Advances in Visual Immunoassays for Sensitive Detection of Mycotoxins in Food—A Review †
Abstract
:1. Introduction
2. The Signal Amplified Strategies
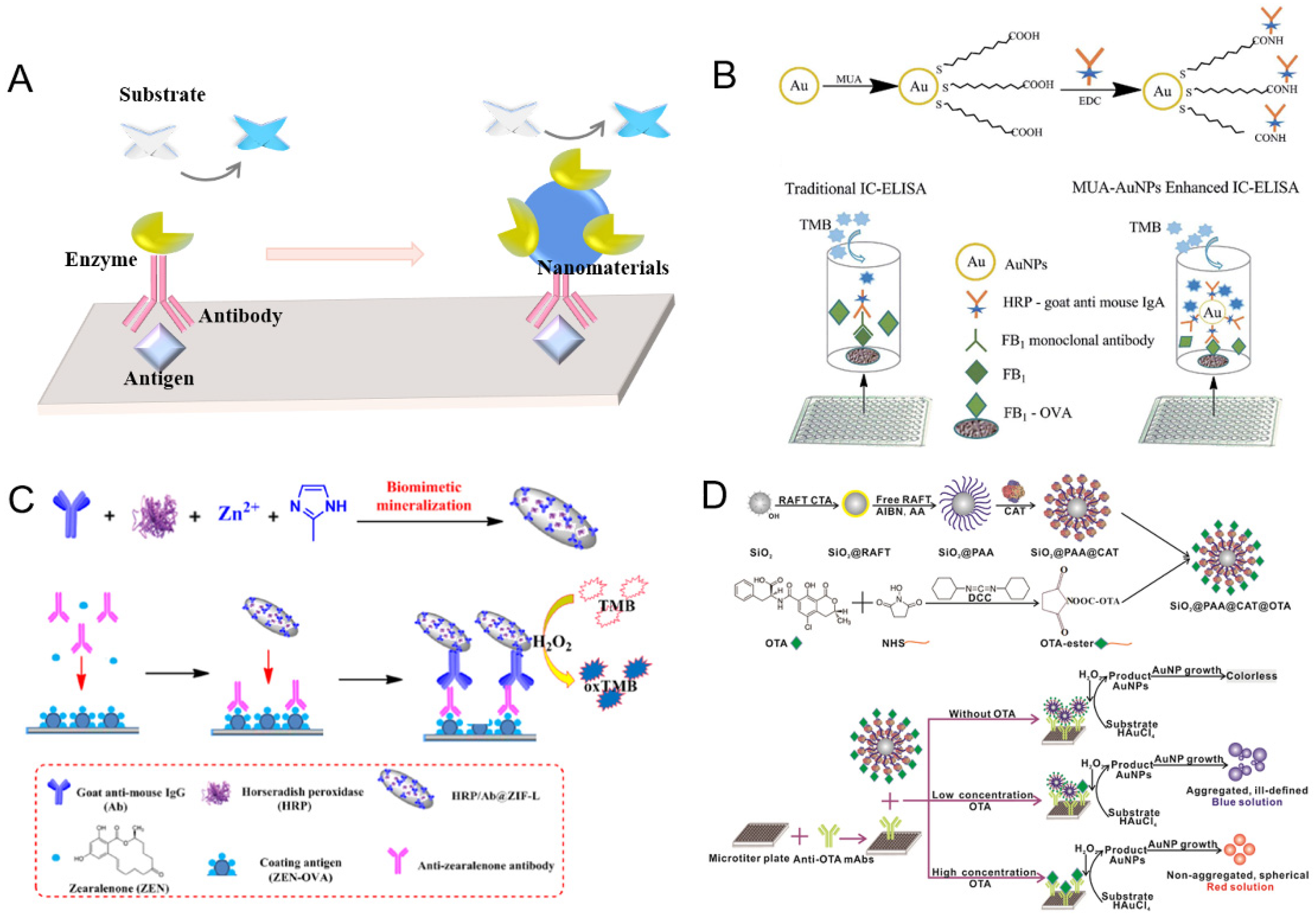
2.1. Immobilized Natural Enzymes on Nanomaterials for Amplification
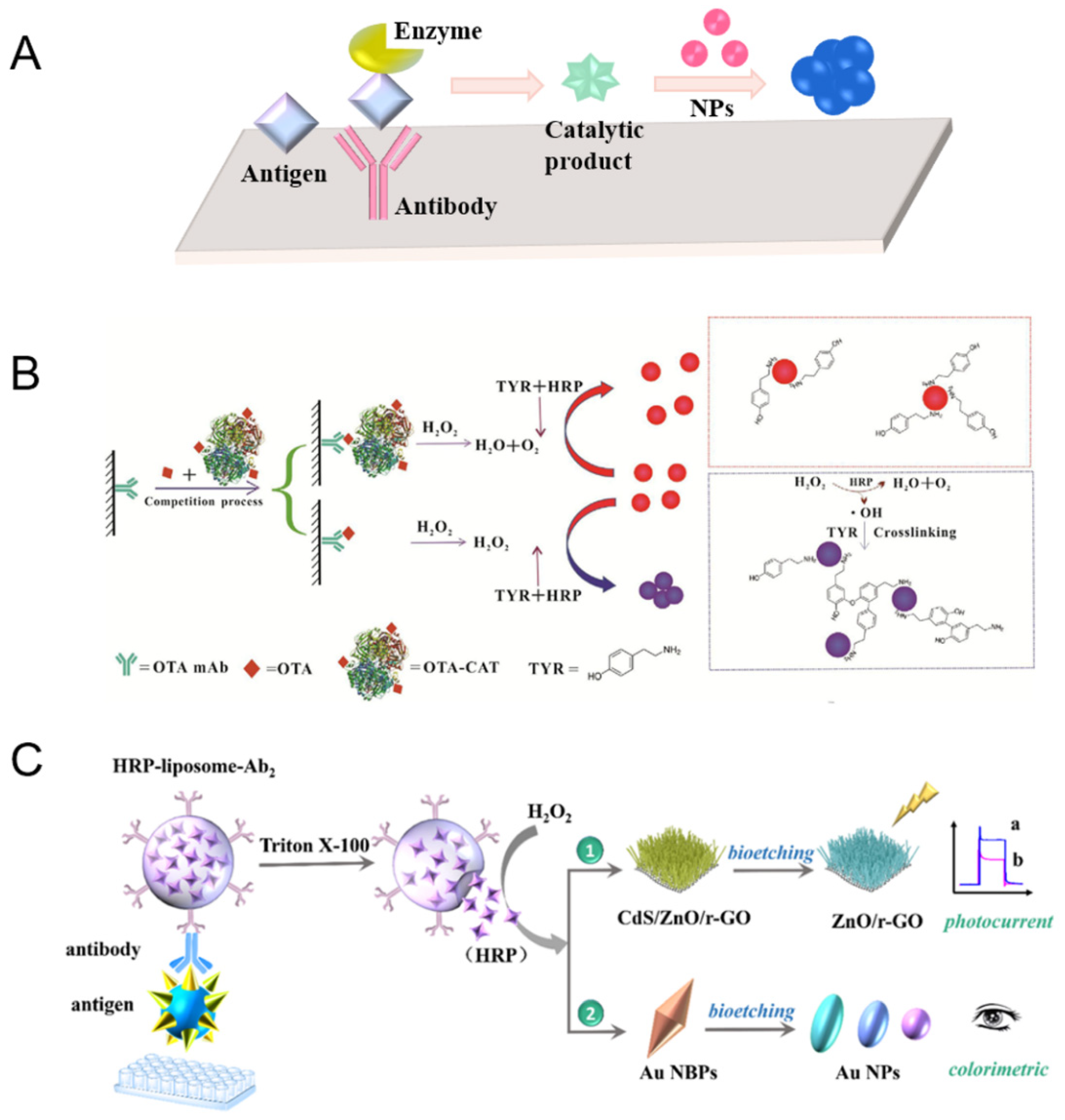
2.2. Natural Enzyme-Mediated Nanomaterials for Amplified Signal Readout
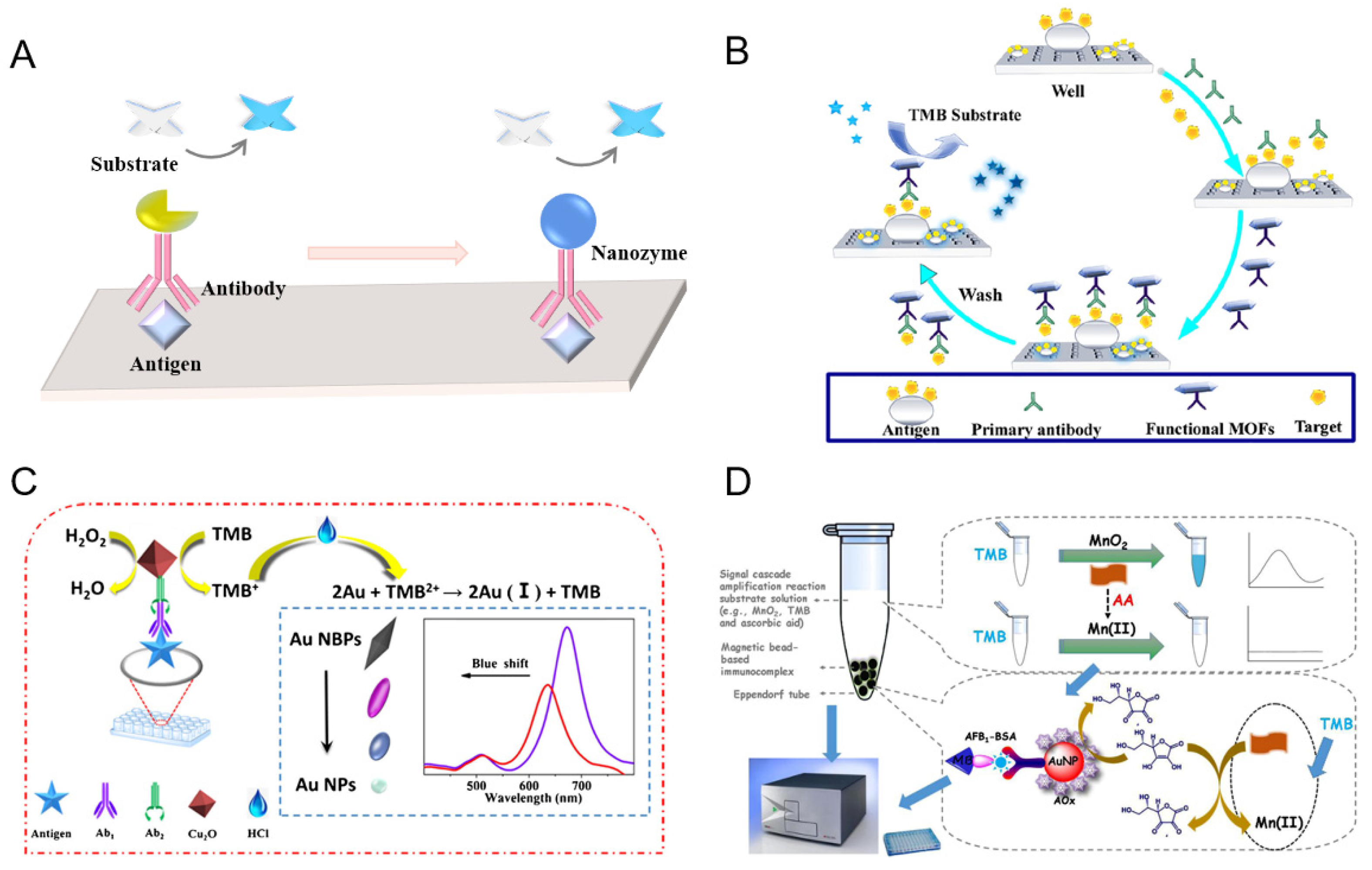
2.3. Nanozyme for Signal Amplification
3. Conclusions and Outlook
Author Contributions
Funding
Conflicts of Interest
References
- Alshannaq, A.; Yu, J.-H. Occurrence, toxicity, and analysis of major mycotoxins in food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sweeney, M.J.; Dobson, A.D.W. Mycotoxin production by Aspergillus, Fusarium and Penicillium species. Int. J. Food Microbiol. 1998, 43, 141–158. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, Q.; Han, M.; Zhou, J.; Gong, L.; Niu, Y.; Zhang, Y.; He, L.; Zhang, L. Development and optimization of a multiplex lateral flow immunoassay for the simultaneous determination of three mycotoxins in corn, rice and peanut. Food Chem. 2016, 213, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Liu, X.; Li, J. Updating techniques on controlling mycotoxins—A review. Food Control 2018, 89, 123–132. [Google Scholar] [CrossRef]
- Yang, Y.; Li, G.; Wu, D.; Liu, J.; Li, X.; Luo, P.; Hu, N.; Wang, H.; Wu, Y. Recent advances on toxicity and determination methods of mycotoxins in foodstuffs. Trends Food Sci. Technol. 2020, 96, 233–252. [Google Scholar] [CrossRef]
- Rossi, C.N.; Takabayashi, C.R.; Ono, M.A.; Saito, G.H.; Itano, E.N.; Kawamura, O.; Hirooka, E.Y.; Ono, E.Y.S. Immunoassay based on monoclonal antibody for aflatoxin detection in poultry feed. Food Chem. 2012, 132, 2211–2216. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Xu, L.; Kuang, H.; Xiao, J.; Xu, C. Immunoassays for rapid mycotoxin detection: State of the art. Analyst 2020, 145, 7088–7102. [Google Scholar] [CrossRef]
- Singh, J.; Mehta, A. Rapid and sensitive detection of mycotoxins by advanced and emerging analytical methods: A review. Food Sci. Nutr. 2020, 8, 2183–2204. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Ma, F.; Zhang, L.; Li, P. Determination of aflatoxin B-1 and B-2 in vegetable oils using Fe3O4/rGO magnetic solid phase extraction coupled with high-performance liquid chromatography fluorescence with post-column photochemical derivatization. Toxins 2019, 11, 621. [Google Scholar] [CrossRef] [Green Version]
- Andrade, P.D.; Dantas, R.R.; Moura-Alves, T.L.d.S.d.; Caldas, E.D. Determination of multi-mycotoxins in cereals and of total fumonisins in maize products using isotope labeled internal standard and liquid chromatography/tandem mass spectrometry with positive ionization. J. Chromatogr. A 2017, 1490, 138–147. [Google Scholar] [CrossRef]
- Zhang, Y.; Pei, F.; Fang, Y.; Li, P.; Zhao, Y.; Shen, F.; Zou, Y.; Hu, Q. Comparison of concentration and health risks of 9 Fusarium mycotoxins in commercial whole wheat flour and refined wheat flour by multi-IAC-HPLC. Food Chem. 2019, 275, 763–769. [Google Scholar] [CrossRef] [PubMed]
- Medina, D.A.V.; Borsatto, J.V.B.; Maciel, E.V.S.; Lancas, F.M. Current role of modern chromatography and mass spectrometry in the analysis of mycotoxins in food. TrAC Trends Anal. Chem. 2021, 135, 116156. [Google Scholar] [CrossRef]
- Alsharif, A.M.A.; Choo, Y.-M.; Tan, G.-H. Detection of five mycotoxins in different food matrices in the Malaysian market by using validated liquid chromatography electrospray ionization triple quadrupole mass spectrometry. Toxins 2019, 11, 196. [Google Scholar] [CrossRef] [Green Version]
- Yan, T.T.; Zhang, Z.W.; Zhang, Q.; Tang, X.Q.; Wang, D.; Hu, X.F.; Zhang, W.; Chen, X.M.; Li, P.W. Simultaneous determination for A. flavus-metabolizing mycotoxins by time-resolved fluorescent microbead or gold-enabling test strip in agricultural products based on monoclonal antibodies. Microchim. Acta 2020, 187, 653. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.L.; Zhang, Q.; Liu, X.L.; Yang, Y.Y.; Yang, Y.; Liu, M.Y.; Li, P.W.; Zhou, Y. Antibody-biotin-streptavidin-horseradish peroxidase (HRP) sensor for rapid and ultra-sensitive detection of fumonisins. Food Chem. 2020, 316, 126356. [Google Scholar] [CrossRef]
- Beloglazova, N.V.; Graniczkowska, K.; Ediage, E.N.; Averkieva, O.; De Saeger, S. Sensitive flow-through immunoassay for rapid multiplex determination of cereal-borne mycotoxins in feed and feed ingredients. J. Agric. Food Chem. 2017, 65, 7131–7137. [Google Scholar] [CrossRef]
- He, Q.-H.; Xu, Y.; Wang, D.; Kang, M.; Huang, Z.-B.; Li, Y.-P. Simultaneous multiresidue determination of mycotoxins in cereal samples by polyvinylidene fluoride membrane based dot immunoassay. Food Chem. 2012, 134, 507–512. [Google Scholar] [CrossRef]
- Li, M.; Wang, H.M.; Sun, J.D.; Ji, J.; Ye, Y.L.; Lu, X.; Zhang, Y.Z.; Sun, X.L. Rapid, on-site, and sensitive detection of aflatoxin M1 in milk products by using time-resolved fluorescence microsphere test strip. Food Control 2021, 121, 107616. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, B.; Chen, E.J.; Yu, X.P.; Ye, Z.H.; Sun, C.X.; Zhang, M.Z. Dual fluorescent immunochromatographic assay for simultaneous quantitative detection of citrinin and zearalenone in corn samples. Food Chem. 2021, 336, 127713. [Google Scholar] [CrossRef]
- Li, H.; Wang, D.; Tang, X.; Zhang, W.; Zhang, Q.; Li, P. Time-resolved fluorescence immunochromatography assay (TRFICA) for aflatoxin: Aiming at increasing strip method sensitivity. Front. Microbiol. 2020, 11, 676. [Google Scholar] [CrossRef]
- Kaminiaris, M.D.; Mavrikou, S.; Georgiadou, M.; Paivana, G.; Tsitsigiannis, D.I.; Kintzios, S. An impedance based electrochemical immunosensor for aflatoxin B-1 monitoring in pistachio matrices. Chemosensors 2020, 8, 121. [Google Scholar] [CrossRef]
- Kudr, J.; Zhao, L.; Nguyen, E.P.; Arola, H.; Nevanen, T.K.; Adam, V.; Zitka, O.; Merkoci, A. Inkjet-printed electrochemically reduced graphene oxide microelectrode as a platform for HT-2 mycotoxin immunoenzymatic biosensing. Biosens. Bioelectron. 2020, 156, 112109. [Google Scholar] [CrossRef] [PubMed]
- Kunene, K.; Weber, M.; Sabela, M.; Voiry, D.; Kanchi, S.; Bisetty, K.; Bechelany, M. Highly-efficient electrochemical label-free immunosensor for the detection of ochratoxin A in coffee samples. Sens. Actuators B Chem. 2020, 305, 127438. [Google Scholar] [CrossRef]
- Hou, S.; Ma, Z.; Meng, H.; Xu, Y.; He, Q. Ultrasensitive and green electrochemical immunosensor for mycotoxin ochratoxin A based on phage displayed mimotope peptide. Talanta 2019, 194, 919–924. [Google Scholar] [CrossRef]
- Quan, Y.; Zhang, Y.; Wang, S.; Lee, N.; Kennedy, I.R. A rapid and sensitive chemiluminescence enzyme-linked immunosorbent assay for the determination of fumonisin B1 in food samples. Anal. Chim. Acta 2006, 580, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Lu, P.; Feng, R.; Zhang, T.; Zhang, Y.; Wu, D.; Wei, Q. An ITO-based point-of-care colorimetric immunosensor for ochratoxin A detection. Talanta 2018, 188, 593–599. [Google Scholar] [CrossRef]
- Bazin, I.; Nabais, E.; Lopez-Ferber, M. Rapid visual tests: Fast and reliable detection of ochratoxin A. Toxins 2010, 2, 2230–2241. [Google Scholar] [CrossRef]
- Garden, S.R.; Strachan, N.J.C. Novel colorimetric immunoassay for the detection of aflatoxin B1. Anal. Chim. Acta 2001, 444, 187–191. [Google Scholar] [CrossRef]
- Yu, Z.; Cai, G.; Liu, X.; Tang, D. Pressure-based biosensor integrated with a flexible pressure sensor and an electrochromic device for visual detection. Anal. Chem. 2021, 93, 2916–2925. [Google Scholar] [CrossRef]
- Wang, L.; He, K.; Wang, X.; Wang, Q.; Quan, H.; Wang, P.; Xu, X. Recent progress in visual methods for aflatoxin detection. Crit. Rev. Food Sci. Nutr. 2021. [Google Scholar] [CrossRef]
- Majdinasab, M.; Ben Aissa, S.; Marty, J.L. Advances in colorimetric strategies for mycotoxins detection: Toward rapid industrial monitoring. Toxins 2021, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.-H.; Chu, K.C.; Yu, F.-Y. Novel monoclonal antibody-based sensitive enzyme-linked immunosorbent assay and rapid immunochromatographic strip for detecting aflatoxin M1 in milk. Food Control 2016, 66, 1–7. [Google Scholar] [CrossRef]
- Wang, Y.; Li, P.; Zhang, Q.; Hu, X.; Zhang, W. A toxin-free enzyme-linked immunosorbent assay for the analysis of aflatoxins based on a VHH surrogate standard. Anal. Bioanal. Chem. 2016, 408, 6019–6026. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Jin, G.; Liu, L.; Xiao, J.; Kuang, H. Fast determination of citreoviridin residues in rice using a monoclonal antibody-based immunochromatographic strip assay. Food Agric. Immunol. 2020, 31, 893–906. [Google Scholar] [CrossRef]
- Badea, M.; Micheli, L.; Messia, M.C.; Candigliota, T.; Marconi, E.; Mottram, T.; Velasco-Garcia, M.; Moscone, D.; Palleschi, G. Aflatoxin M1 determination in raw milk using a flow-injection immunoassay system. Anal. Chim. Acta 2004, 520, 141–148. [Google Scholar] [CrossRef]
- Qu, J.W.; Xie, H.J.; Zhang, S.Y.; Luo, P.J.; Guo, P.; Chen, X.X.; Ke, Y.B.; Zhuang, J.Y.; Zhou, F.M.; Jiang, W.X. Multiplex flow cytometric immunoassays for high-throughput screening of multiple mycotoxin residues in milk. Food Anal. Methods 2019, 12, 877–886. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, N.; Wang, H.; Zhao, Q. An immunoassay for ochratoxin a using tetramethylrhodamine-labeled ochratoxin a as a probe based on a binding-induced change in fluorescence intensity. Analyst 2020, 145, 651–655. [Google Scholar] [CrossRef]
- Tian, Y.; Bu, T.; Zhang, M.; Sun, X.; Jia, P.; Wang, Q.; Liu, Y.; Bai, F.; Zhao, S.; Wang, L. Metal-polydopamine framework based lateral flow assay for high sensitive detection of tetracycline in food samples. Food Chem. 2021, 339, 127854. [Google Scholar] [CrossRef]
- Tan, X.; Wang, X.; Zhang, L.; Liu, L.; Zheng, G.; Li, H.; Zhou, F. Stable and photothermally efficient antibody-covered Cu3(PO4)2@polydopamine nanocomposites for sensitive and cost-effective immunoassays. Anal. Chem. 2019, 91, 8274–8279. [Google Scholar] [CrossRef]
- Cheng, N.; Song, Y.; Zeinhom, M.M.A.; Chang, Y.-C.; Sheng, L.; Li, H.; Du, D.; Li, L.; Zhu, M.-J.; Luo, Y.; et al. Nanozyme-mediated dual immunoassay integrated with smartphone for use in simultaneous detection of pathogens. ACS Appl. Mater. Interfaces 2017, 9, 40671–40680. [Google Scholar] [CrossRef]
- Yao, X.; Wang, Z.; Zhao, M.; Liu, S.; Su, L.; Dou, L.; Li, T.; Wang, J.; Zhang, D. Graphite-like carbon nitride-laden gold nanoparticles as signal amplification label for highly sensitive lateral flow immunoassay of 17β-estradiol. Food Chem. 2021, 347, 129001. [Google Scholar] [CrossRef]
- Oh, S.; Kim, J.; Tran, V.T.; Lee, D.K.; Ahmed, S.R.; Hong, J.C.; Lee, J.; Park, E.Y.; Lee, J. Magnetic nanozyme-linked immunosorbent assay for ultrasensitive influenza a virus detection. ACS Appl. Mater. Interfaces 2018, 10, 12534–12543. [Google Scholar] [CrossRef]
- Zhang, X.; Song, M.; Yu, X.; Wang, Z.; Ke, Y.; Jiang, H.; Li, J.; Shen, J.; Wen, K. Development of a new broad-specific monoclonal antibody with uniform affinity for aflatoxins and magnetic beads-based enzymatic immunoassay. Food Control 2017, 79, 309–316. [Google Scholar] [CrossRef]
- Lai, W.; Wei, Q.; Zhuang, J.; Lu, M.; Tang, D. Fenton reaction-based colorimetric immunoassay for sensitive detection of brevetoxin B. Biosens. Bioelectron. 2016, 80, 249–256. [Google Scholar] [CrossRef] [Green Version]
- Yan, C.; Wang, Q.; Yang, Q.; Wu, W. Recent advances in aflatoxins detection based on nanomaterials. Nanomaterials 2020, 10, 1626. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, A.; Airo, F.; Merkoci, A. Enhanced gold nanoparticle based ELISA for a breast cancer biomarker. Anal. Chem. 2010, 82, 1151–1156. [Google Scholar] [CrossRef]
- Zhou, Y.; Tian, X.L.; Li, Y.S.; Pan, F.G.; Zhang, Y.Y.; Zhang, J.H.; Yang, L.; Wang, X.R.; Ren, H.L.; Lu, S.Y.; et al. An enhanced ELISA based on modified colloidal gold nanoparticles for the detection of Pb(II). Biosens. Bioelectron. 2011, 26, 3700–3704. [Google Scholar] [CrossRef] [PubMed]
- Zha, Y.H.; Zhou, Y. Functional nanomaterials based immunological detection of aflatoxin B-1: A review. World Mycotoxin J. 2020, 13, 151–162. [Google Scholar] [CrossRef]
- Liang, M.; Yan, X. Nanozymes: From new concepts, mechanisms, and standards to applications. Acc. Chem. Res. 2019, 52, 2190–2200. [Google Scholar] [CrossRef]
- Huang, Y.; Ren, J.; Qu, X. Nanozymes: Classification, catalytic mechanisms, activity regulation, and applications. Chem. Rev. 2019, 119, 4357–4412. [Google Scholar] [CrossRef]
- Wu, L.; Li, G.; Xu, X.; Zhu, L.; Huang, R.; Chen, X. Application of nano-ELISA in food analysis: Recent advances and challenges. TrAC Trends Anal. Chem. 2019, 113, 140–156. [Google Scholar] [CrossRef]
- Gao, Y.; Zhou, Y.; Chandrawati, R. Metal and metal oxide nanoparticles to enhance the performance of enzyme-linked immunosorbent assay (ELISA). ACS Appl. Nano Mater. 2020, 3, 1–21. [Google Scholar] [CrossRef]
- Heussner, A.H.; Ausländer, S.; Dietrich, D.R. Development and characterization of a monoclonal antibody against ochratoxin b and its application in ELISA. Toxins 2010, 2, 1582–1594. [Google Scholar] [CrossRef]
- Huang, X.; Liu, Y.; Yung, B.; Xiong, Y.; Chen, X. Nanotechnology-enhanced no-wash biosensors for in vitro diagnostics of cancer. ACS Nano 2017, 11, 5238–5292. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhang, Q.; Zhang, W.; Zhang, J.; Chen, X.; Jiang, J.; Xie, L.; Zhang, D. Development of a class-specific monoclonal antibody-based ELISA for aflatoxins in peanut. Food Chem. 2009, 115, 313–317. [Google Scholar] [CrossRef]
- He, T.; Wang, Y.; Li, P.; Zhang, Q.; Lei, J.; Zhang, Z.; Ding, X.; Zhou, H.; Zhang, W. Nanobody-based enzyme immunoassay for aflatoxin in agro-products with high tolerance to cosolvent methanol. Anal. Chem. 2014, 86, 8873–8880. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, J.; Li, F.; Li, J.; Shen, Z. A Calibration curve implanted enzyme-linked immunosorbent assay for simultaneously quantitative determination of multiplex mycotoxins in cereal samples, Soybean and Peanut. Toxins 2020, 12, 718. [Google Scholar] [CrossRef] [PubMed]
- Guan, D.; Li, P.; Zhang, Q.; Zhang, W.; Zhang, D.; Jiang, J. An ultra-sensitive monoclonal antibody-based competitive enzyme immunoassay for aflatoxin M-1 in milk and infant milk products. Food Chem. 2011, 125, 1359–1364. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, Z.; Noelke, G.; Zhang, J.; Niu, L.; Shen, J. Simultaneous determination of aflatoxin b-1 and aflatoxin m-1 in food matrices by enzyme-linked immunosorbent assay. Food Anal. Methods 2013, 6, 767–774. [Google Scholar] [CrossRef]
- Guesdon, J.-L. Immunoenzymatic techniques applied to the specific detection of nucleic acids: A review. J. Immunol. Methods 1992, 150, 33–49. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, X.; Chen, Q.; Yun, Y.; Tang, Z.; Liu, X. Nanobody-alkaline phosphatase fusion protein-based enzyme-linked immunosorbent assay for one-step detection of ochratoxin A in rice. Sensors 2018, 18, 4044. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.; Wang, X.; Lv, J.; Hu, X.; Liu, X. One-step detection of ochratoxin A in cereal by dot immunoassay using a nanobody-alkaline phosphatase fusion protein. Food Control 2018, 92, 430–436. [Google Scholar] [CrossRef]
- Shu, M.; Xu, Y.; Liu, X.; Li, Y.; He, Q.; Tu, Z.; Fu, J.; Gee, S.J.; Hammock, B.D. Anti-idiotypic nanobody-alkaline phosphatase fusion proteins: Development of a one-step competitive enzyme immunoassay for fumonisin B1 detection in cereal. Anal. Chim. Acta 2016, 924, 53–59. [Google Scholar] [CrossRef]
- Yan, J.; Wang, J.; Zhao, M.P.; Chang, W.B. Determination of papaverine by biotin-avidin amplified ELISA. Anal. Lett. 2004, 37, 2977–2989. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, D.; Zhou, X.; Yu, Y.; Liu, J.; Hu, N.; Wang, H.; Li, G.; Wu, Y. Recent progress in the construction of nanozyme-based biosensors and their applications to food safety assay. TrAC Trends Anal. Chem. 2019, 121, 115668. [Google Scholar] [CrossRef]
- Wang, Q.Q.; Wei, H.; Zhang, Z.Q.; Wang, E.K.; Dong, S.J. Nanozyme: An emerging alternative to natural enzyme for biosensing and immunoassay. TrAC Trends Anal. Chem. 2018, 105, 218–224. [Google Scholar] [CrossRef]
- Niu, X.; Cheng, N.; Ruan, X.; Du, D.; Lin, Y. Review-nanozyme-based immunosensors and immunoassays: Recent developments and future trends. J. Electrochem. Soc. 2020, 167, 037508. [Google Scholar] [CrossRef]
- Xiong, Y.; Leng, Y.; Li, X.; Huang, X.; Xiong, Y. Emerging strategies to enhance the sensitivity of competitive ELISA for detection of chemical contaminants in food samples. TrAC Trends Anal. Chem. 2020, 126, 115861. [Google Scholar] [CrossRef]
- Lei, C.; Xu, C.; Nouwens, A.; Yu, C. Ultrasensitive ELISA(+) enhanced by dendritic mesoporous silica nanoparticles. J. Mater. Chem. B 2016, 4, 4975–4979. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, C.-L.; Xie, Z.-J.; Liu, L.-Q.; Peng, C.-F.; Xue, F. Botryoid-shaped nanoparticles-enhanced ELISA for ochratoxin A. Food Agric. Immunol. 2017, 28, 299–309. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Sheng, W.; Liu, Q.; Li, S.; Shi, Y.; Zhang, Y.; Wang, S. Development of a gold nanoparticle enhanced enzyme linked immunosorbent assay based on monoclonal antibodies for the detection of fumonisin B-1, B-2, and B-3 in maize. Anal. Methods 2018, 10, 3506–3513. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, X.; Dong, F.; Li, Y.; Guo, Y.; Liu, X.; Xu, J.; Wu, X.; Zheng, Y. Ultrasensitive immunoassay for detection of zearalenone in agro-products using enzyme and antibody co-embedded zeolitic imidazolate framework as labels. J. Hazard. Mater. 2021, 412, 125276. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Chen, R.; Xu, H.; Lai, W.; Xiong, Y. Nanospherical brush as catalase container for enhancing the detection sensitivity of competitive plasmonic ELISA. Anal. Chem. 2016, 88, 1951–1958. [Google Scholar] [CrossRef]
- Liang, Y.; Huang, X.; Chen, X.; Zhang, W.; Ping, G.; Xiong, Y. Plasmonic ELISA for naked-eye detection of ochratoxin A based on the tyramine-H2O2 amplification system. Sens. Actuators B Chem. 2018, 259, 162–169. [Google Scholar] [CrossRef]
- Zhan, S.; Zheng, L.; Zhou, Y.; Wu, K.; Duan, H.; Huang, X.; Xiong, Y. A gold growth-based plasmonic ELISA for the sensitive detection of Fumonisin B1 in maize. Toxins 2019, 11, 323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, Y.; Pei, K.; Wu, Y.; Duan, H.; Lai, W.; Xiong, Y. Plasmonic ELISA based on enzyme-assisted etching of Au nanorods for the highly sensitive detection of aflatoxin B-1 in corn samples. Sens. Actuators B Chem. 2018, 267, 320–327. [Google Scholar] [CrossRef]
- Wang, M.; Zhou, X.; Wang, S.; Xie, X.; Wang, Y.; Su, X. Fabrication of bioresource-derived porous carbon-supported iron as an efficient oxidase mimic for dual-channel biosensing. Anal. Chem. 2021, 93, 3130–3137. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ge, J.; Chen, H.; Wang, Z.; Han, J.; Xie, G.; Chen, S. Dual-signal readout aptasensor for electrochemical and colorimetric assay using a bifunctional Ni-Fe PBA probe. Sens. Actuators B Chem. 2021, 327, 128871. [Google Scholar] [CrossRef]
- Chang, J.; Lv, W.; Li, Q.; Li, H.; Li, F. One-step synthesis of methylene blue-encapsulated zeolitic imidazolate framework for dual-signal fluorescent and homogeneous electrochemical biosensing. Anal. Chem. 2020, 92, 8959–8964. [Google Scholar] [CrossRef]
- Wei, J.; Chen, H.; Chen, H.; Cui, Y.; Qileng, A.; Qin, W.; Liu, W.; Liu, Y. Multifunctional peroxidase-encapsulated nanoliposomes: Bioetching-induced photoelectrometric and colorimetric immunoassay for broad-spectrum detection of Ochratoxins. ACS Appl. Mater. Interfaces 2019, 11, 23832–23839. [Google Scholar] [CrossRef]
- Payal, A.; Krishnamoorthy, S.; Elumalai, A.; Moses, J.A.; Anandharamakrishnan, C. A Review on recent developments and applications of nanozymes in food safety and quality analysis. Food Anal. Methods 2021, 14, 1537–1558. [Google Scholar] [CrossRef]
- Jia, M.; Liao, X.; Fang, L.; Jia, B.; Liu, M.; Li, D.; Zhou, L.; Kong, W. Recent advances on immunosensors for mycotoxins in foods and other commodities. TrAC Trends Anal. Chem. 2021, 136, 116193. [Google Scholar] [CrossRef]
- Xu, W.Q.; Jiao, L.; Wu, Y.; Hu, L.Y.; Gu, W.L.; Zhu, C.Z. Metal-organic frameworks enhance biomimetic cascade catalysis for biosensing. Adv. Mater. 2021, 33, 2005172. [Google Scholar] [CrossRef]
- Gao, L.Z.; Zhuang, J.; Nie, L.; Zhang, J.B.; Zhang, Y.; Gu, N.; Wang, T.H.; Feng, J.; Yang, D.L.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Zhi, L.-J.; Sun, A.-L. Platinum nanozyme-encapsulated poly(amidoamine) dendrimer for voltammetric immunoassay of pro-gastrin-releasing peptide. Anal. Chim. Acta 2020, 1134, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Yao, S.; Li, J.; Pang, B.; Wang, X.; Shi, Y.; Song, X.; Xu, K.; Wang, J.; Zhao, C. Colorimetric immunoassay for rapid detection of Staphylococcus aureus based on etching-enhanced peroxidase-like catalytic activity of gold nanoparticles. Microchim. Acta 2020, 187, 504. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Cao, Y.; Hinman, S.S.; McKeating, K.S.; Guan, Y.; Hu, X.; Cheng, Q.; Yang, Z. Efficient label-free chemiluminescent immunosensor based on dual functional cupric oxide nanorods as peroxidase mimics. Biosens. Bioelectron. 2018, 100, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Lian, J.; Liu, P.; Jin, C.; Shi, Z.; Luo, X.; Liu, Q. Perylene diimide-functionalized CeO2 nanocomposite as a peroxidase mimic for colorimetric determination of hydrogen peroxide and glutathione. Microchim. Acta 2019, 186, 332. [Google Scholar] [CrossRef]
- Wu, J.; Yang, Q.; Li, Q.; Li, H.; Li, F. Two-dimensional MnO2 nanozyme-mediated homogeneous electrochemical detection of organophosphate pesticides without the interference of H2O2 and color. Anal. Chem. 2021, 93, 4084–4091. [Google Scholar] [CrossRef]
- Ge, J.; Yu, J.-H.; Yang, H.; Yang, D.; Cai, R. Human serum albumin templated MnO2 nanosheets as an efficient biomimetic oxidase for biomolecule sensing. J. Mater. Chem. B 2020, 8, 11090–11095. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Xia, F.; Nan, Z. Fabrication of FeS2/SiO2 double mesoporous hollow spheres as an artificial peroxidase and rapid determination of H2O2 and glutathione. ACS Appl. Mater. Interfaces 2020, 12, 46539–46548. [Google Scholar] [CrossRef]
- Chen, W.; Chen, J.; Liu, A.-L.; Wang, L.-M.; Li, G.-W.; Lin, X.-H. Peroxidase-like activity of cupric oxide nanoparticle. ChemCatChem 2011, 3, 1151–1154. [Google Scholar] [CrossRef]
- Song, Y.; Qu, K.; Zhao, C.; Ren, J.; Qu, X. Graphene oxide: Intrinsic peroxidase catalytic activity and its application to glucose detection. Adv. Mater. 2010, 22, 2206–2210. [Google Scholar] [CrossRef]
- Tian, J.; Liu, Q.; Asiri, A.M.; Qusti, A.H.; Al-Youbi, A.O.; Sun, X. Ultrathin graphitic carbon nitride nanosheets: A novel peroxidase mimetic, Fe doping-mediated catalytic performance enhancement and application to rapid, highly sensitive optical detection of glucose. Nanoscale 2013, 5, 11604–11609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Lu, X.; Tang, D.; Wu, S.; Hou, X.; Liu, J.; Wu, P. Phosphorescent carbon dots for highly efficient oxygen photosensitization and as photo-oxidative nanozymes. ACS Appl. Mater. Interfaces 2018, 10, 40808–40814. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Wang, Q.; Long, Y.; Cheng, Z.; Chen, S.; Zheng, H.; Huang, Y. Carbon nanodots as peroxidase mimetics and their applications to glucose detection. Chem. Commun. 2011, 47, 6695–6697. [Google Scholar] [CrossRef]
- Xia, H.; Li, N.; Huang, W.; Song, Y.; Jiang, Y. Enzymatic cascade reactions mediated by highly efficient biomimetic quasi metal-organic frameworks. ACS Appl. Mater. Interfaces 2021, 13, 22240–22253. [Google Scholar] [CrossRef]
- Yuan, A.; Lu, Y.; Zhang, X.; Chen, Q.; Huang, Y. Two-dimensional iron MOF nanosheet as a highly efficient nanozyme for glucose biosensing. J. Mater. Chem. B 2020, 8, 9295–9303. [Google Scholar] [CrossRef]
- Guo, J.; Wu, S.; Wang, Y.; Zhao, M. A label-free fluorescence biosensor based on a bifunctional MIL-101(Fe) nanozyme for sensitive detection of choline and acetylcholine at nanomolar level. Sens. Actuators B Chem. 2020, 312, 128021. [Google Scholar] [CrossRef]
- Xu, Z.; Long, L.-l.; Chen, Y.-q.; Chen, M.-L.; Cheng, Y.-H. A nanozyme-linked immunosorbent assay based on metal-organic frameworks (MOFs) for sensitive detection of aflatoxin B-1. Food Chem. 2021, 338, 128039. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Liu, C.; Liu, X.; Quan, Z.; Liu, W.; Liu, Y. A multi-colorimetric immunosensor for visual detection of ochratoxin A by mimetic enzyme etching of gold nanobipyramids. Microchim. Acta 2021, 188, 62. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-González, M.; Wang, C.; Willner, I. Biocatalytic cascades operating on macromolecular scaffolds and in confined environments. Nat. Catal. 2020, 3, 256–273. [Google Scholar] [CrossRef]
- Kuzmak, A.; Carmali, S.; von Lieres, E.; Russell, A.J.; Kondrat, S. Can enzyme proximity accelerate cascade reactions? Sci. Rep. 2019, 9, 455. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Hou, L.; Xu, M.; Tang, D. Enhanced colorimetric immunoassay accompanying with enzyme cascade amplification strategy for ultrasensitive detection of low-abundance protein. Sci. Rep. 2014, 4, 3966. [Google Scholar] [CrossRef] [Green Version]
- Lai, W.; Wei, Q.; Xu, M.; Zhuang, J.; Tang, D. Enzyme-controlled dissolution of MnO2 nanoflakes with enzyme cascade amplification for colorimetric immunoassay. Biosens. Bioelectron. 2017, 89, 645–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, W.; Zeng, Q.; Tang, J.; Zhang, M.; Tang, D. A conventional chemical reaction for use in an unconventional assay: A colorimetric immunoassay for aflatoxin B-1 by using enzyme-responsive just-in-time generation of a MnO2 based nanocatalyst. Microchim. Acta 2018, 185, 92. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhan, L.; Qin, Z.; Sackrison, J.; Bischof, J.C. Ultrasensitive and highly specific lateral flow assays for point-of-care diagnosis. ACS Nano 2021, 15, 3593–3611. [Google Scholar] [CrossRef]
- Bishop, J.D.; Hsieh, H.V.; Gasperino, D.J.; Weigl, B.H. Sensitivity enhancement in lateral flow assays: A systems perspective. Lab Chip 2019, 19, 2486. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.; He, L.; Yu, F.; Liu, L.; Qu, C.; Qu, L.; Liu, J.; Wu, Y.; Wu, Y. A lateral flow assay for simultaneous detection of Deoxynivalenol, Fumonisin B-1 and Aflatoxin B-1. Toxicon 2018, 156, 23–27. [Google Scholar] [CrossRef]
- Bahadır, E.B.; Sezgintürk, M.K. Lateral flow assays: Principles, designs and labels. TrAC Trends Anal. Chem. 2016, 82, 286–306. [Google Scholar] [CrossRef]
- Mahmoudi, T.; de la Guardia, M.; Shirdel, B.; Mokhtarzadeh, A.; Baradaran, B. Recent advancements in structural improvements of lateral flow assays towards point-of-care testing. TrAC Trends Anal. Chem. 2019, 116, 13–30. [Google Scholar] [CrossRef]
- Xing, K.-Y.; Shan, S.; Liu, D.-F.; Lai, W.-H. Recent advances of lateral flow immunoassay for mycotoxins detection. TrAC Trends Anal. Chem. 2020, 133, 116087. [Google Scholar] [CrossRef]
- Wu, L.; Wang, M.; Wei, D. Advances in gold nanoparticles for mycotoxin analysis. Analyst 2021, 146, 1793–1806. [Google Scholar] [CrossRef]
- Ren, W.; Huang, Z.; Xu, Y.; Li, Y.; Ji, Y.; Su, B. Urchin-like gold nanoparticle-based immunochromatographic strip test for rapid detection of fumonisin B-1 in grains. Anal. Bioanal. Chem. 2015, 407, 7341–7348. [Google Scholar] [CrossRef]
- Ji, Y.; Ren, M.; Li, Y.; Huang, Z.; Shu, M.; Yang, H.; Xiong, Y.; Xu, Y. Detection of aflatoxin B-1 with immunochromatographic test strips: Enhanced signal sensitivity using gold nanoflowers. Talanta 2015, 142, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Anfossi, L.; Di Nardo, F.; Giovannoli, C.; Passini, C.; Baggiani, C. Increased sensitivity of lateral flow immunoassay for ochratoxin A through silver enhancement. Anal. Bioanal. Chem. 2013, 405, 9859–9867. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Zhang, G.; Fang, B.; Xiong, Q.; Duan, H.; Lai, W. Lateral flow immunoassay based on polydopamine-coated gold nanoparticles for the sensitive detection of zearalenone in maize. ACS Appl. Mater. Interfaces 2019, 11, 31283–31290. [Google Scholar] [CrossRef]
- Mirasoli, M.; Buragina, A.; Dolci, L.S.; Simoni, P.; Anfossi, L.; Giraudi, G.; Roda, A. Chemiluminescence-based biosensor for fumonisins quantitative detection in maize samples. Biosens. Bioelectron. 2012, 32, 283–287. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, G.P.; Correa, C.C.; Kubota, L.T. A simple, sensitive and reduced cost paper-based device with low quantity of chemicals for the early diagnosis of Plasmodium falciparum malaria using an enzyme-based colorimetric assay. Sens. Actuators B Chem. 2018, 255, 2113–2120. [Google Scholar] [CrossRef]
- Duan, D.; Fan, K.; Zhang, D.; Tan, S.; Liang, M.; Liu, Y.; Zhang, J.; Zhang, P.; Liu, W.; Qiu, X.; et al. Nanozyme-strip for rapid local diagnosis of Ebola. Biosens. Bioelectron. 2015, 74, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, Q.C.; Qiu, W.W.; Li, K.; Qian, L.S.; Zhang, X.J.; Liu, G.D. Gold-platinum nanoflowers as a label and as an enzyme mimic for use in highly sensitive lateral flow immunoassays: Application to detection of rabbit IgG. Microchim. Acta 2019, 186, 357. [Google Scholar] [CrossRef]
- Loynachan, C.N.; Thomas, M.R.; Gray, E.R.; Richards, D.A.; Kim, J.; Miller, B.S.; Brookes, J.C.; Agarwal, S.; Chudasama, V.; McKendry, R.A.; et al. Platinum nanocatalyst amplification: Redefining the gold standard for lateral flow immunoassays with ultrabroad dynamic range. ACS Nano 2018, 12, 279–288. [Google Scholar] [CrossRef]
- Cheng, N.; Shi, Q.; Zhu, C.; Li, S.; Lin, Y.; Du, D. Pt-Ni(OH)2 nanosheets amplified two-way lateral flow immunoassays with smartphone readout for quantification of pesticides. Biosens. Bioelectron. 2019, 142, 111498. [Google Scholar] [CrossRef]
- Liu, S.; Dou, L.; Yao, X.; Zhang, W.; Zhao, M.; Yin, X.; Sun, J.; Zhang, D.; Wang, J. Nanozyme amplification mediated on-demand multiplex lateral flow immunoassay with dual-readout and broadened detection range. Biosens. Bioelectron. 2020, 169, 112610. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Xie, W.; Zhang, T.; Liu, Y.; Lu, Z.; Li, C.M.; Liu, Y. A sensitive lateral flow immunochromatographic strip with prussian blue nanoparticles mediated signal generation and cascade amplification. Sens. Actuators B Chem. 2020, 309, 127728. [Google Scholar] [CrossRef]
- Liu, H.; Li, Z.; Shen, R.; Li, Z.; Yang, Y.; Yuan, Q. Point-of-care pathogen testing using photonic crystals and machine vision for diagnosis of urinary tract infections. Nano Lett. 2021, 21, 2854–2860. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, M.; Zhang, Q.; Li, P. Advances in Visual Immunoassays for Sensitive Detection of Mycotoxins in Food—A Review. Chem. Proc. 2021, 5, 25. https://doi.org/10.3390/CSAC2021-10443
Liang M, Zhang Q, Li P. Advances in Visual Immunoassays for Sensitive Detection of Mycotoxins in Food—A Review. Chemistry Proceedings. 2021; 5(1):25. https://doi.org/10.3390/CSAC2021-10443
Chicago/Turabian StyleLiang, Meijuan, Qi Zhang, and Peiwu Li. 2021. "Advances in Visual Immunoassays for Sensitive Detection of Mycotoxins in Food—A Review" Chemistry Proceedings 5, no. 1: 25. https://doi.org/10.3390/CSAC2021-10443
APA StyleLiang, M., Zhang, Q., & Li, P. (2021). Advances in Visual Immunoassays for Sensitive Detection of Mycotoxins in Food—A Review. Chemistry Proceedings, 5(1), 25. https://doi.org/10.3390/CSAC2021-10443







