Simultaneous Quantification of Four Principal NSAIDs through Voltammetry and Artificial Neural Networks Using a Modified Carbon Paste Electrode in Pharmaceutical Samples †
Abstract
:1. Introduction
2. Methods and Materials
2.1. Instrumentation and Reagents
2.2. Electrochemical Characterization
2.2.1. Electrode Preparation
2.2.2. Electrochemical Analysis of the NSAIDs in the Proposed Working Electrode
2.3. Quantification of NSAIDs by ANN
2.3.1. Data Processing
2.3.2. ANN Modeling
3. Results and Discussion
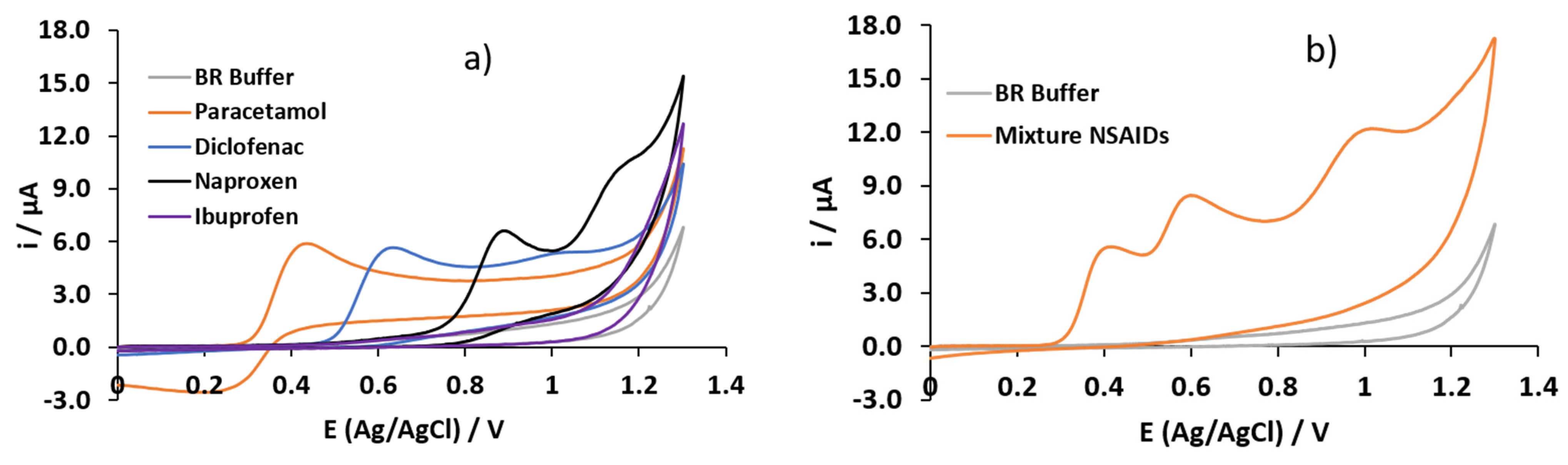
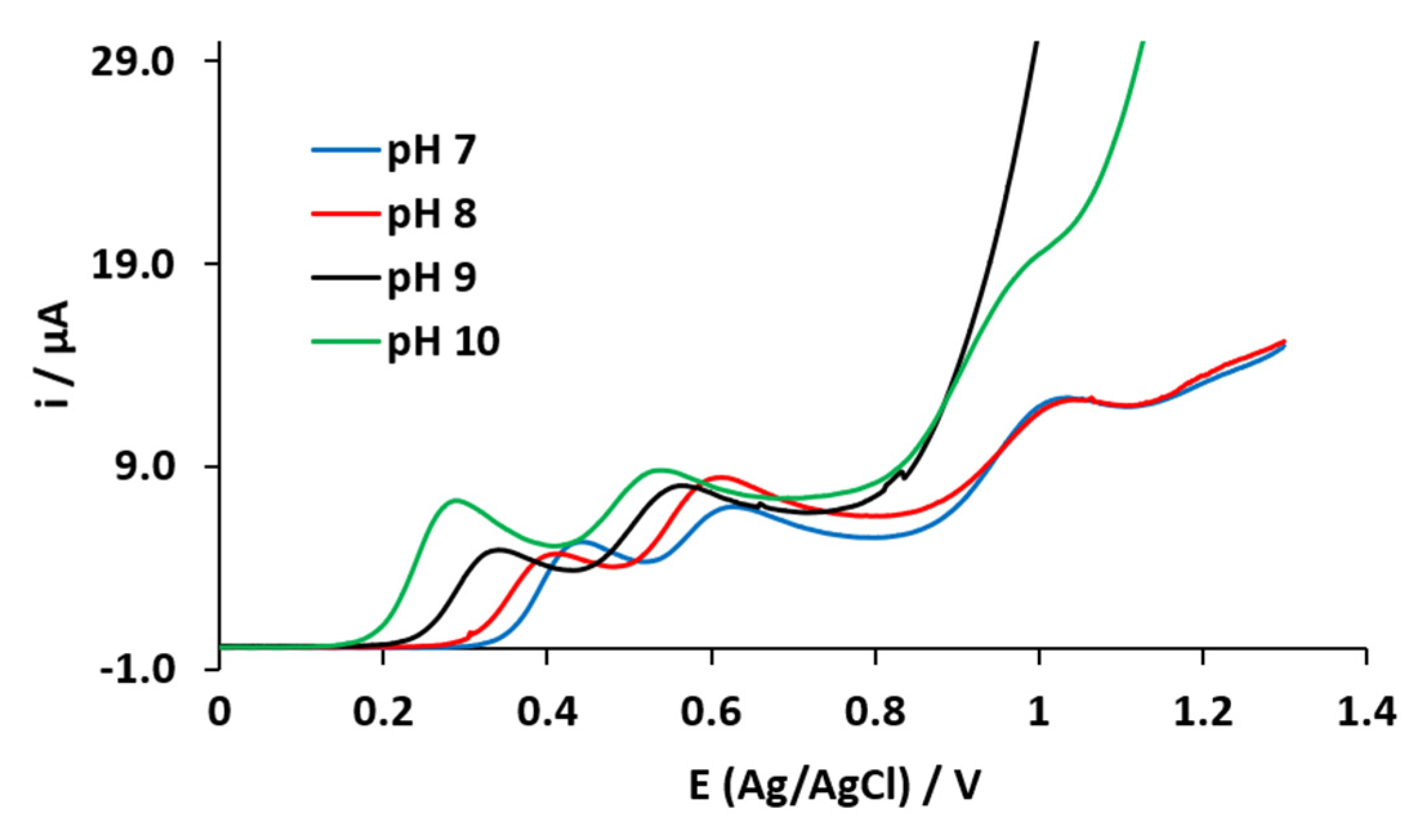
3.1. Electrochemical Characterization
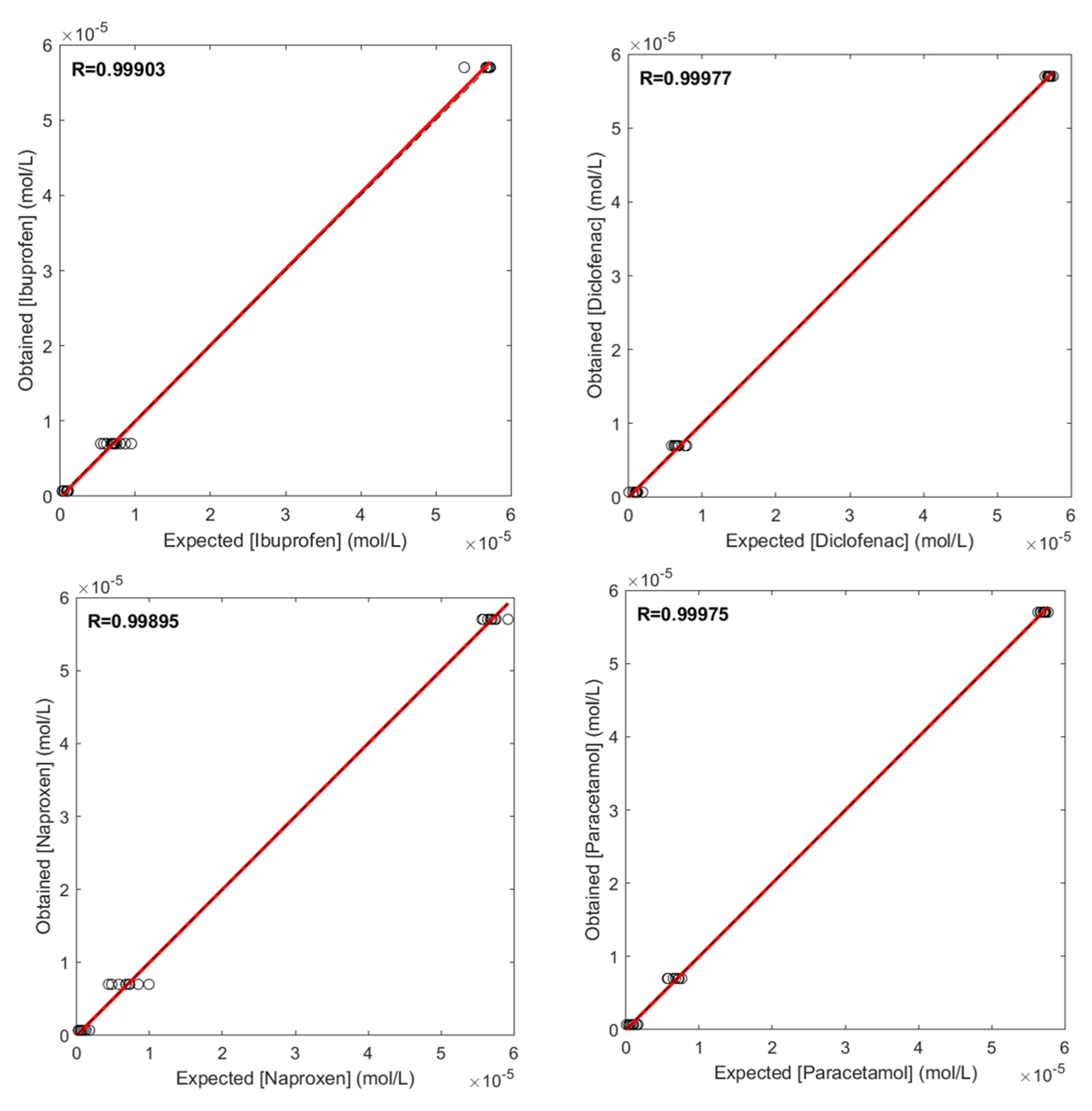
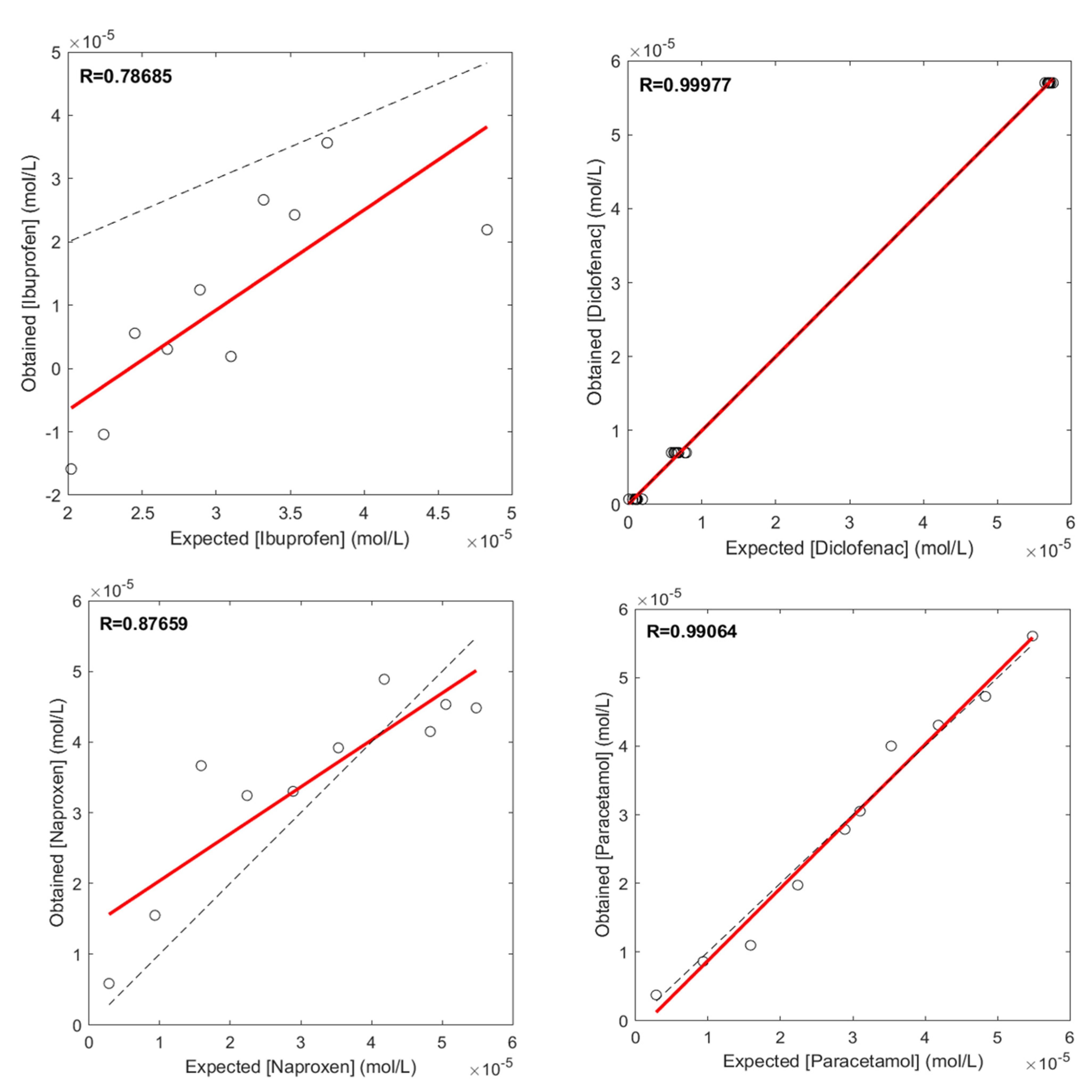
3.2. Quantification of NSAIDs Using ANN
4. Conclusions
Supplementary Materials
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Villoria, C.M. Tratamiento de Dolor Crónico, Diagnóstico, Clínica y Tratamiento; Arán Ediciones, S.L.: Madrid, Spain, 2007. [Google Scholar]
- Aguilar-Lira, G.Y.; Rojas-Hernández, A.; Rodriguez, J.A.; Páez-Hernández, M.E.; Álvarez-Romero, G.A. Optimized quantification of naproxen based on DPV and a multiwalled MWCNT-carbon paste electrode. J. Electrochem. Soc. 2020, 167, 166510. [Google Scholar] [CrossRef]
- Sondergaard, K.B.; Weeke, P.; Wissenberg, M.; Olsen, A.-M.S.; Fosbol, E.L.; Lippert, F.K.; Torp-Pedersen, C.; Gislason, G.H.; Folke, F. Non-steroidal anti-inflammatory drug use is associated with increased risk of out-of-hospital cardiac arrest: A nationwide case–time–control study. Eur. Heart J. Cardiovasc. Pharmacother. 2017, 3, 100–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguilar-Arteaga, K.; Rodriguez, J.; Miranda, J.M.; Medina, J.; Barrado, E. Determination of non-steroidal anti-inflammatory drugs in wastewaters by magnetic matrix solid phase dispersion-HPLC. Talanta 2010, 80, 1152. [Google Scholar] [CrossRef] [PubMed]
- Alcañiz, M.; Vivancos, J.-L.; Masot, R.; Ibañez, J.; Raga, M.; Soto, J.; Martínez-Máñez, R. Design of an electronic system and its application to electronic tongues using variable amplitude pulse voltammetry and impedance spectroscopy. J. Food Eng. 2012, 111, 122–128. [Google Scholar] [CrossRef]
- Cosio, M.S.; Ballabio, D.; Benedetti, S.; Gigliotti, C. Evaluation of different storage conditions of extra virgin olive oils with an innovative recognition tool built by means of electronic nose and electronic tongue. Food Chem. 2007, 101, 485–491. [Google Scholar] [CrossRef]
- Vlasov, Y.G.; Legin, A.V.; Rudnitskaya, A.M.; D’Amico, A.; Natale, C.D. Sens. Actuators B 2000, 65, 235–236. [Google Scholar] [CrossRef]
- Martínez-Máñez, R.; Soto, J.; Garcia-Breijo, E.; Gil, L.; Ibáñez, J.; Llobet, E. An “electronic tongue” design for the qualitative analysis of natural waters. Sens. Actuators B 2005, 104, 302–307. [Google Scholar] [CrossRef]
- Lvova, L.; Martinelli, E.; Mazzone, E.; Pede, A.; Paolesse, R.; Natale, C.D.; D’Amico, A. Electronic tongue based on an array of metallic potentiometric sensors. Talanta 2006, 70, 833–839. [Google Scholar] [CrossRef] [PubMed]
- Bessant, C.; Saini, S. Simultaneous Determination of Ethanol, Fructose, and Glucose at an Unmodified Platinum Electrode Using Artificial Neural Networks. Anal. Chem. 1999, 71, 2806–28013. [Google Scholar] [CrossRef]
- Martina, V.; Ionescu, K.; Pigani, L.; Terzi, F.; Ulrici, A.; Zanardi, C.; Seeber, R. Development of an electronic tongue based on a PEDOT-modified voltammetric sensor. Anal. Bioanal. Chem. 2007, 387, 2101–2110. [Google Scholar] [CrossRef] [PubMed]
- Garcia, B.; Olguin, P.; Masot, P.; Alcañiz, F.; Martínez-Máñeza, R.; Soto, C. TNT detection using a voltammetric electronic tongue based on neural networks. Sens. Actuator A Phys. 2013, 192, 1–8. [Google Scholar] [CrossRef]
- Aguilar-Lira, G.Y.; Gutiérrez-Salgado, J.M.; Rojas-Hernández, A.; Rodríguez-Ávila, J.A.; Páez-Hernández, M.E.; Álvarez-Romero, G.A. Artificial neural network for the voltamperometric quantification of diclofenac in presence of other nonsteroidal anti-inflammatory drugs and some commercial excipients. J. Electroanal. Chem. 2017, 801, 527–535. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments, 8th ed.; Wiley: Hoboken, NJ, USA, 2012. [Google Scholar]
| X1 (V) | X2 (s) | X3 (s) | X4 (V) | Y (µA) |
|---|---|---|---|---|
| 0.00585 | 0.75 | 0.05 | 0.05 | 5.24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar-Lira, G.Y.; Hernandez, P.; Álvarez-Romero, G.A.; Gutiérrez, J.M. Simultaneous Quantification of Four Principal NSAIDs through Voltammetry and Artificial Neural Networks Using a Modified Carbon Paste Electrode in Pharmaceutical Samples. Chem. Proc. 2021, 5, 3. https://doi.org/10.3390/CSAC2021-10450
Aguilar-Lira GY, Hernandez P, Álvarez-Romero GA, Gutiérrez JM. Simultaneous Quantification of Four Principal NSAIDs through Voltammetry and Artificial Neural Networks Using a Modified Carbon Paste Electrode in Pharmaceutical Samples. Chemistry Proceedings. 2021; 5(1):3. https://doi.org/10.3390/CSAC2021-10450
Chicago/Turabian StyleAguilar-Lira, Guadalupe Yoselin, Prisciliano Hernandez, Giaan Arturo Álvarez-Romero, and Juan Manuel Gutiérrez. 2021. "Simultaneous Quantification of Four Principal NSAIDs through Voltammetry and Artificial Neural Networks Using a Modified Carbon Paste Electrode in Pharmaceutical Samples" Chemistry Proceedings 5, no. 1: 3. https://doi.org/10.3390/CSAC2021-10450
APA StyleAguilar-Lira, G. Y., Hernandez, P., Álvarez-Romero, G. A., & Gutiérrez, J. M. (2021). Simultaneous Quantification of Four Principal NSAIDs through Voltammetry and Artificial Neural Networks Using a Modified Carbon Paste Electrode in Pharmaceutical Samples. Chemistry Proceedings, 5(1), 3. https://doi.org/10.3390/CSAC2021-10450







