Review of the Recent Advances in Nano-Biosensors and Technologies for Healthcare Applications †
Abstract
:1. Introduction
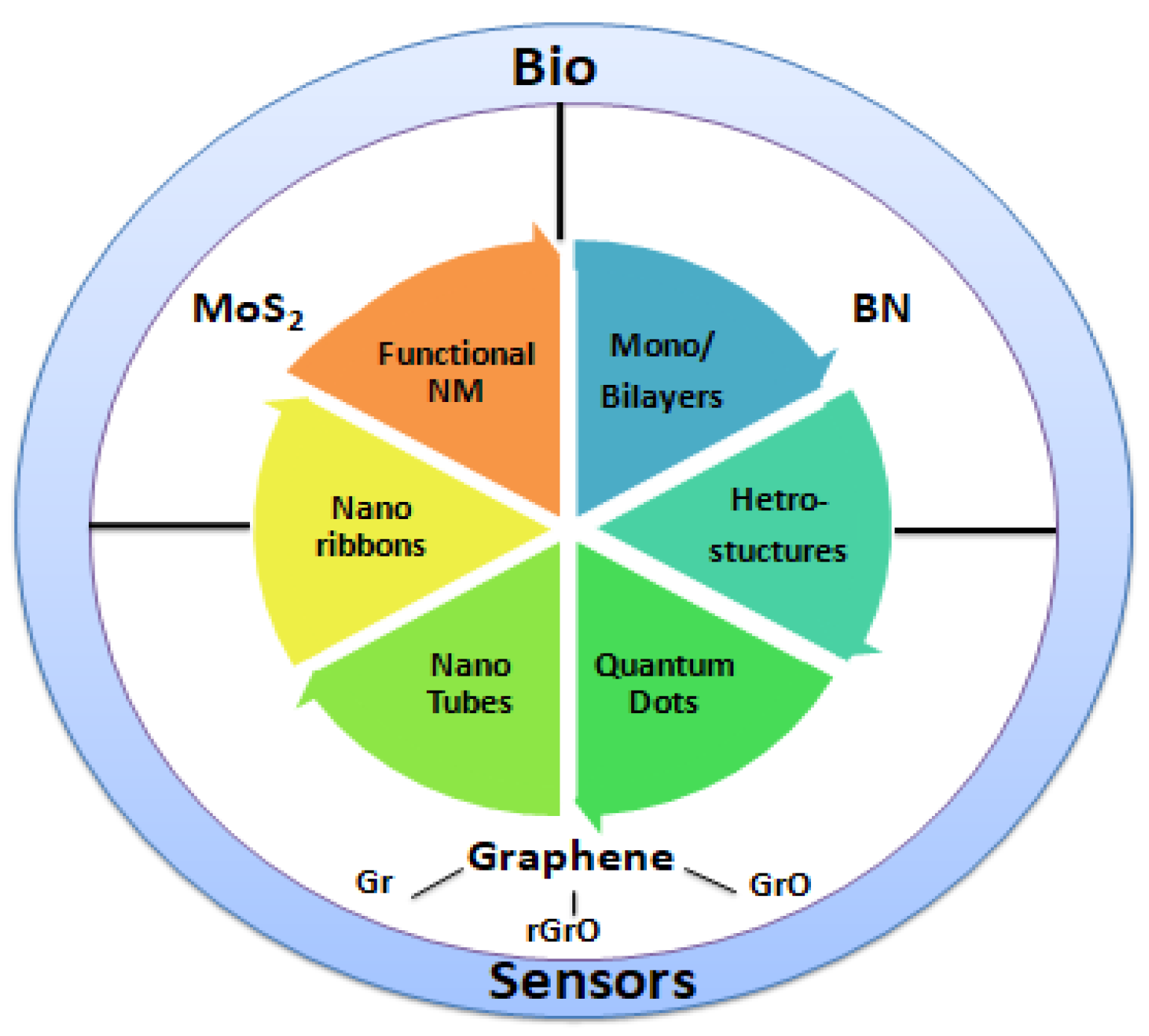
2. Nano-Biosensors
2.1. Biosensor Types
2.2. Nanostructured Materials for Biosensing
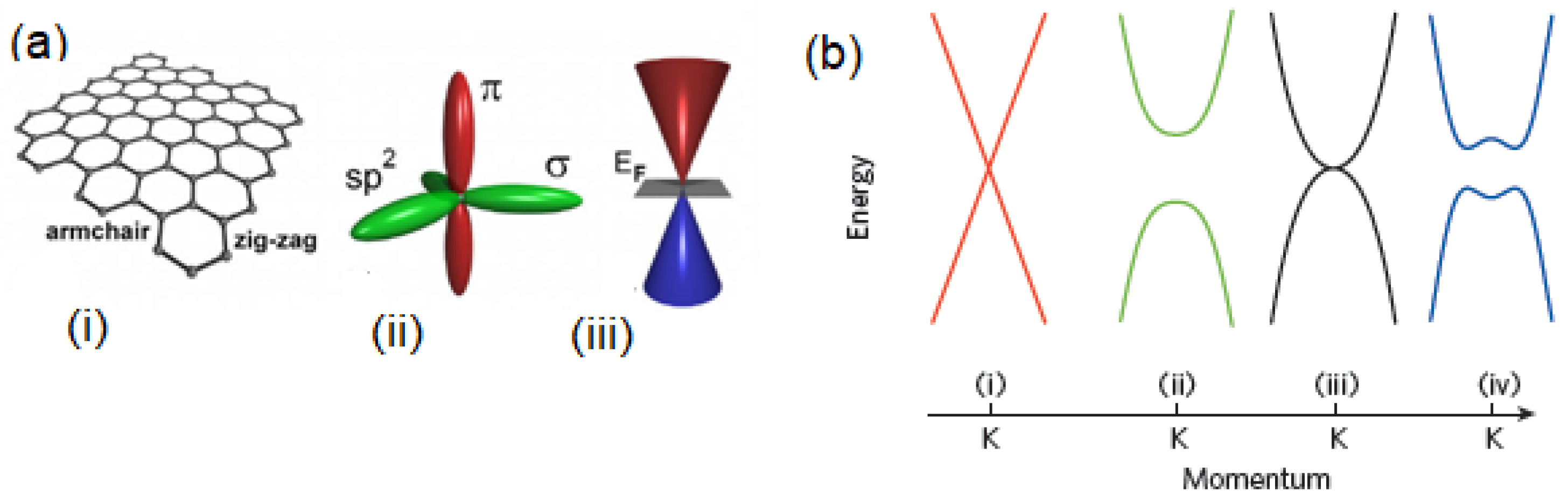
2.2.1. Gr Nano-Biosensors
2.2.2. MoS2 and BN Nano-Biosensors
2.2.3. Hetrostructures
3. Smart Technologies
4. Challenges or Opportunities
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tricoli, A.; Nasiri, N.; De, S. Wearable and Miniaturized Sensor Technologies for Personalized and Preventive Medicine. Adv. Funct. Mater. 2017, 27, 1605271. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Z.; Zhang, T. Flexible sensing electronic for wearable/attachable health monitoring. Adv. Sci. 2017, 13, 1602790. [Google Scholar] [CrossRef]
- Yao, S.; Swetha, P.; Zhu, Y. Nanomaterial-enabled wearable sensors for healthcare. Adv. Healthc. Mater. 2017, 7, 1700889. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, J. First-principles design of spintronics materials. Natl. Sci. Rev. 2016, 3, 365–381. [Google Scholar] [CrossRef]
- Ramanathan, A.A.; Khalifeh, J.M. Electronic, magnetic and optical properties of XScO3 (X = Mo, W) perovskites. PeerJ Mater. Sci. 2021, 3, e15. [Google Scholar] [CrossRef]
- Pantelopoulos, A.; Bourbakis, N.G. Prognosis—A wearable health monitoring system for people at risk: Methodology and modeling. IEEE Trans. Inf. Technol. Biomed. 2010, 14, 613–621. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Huang, P.J.J.; Liu, B.; Ying, Y.; Liu, J. Comparison of Graphene Oxide and Reduced Graphene Oxide for DNA Adsorption and Sensing. Langmuir 2016, 32, 10776–10783. [Google Scholar] [CrossRef] [PubMed]
- Hu, K.; Tsukruk, V.V. Tuning the Electronic Properties of Robust Bio-Bond Graphene Papers by Spontaneous Electrochemical Reduction: From Insulators to Flexible Semi-Metals. Chem. Mater. 2015, 27, 6717–6729. [Google Scholar] [CrossRef]
- Chong, Y.; Ge, C.; Yang, Z.; Garate, J.A.; Gu, Z.; Weber, J.K.; Liu, J.; Zhou, R. Reduced Cytotoxicity of Graphene Nanosheets Mediated by Blood-Protein Coating. ACS Nano 2015, 9, 5713–5724. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, A.A. Defect Functionalization of MoS2 nanostructures as toxic gas sensors: A review. IOP Conf. Ser. Mater. Sci. Eng. 2018, 305, 012001. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.H.; Huang, K.J.; Wu, X. Recent advances in transition-metal dichalcogenides based electrochemical biosensors: A review. Biosens. Bioelectron. 2017, 97, 305. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tan, C.; Zhang, H.; Wang, L. Two-Dimensional Graphene Analogues for Biomedical Applications. Chem. Soc. Rev. 2015, 44, 2681–2701. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.K.; Ménard-Moyon, C.; Bianco, A. Physically-triggered nanosystems based on two-dimensional materials for cancer theranostics. Adv. Drug Deliv. Rev. 2019, 138, 211–232. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, A.A.; Aqra, M.W.; Al-Rawajfeh, A.E. Recent advances in 2D-nanopores for desalination. Environ. Chem. Lett. 2018, 16, 1217–1231. [Google Scholar] [CrossRef]
- Yang, T.; Lin, H.; Loh, K.P.; Jia, B. Fundamental Transport Mechanisms and Advancements of Graphene Oxide Membranes for Molecular Separation. Chem. Mater. 2019, 31, 1829–1846. [Google Scholar] [CrossRef]
- Li, M.; Liu, C.; Zhao, H.; An, H.; Cao, H.; Zhang, Y.; Fan, Z. Tuning Sulfur Doping in Graphene for Highly Sensitive Dopamine Biosensors. Carbon 2015, 86, 197–206. [Google Scholar] [CrossRef]
- Aqra, M.W.; Ramanathan, A.A. Graphene and related 2D materials for desalination: A review of recent patents. Jordan J. Phys. 2020, 13, 233–242. [Google Scholar]
- Szunerits, S.; Boukherroub, R. Graphene-based biosensors. Interface Focus 2018, 8, 20160132. [Google Scholar] [CrossRef] [PubMed]
- Lemme, M.C. Current Status of Graphene Transistors. Solid State Phenom. 2010, 156–158, 499–509. [Google Scholar] [CrossRef]
- Xiang, L.; Ma, S.Y.; Wang, F.; Zhang, K. Nano indentation models and young’s modulus of few-layer graphene: A molecular dynamics simulation study. J. Phys. D Appl. Phys. 2015, 48, 395305. [Google Scholar] [CrossRef]
- Yang, H.; Xue, T.; Li, F.; Liu, W.; Song, Y. Graphene: Diversified flexible 2D material for wearable vital signs monitoring. Adv. Mater. Technol. 2018, 4, 1800574. [Google Scholar] [CrossRef]
- Schwierz, F. Graphene Transistors. Nat. Nanotechnol. 2010, 5, 487–496. [Google Scholar] [CrossRef] [PubMed]
- Trung, T.Q.; Le, H.S.; Dang, T.M.L.; Ju, S.; Park, S.Y.; Lee, N.-E. Freestanding, fiber-based, wearable temperature sensor with tunable thermal index for healthcare monitoring. Adv. Healthc. Mater. 2018, 7, 1800074. [Google Scholar] [CrossRef]
- Wang, Z.; Gao, W.; Zhang, Q.; Zheng, K.; Xu, J.; Xu, W.; Shang, E.; Jiang, J.; Zhang, J.; Liu, Y. 3D printed graphene/polydimethylsiloxane composites for stretchable and strain insensitive temperature sensors. ACS Appl. Mater. Interfaces 2018, 11, 1344–1352. [Google Scholar] [CrossRef] [PubMed]
- Kou, H.; Zhang, L.; Tan, Q.; Liu, G.; Lv, W.; Lu, F.; Dong, H.; Xiong, J. Wireless flexible pressure sensor based on micro-patterned Graphene/PDMS composite. Sens. Actuators A Phys. 2018, 277, 150–156. [Google Scholar] [CrossRef]
- Yang, Z.; Pang, Y.; Han, X.; Yang, Y.; Ling, J.; Jian, M.; Zhang, Y.; Yang, Y.; Ren, T.L. Graphene textile strain sensor with negative resistance variation for human motion detection. ACS Nano 2018, 12, 9134–9141. [Google Scholar] [CrossRef]
- Karim, N.; Afroj, S.; Malandraki, A.; Butterworth, S.; Beach, C.; Rigout, M.; Novoselov, K.S.; Casson, A.J.; Yeates, S.G. All inkjet-printed graphene-based conductive patterns for wearable e-textile applications. J. Mater. Chem. C 2017, 5, 11640–11648. [Google Scholar] [CrossRef] [Green Version]
- Yang, T.; Jiang, X.; Zhong, Y.; Zhao, X.; Lin, S.; Li, J.; Li, X.; Xu, J.; Li, Z.; Zhu, H. A wearable and highly sensitive graphene strain sensor for precise home-based pulse wave monitoring. ACS Sens. 2017, 2, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Pang, Y.; Zhang, K.; Yang, Z.; Jiang, S.; Ju, Z.; Li, Y.; Wang, X.; Wang, D.; Jian, M.; Zhang, Y.; et al. Epidermis microstructure inspired graphene pressure sensor with random distributed spinosum for high sensitivity and large linearity. ACS Nano 2018, 12, 2346–2354. [Google Scholar] [CrossRef] [PubMed]
- Boland, C.S.; Khan, U.; Backes, C.; O’Neill, A.; McCauley, J.; Duane, S.; Shanker, R.; Liu, Y.; Jurewicz, I.; Dalton, A.B.; et al. Sensitive, high-strain, high-rate bodily motion sensors based on graphene–rubber composites. ACS Nano 2014, 8, 8819–8830. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Qi, J.; Li, F.; Zhang, Y. Highly stretchable strain sensors with reduced graphene oxide sensing liquids for wearable electronics. Nanoscale 2018, 10, 5264–5271. [Google Scholar] [CrossRef]
- Pang, Y.; Tian, H.; Tao, L.; Li, Y.; Wang, X.; Deng, N.; Yang, Y.; Ren, T.L. Flexible, highly sensitive, and wearable pressure and strain sensors with graphene porous network structure. ACS Appl. Mater. Interfaces 2016, 8, 26458–26462. [Google Scholar] [CrossRef] [PubMed]
- Pu, Z.; Tu, J.; Han, R.; Zhang, X.; Wu, J.; Fang, C.; Wu, H.; Zhang, X.; Yu, H.; Li, D. A flexible enzyme-electrode sensor with cylindrical working electrode modified with a 3D nanostructure for implantable continuous glucose monitoring. Lab Chip 2018, 18, 3570–3577. [Google Scholar] [CrossRef]
- Toi, P.T.; Trung, T.Q.; Dang, T.M.L.; Bae, C.W.; Lee, N.E. Highly electrocatalytic, durable, and stretchable nano hybrid fiber for on body sweat glucose detection. ACS Appl. Mater. Interfaces 2019, 11, 10707–10717. [Google Scholar] [CrossRef]
- Ameri, S.K.; Singh, P.K.; D’Angelo, R.; Stoppel, W.; Black, L.; Sonkusale, S.R. Three dimensional graphene scaffold for cardiac tissue engineering and in-situ electrical recording. In Proceedings of the 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 16–20 August 2016; pp. 4201–4203. [Google Scholar] [CrossRef]
- Yun, Y.J.; Ju, J.; Lee, J.H.; Moon, S.-H.; Park, S.-J.; Kim, Y.H.; Hong, W.G.; Ha, D.H.; Jang, H.; Lee, G.H.; et al. Highly elastic graphene-based electronics toward electronic skin. Adv. Funct. Mater. 2017, 27, 1701510–1701513. [Google Scholar] [CrossRef]
- Sun, B.; McCay, R.N.; Goswami, S.; Xu, Y.; Zhang, C.; Ling, Y.; Lin, J.; Yan, Z. Gas-permeable, multifunctional on-skin electronics based on laser-induced porous graphene and sugar-templated elastomer sponges. Adv. Mater. 2018, 30, 1804327–1804328. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Sakthivel, T.; Seal, S. Recent development in 2D materials beyond graphene. Prog. Mater. Sci. 2015, 73, 44–126. [Google Scholar] [CrossRef]
- Kadantsev, E.S.; Hawrylak, P. Electronic structure of a single MoS2 monolayer. Solid State Commun. 2012, 152, 909–913. [Google Scholar] [CrossRef]
- Cassabois, G.; Valvin, P.; Gil, B. Hexagonal boron nitride is an indirect bandgap semiconductor. Nat. Photon 2016, 10, 262–266. [Google Scholar] [CrossRef] [Green Version]
- Elias, C.; Valvin, P.; Pelini, T.; Summerfield, A.; Mellor, C.J.; Cheng, T.S.; Eaves, L.; Foxon, C.T.; Beton, P.H.; Novikov, S.V.; et al. Direct band-gap crossover in epitaxial monolayer boron nitride. Nat. Commun. 2019, 10, 2639. [Google Scholar] [CrossRef] [Green Version]
- Geim, A.K.; Grigorieva, I.V. Van der Waals heterostructures. Nature 2013, 499, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Panahi, S.F.K.S.; Namiranian, A.; Jamaati, M. Graphene-hBN hybrid nanogap for boosting DNA nucleobases recognition sensitivity. ChemNanoMat 2019, 5, 488–498. [Google Scholar] [CrossRef]
- de Souza, F.A.; Amorim, R.G.; Scopel, W.L.; Scheicher, R.H. Electrical detection of nucleotides via nanopores in a hybrid graphene/h-BN sheet. Nanoscale 2017, 9, 2207–2212. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Choudhury, S.; Kudyshev, Z.A.; Wang, D.; Prokopeva, L.J.; Xiao, P.; Jiang, Y.; Kildishev, A.V. Enhancing sensitivity to ambient refractive index with tunable few-layer graphene/hBN nanoribbons. Photonics Res. 2019, 7, 815–822. [Google Scholar] [CrossRef]
- Zeng, S.; Hu, S.; Xia, J.; Anderson, T.; Dinh, X.-Q.; Meng, X.-M.; Coquet, P.; Yong, K.-T. Graphene-MoS2 hybrid nanostructures enhanced surface plasmon resonance biosensors. Sens. Actuators B 2015, 207, 801–810. [Google Scholar] [CrossRef]
- Wu, L.; Jia, Y.; Jiang, L.; Guo, J.; Dai, X.; Xiang, Y.; Fan, D. Sensitivity improved SPR biosensor based on the MoS2/graphene–aluminum hybrid structure. J. Lightwave Technol. 2016, 35, 82–87. [Google Scholar] [CrossRef]
- Huang, Y.; Shi, Y.; Yang, H.Y.; Ai, Y. A novel single-layered MoS2 nanosheet based microfluidic biosensor for ultrasensitive detection of DNA. Nanoscale 2015, 7, 2245–2249. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.-J.; Wang, L.; Li, J.; Liu, Y.-M. Electrochemical sensing based on layered MoS2-graphene composites. Sens. Actuators B 2013, 178, 671–677. [Google Scholar] [CrossRef]
- Loan, P.T.K.; Zhang, W.; Lin, C.-T.; Wei, K.-H.; Li, L.-J.; Chen, C.-H. Graphene/MoS2 heterostructures for ultrasensitive detection of DNA hybridization. Adv. Mater. 2014, 26, 4838–4844. [Google Scholar] [CrossRef]
- Li, Y.; Yan, H.; Farmer, D.B.; Meng, X.; Zhu, W.; Osgood, R.M.; Heinz, T.F.; Avouris, P. Graphene plasmon enhanced vibrational sensing of surface-adsorbed layers. Nano Lett. 2014, 14, 1573–1577. [Google Scholar] [CrossRef]
- Zeng, S.; Sreekanth, K.V.; Shang, J.; Yu, T.; Chen, C.K.; Yin, F.; Baillargeat, D.; Coquet, P.; Ho, H.P.; Kabashin, A.V.; et al. Graphene-gold metasurface architectures for ultrasensitive plasmonic biosensing. Adv. Mater. 2015, 27, 6163–6169. [Google Scholar] [CrossRef]
- Zhang, M.; Liao, C.; Mak, C.H.; You, P.; Mak, C.L.; Yan, F. Highly sensitive glucose sensors based on enzyme-modified whole-graphene solution-gated transistors. Sci. Rep. 2015, 5, 8311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Mao, H.; Wu, C.; Tang, L.; Wu, Z.; Sun, H.; Zhang, H.; Zhou, H.; Jia, C.; Jin, Q.; et al. Label-free graphene biosensor targeting cancer molecules based on non-covalent modification Biosens. Bioelectron. 2017, 87, 701–707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.; Autore, M.; Li, J.; Li, P.; Alonso-Gonzalez, P.; Yang, Z.; Martin-Moreno, L.; Hillenbrand, R.; Nikitin, A.Y. Acoustic graphene plasmon nanoresonators for field-enhanced infrared molecular spectroscopy. ACS Photonics 2017, 4, 3089–3097. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Zhan, J.; Man, B.; Jiang, S.; Yue, W.; Gao, S.; Guo, C.; Liu, H.; Li, Z.; Wang, J.; et al. Real-time reliable determination of binding kinetics of DNA hybridization using a multi-channel graphene biosensor. Nat. Commun. 2017, 8, 14902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seber, G.; Muñoz, J.; Sandoval, S.; Rovira, C.; Tobias, G.; Mas-Torrent, M.; Crivillers, N. Synergistic exploitation of the superoxide scavenger properties of reduced graphene oxide and a trityl organic radical for the impedimetric sensing of xanthine. Adv. Mater. Interface 2018, 5, 1701072. [Google Scholar] [CrossRef] [Green Version]
- Chiu, N.-F.; Kuo, C.-T.; Lin, T.-L.; Chang, C.-C.; Chen, C.-Y. Ultra-high sensitivity of the non-immunological affinity of graphene oxide-peptide-based surface plasmon resonance biosensors to detect human chorionic gonadotropin. Biosens. Bioelectron. 2017, 94, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Nurunnabi, M.; Nafiujjaman, M.; Lee, S.-J.; Park, I.-K.; Huh, K.M.; Lee, Y.-k. Preparation of ultra-thin hexagonal boron nitride nanoplates for cancer cell imaging and neurotransmitter sensing. Chem. Commun. 2016, 52, 6146–6149. [Google Scholar] [CrossRef] [PubMed]
- Autore, M.; Li, P.; Dolado, I.; Alfaro-Mozaz, F.J.; Esteban, R.; Atxabal, A.; Casanova, F.; Hueso, L.E.; Alonso-González, P.; Aizpurua, J.; et al. Boron nitride nanoresonators for phonon-enhanced molecular vibrational spectroscopy at the strong coupling limit. Light Sci. Appl. 2018, 7, 17172. [Google Scholar] [CrossRef] [PubMed]
- Kostarelos, K.; Vincent, M.; Hebert, C.; Garrido, J.A. Graphene in the Design and Engineering of Next-Generation Neural Interfaces. Adv. Mater. 2017, 29, 1700909. [Google Scholar] [CrossRef] [PubMed]
- Sazonov, E. Wearable Sensors: Fundamentals, Implementation and Applications; Technology & Engineering; Academic Press: Cambridge, MA, USA, 2020; 660p. [Google Scholar]
- Belizário, J.E.; Faintuch, J.; Malpartida, M.G. Breath Biopsy and Discovery of Exclusive Volatile Organic Compounds for Diagnosis of Infectious Diseases. Front. Cell. Infect. Microbiol. 2021, 10, 564194. [Google Scholar] [CrossRef]
- Scarlata, S.; Finamore, P.; Meszaros, M.; Dragonieri, S.; Bikov, A. The Role of Electronic Noses in Phenotyping Patients with Chronic Obstructive Pulmonary Disease. Biosensors 2020, 10, 171. [Google Scholar] [CrossRef] [PubMed]
- Al Ramahi, R.; Zaid, A.N.; Abu-Khalaf, N. Evaluating the potential use of electronic tongue in early identification and diagnosis of bacterial infections. Infect. Drug Resist. 2019, 12, 2445–2451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.C.; Mun, J.; Kwon, S.Y.; Park, S.; Bao, Z.; Park, S. Electronic Skin: Recent Progress and Future Prospects for Skin-Attachable Devices for Health Monitoring, Robotics, and Prosthetics. Adv. Mater. 2019, 31, 1904765. [Google Scholar] [CrossRef] [Green Version]
- Behera, B.; Joshi, R.; Anil Vishnu, G.K.; Bhalerao, S.; Pandya, H.J. Electronic nose: A non-invasive technology for breath analysis of diabetes and lung cancer patients. J. Breath Res. 2019, 13, 024001–024023. [Google Scholar] [CrossRef] [PubMed]
- Briggs, N.; Subramanian, S.; Lin, Z.; Li, X.; Zhang, X.; Zhang, K.; Xiao, K.; Geohegan, D.; Wallace, R.; Chen, L.Q.; et al. A Roadmap for Electronic Grade 2Dimensional Materials. 2D Mater. 2019, 6, 022001. [Google Scholar] [CrossRef]
- Magda, G.Z.; Peto, J.; Dobrik, G.; Hwang, C.; Biro, L.P.; Tapaszto, L. Exfoliation of Large-Area Transition Metal Chalcogenide Single Layers. Sci. Rep. 2015, 5, 14714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramanathan, A.A. and Aqra, M.W. An Overview of the Green Road to the Synthesis of Nanoparticles. J. Mater. Sci. Res. Rev. 2019, 2, 1–11. [Google Scholar]
- Wang, S.; Li, K.; Chen, Y.; Chen, H.; Ma, M.; Feng, J.; Zhao, Q.; Shi, J. Biocompatible PEGylated MoS2 nanosheets: Controllable bottom-up synthesis and highly efficient photothermal regression of tumor. Biomaterials 2015, 39, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, A.; Costa, B.; Ferreira, M.V.; Miguéis, D.; Louros, J.; Durazzo, A.; Lucarini, M.; Eder, P.; Chaud, M.V.; Morsink, M.; et al. Nanotoxicology and Nanosafety: Safety-By-Design and Testing at a Glance. Int. J. Environ. Res. Public Health 2020, 17, 4657. [Google Scholar] [CrossRef]
- Ramanathan, A.A. Toxicity of nanoparticles_challenges and opportunities. Appl. Microsc. 2019, 49, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| NM | Body Function | Sensing Mechanism | Sensitivity | Range | Reference |
|---|---|---|---|---|---|
| Freestanding single reduced graphene oxide (rGrO); 3D Gr–PDS composite | Body temperature | Resistance-based | - | - - | [23] [24] |
| Gr/PDMS; Gr | Body movements | Piezo-capacitive strain; textile strain | 0.24 kPa−1 0.0078 kPa−1 | 0–10 kPa 10–100 kPa | [25] [26] |
| Inkjet-printed Gr | Heart rate | Electronic | - | - | [27] |
| Wrist pulse | Strain; pressure | - | - | [28] [29] | |
| Gr–rubber composite; rGrO | Body movements + respiration rate | Strain | - - | - - | [30] [31] |
| Gr porous network | Blood pressure | Pressure + strain | - | - | [32] |
| 3D nano-implant; Nano-hybrid fiber | Blood glucose Sweat glucose | Electrode Electrocatalytic | - - | - - | [33] [34] |
| 3D Gr scaffold | ECG | Implant | - | - | [35] |
| Gr; Porous Gr | EMG | Electronic skin | - - | - - | [36] [37] |
| 3D Gr scaffold; Gr | EEG | Implant Electronic skin | - - | - - | [35] [36] |
| Nanomaterial (NM) | Analyte | Sensing Mechanism | Detection Limit | Range | Reference + Year |
|---|---|---|---|---|---|
| MoS2 | DNA | Fluorescence quenching | 500 pM | 0–50 nM | [48]; 2014 |
| MoS2/Gr | Acetaminophen | Electrochemical | 20 nM | 0.1–100 μM | [49]; 2013 |
| Gr/MoS2 | DNA hybridization | Photoluminescence | 1 attomolar | [50]; 2014 | |
| MoS2/Gr on Au | Biomolecule | Surface plasmon resonance (SPR) | 10−6 RIU | [46]; 2015 | |
| MoS2/Gr–Al hybrid | Biomolecule | Angle-based SPR | 190.83° RIU−1 | [47]; 2017 | |
| Gr | PMMA, PVP | IR transmission spectroscopy | - | - | [51]; 2014 |
| Gr | ssDNA | Phase-based SPR | 1 attomolar | - | [52]; 2015 |
| Gr | Glucose | FET | 0.5 μM | - | [53]; 2015 |
| Gr | Carcinoembryonic antigen (CEA) | FET | 100 pg mL−1 | [54]; 2016 | |
| Gr | Protein | Acoustic Gr plasmons | - | - | [55]; 2017 |
| Multichannel Gr | DNA | FET | 10 pM | - | [56]; 2017 |
| rGrO + trityl organic radical | Xanthine | Electrode-based | 0.52 nM | - | [57]; 2017 |
| GrO | hCG | Angle-based SPR | 0.06 mM | - | [58]; 2017 |
| hBN | Dopamine | Neurotransmittor | 10 μM | - | [59]; 2016 |
| hBN | CBP | IR vibrational spectroscopy | - | - | [60]; 2018 |
| Gr/hBN | DNA sequencing | Current Modulation | - | - | [44]; 2017 |
| Gr/hBN | DNA sequencing | Current Modulation | - | - | [43]; 2019 |
| Gr/hBN | Biomolecule | SPR | 4.207 µm RIU−1 | [45]; 2019 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aqra, M.W.; Ramanathan, A.A. Review of the Recent Advances in Nano-Biosensors and Technologies for Healthcare Applications. Chem. Proc. 2021, 5, 76. https://doi.org/10.3390/CSAC2021-10473
Aqra MW, Ramanathan AA. Review of the Recent Advances in Nano-Biosensors and Technologies for Healthcare Applications. Chemistry Proceedings. 2021; 5(1):76. https://doi.org/10.3390/CSAC2021-10473
Chicago/Turabian StyleAqra, Maha Wajeeh, and Amall Ahmed Ramanathan. 2021. "Review of the Recent Advances in Nano-Biosensors and Technologies for Healthcare Applications" Chemistry Proceedings 5, no. 1: 76. https://doi.org/10.3390/CSAC2021-10473
APA StyleAqra, M. W., & Ramanathan, A. A. (2021). Review of the Recent Advances in Nano-Biosensors and Technologies for Healthcare Applications. Chemistry Proceedings, 5(1), 76. https://doi.org/10.3390/CSAC2021-10473






