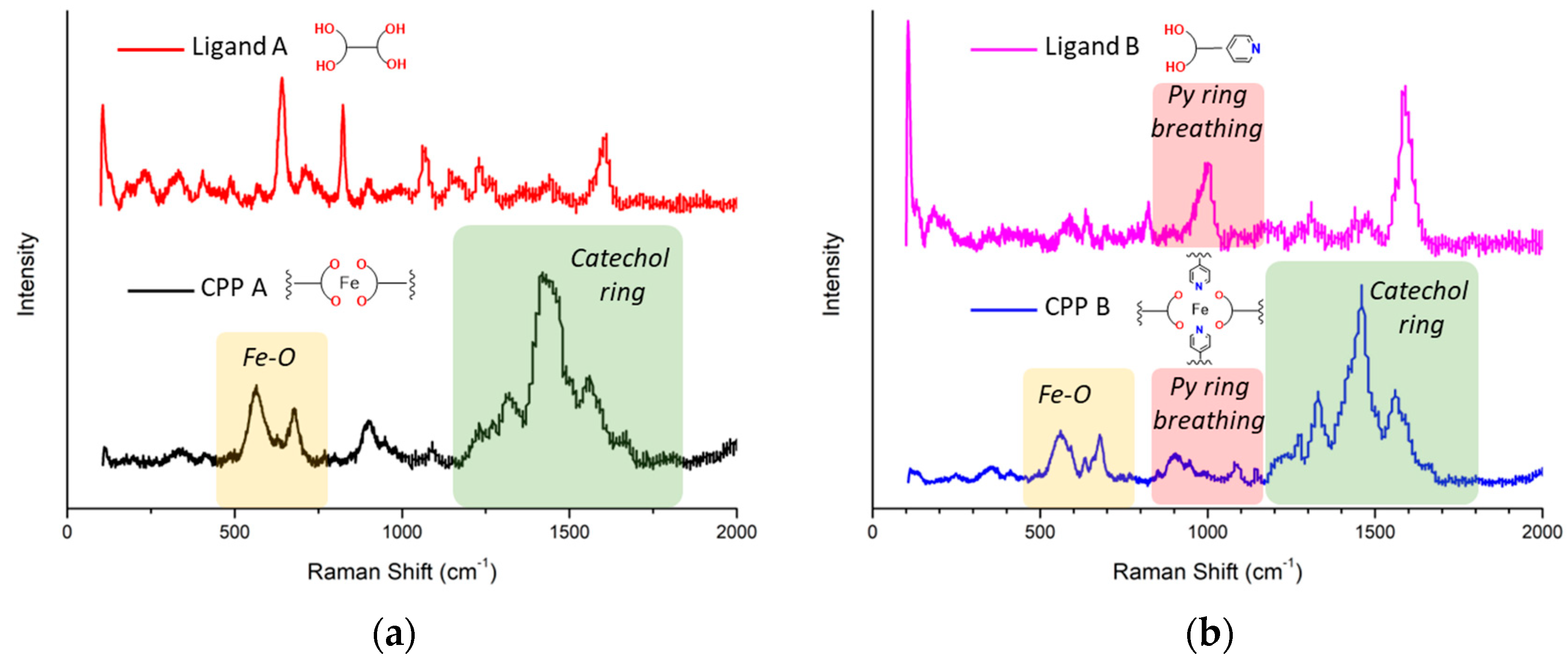
3.2. Raman Spectral Assignment
- Region from 2000 to 1200 cm−1
- Region from 1200 to 800 cm−1
- Region from 800 to 400 cm−1
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, W.; Wang, R.; Sun, Z.; Zhu, X.; Zhao, Q.; Zhang, T.; Cholewinski, A.; Yang, F.; Zhao, B.; Pinnaratip, R.; et al. Catechol Functionalized Hydrogels: Biomimetic Design, Adhesion Mechanism, and Biomedical Applications. Chem. Soc. Rev. 2020, 49, 433–464. [Google Scholar] [CrossRef] [PubMed]
- Saiz-Poseu, J.; Mancebo-Aracil, J.; Nador, F.; Busqué, F.; Ruiz-Molina, D. The Chemistry behind Catechol-Based Adhesion. Angew. Chem. Int. Ed. 2019, 58, 696–714. [Google Scholar] [CrossRef] [PubMed]
- Suárez-García, S.; Solórzano, R.; Novio, F.; Alibés, R.; Busqué, F.; Ruiz-Molina, D. Coordination Polymers Nanoparticles for Bioimaging. Coord. Chem. Rev. 2021, 432, 213716. [Google Scholar] [CrossRef]
- Nador, F.; Mancebo-Aracil, J. Thiol-yne click reaction: An interesting way to derive thiol-provided catechols. RSC Adv. 2021, 11, 2074–2082. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Perdew, J. Density-functional approximation for the correlation energy of the inhomogeneous electron gas. Phys. Rev. B 1986, 33, 8822–8824. [Google Scholar] [CrossRef] [PubMed]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A Consistent and Accurate Ab Initio Parametrization of Density Functional Dispersion Correction (DFT-D) for the 94 Elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Neese, F. Accurate Modeling of Spin-State Energetics in Spin-Crossover Systems with Modern Density Functional Theory. Inorg. Chem. 2010, 49, 772–774. [Google Scholar] [CrossRef] [PubMed]
- Becke, D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785. [Google Scholar] [CrossRef] [PubMed]
| Vibrations | υ =C-H | υ C-H * | υ C=C | υ C-O | Py Ring breathing | γ C-H | γ C-H | δ C-H * | Fe-O |
|---|---|---|---|---|---|---|---|---|---|
| Fe(cat)2 [DFT] | 3101 | - | 1485 | 1254 | - | 956 | 871 | - | 608 |
| CPP A [Exp] | 2930 | 2855 | 1440 | 1250 | - | 920 | 840 | 730 | 626 |
| Fe(cat)(py) [DFT] | 3095 | - | 1475 | 1267 | 1042 | 945 | 869 | - | 643 |
| CPP B [Exp] | 2930 | 2855 | 1435 | 1250 | 1000 | 920 | 840 | 730 | 623 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capurso, M.; Radivoy, G.; Nador, F.; Dorn, V. A Computational and Spectroscopic Approach to Elucidate the Coordination Structures in Iron–Catechol Polymers. Chem. Proc. 2024, 16, 7. https://doi.org/10.3390/ecsoc-28-20208
Capurso M, Radivoy G, Nador F, Dorn V. A Computational and Spectroscopic Approach to Elucidate the Coordination Structures in Iron–Catechol Polymers. Chemistry Proceedings. 2024; 16(1):7. https://doi.org/10.3390/ecsoc-28-20208
Chicago/Turabian StyleCapurso, Matías, Gabriel Radivoy, Fabiana Nador, and Viviana Dorn. 2024. "A Computational and Spectroscopic Approach to Elucidate the Coordination Structures in Iron–Catechol Polymers" Chemistry Proceedings 16, no. 1: 7. https://doi.org/10.3390/ecsoc-28-20208
APA StyleCapurso, M., Radivoy, G., Nador, F., & Dorn, V. (2024). A Computational and Spectroscopic Approach to Elucidate the Coordination Structures in Iron–Catechol Polymers. Chemistry Proceedings, 16(1), 7. https://doi.org/10.3390/ecsoc-28-20208






