Colorimetric Determination of Nitrate after Reduction to Nitrite in a Paper-Based Dip Strip †
Abstract
:1. Introduction
2. Methods
2.1. Materials
2.2. Methods
3. Results and Discussion
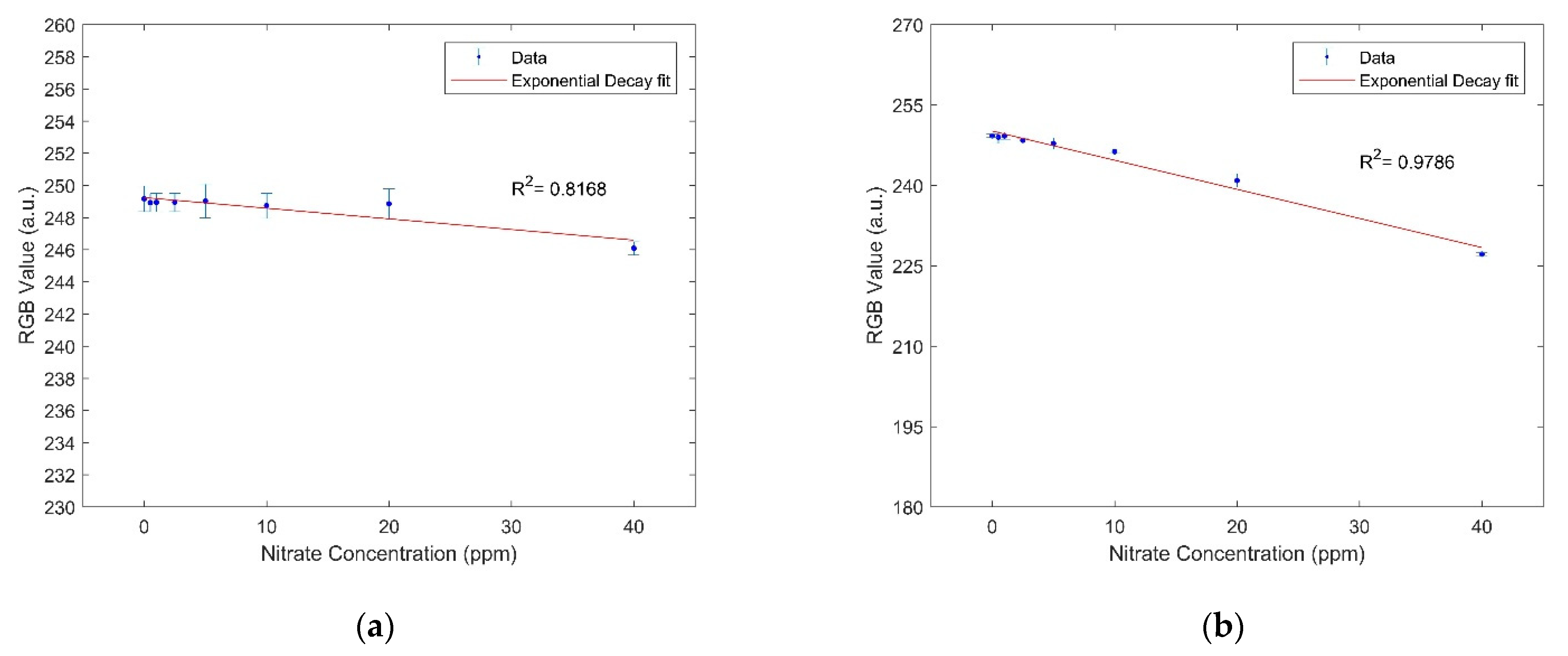
3.1. Nitrate and Nitrite Analysis
3.2. Reduction Efficiency
3.3. Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bouhoun, M.; Blondeau, P.; Louafi, Y.; Andrade, F. A Paper-Based Potentiometric Platform for Determination of Water Hardness. Chemosensors 2021, 9, 96. [Google Scholar] [CrossRef]
- Firdaus, M.L.; Aprian, A.; Meileza, N.; Hitsmi, M.; Elvia, R.; Rahmidar, L.; Khaydarov, R. Smartphone Coupled with a Paper-Based Colorimetric Device for Sensitive and Portable Mercury Ion Sensing. Chemosensors 2019, 7, 25. [Google Scholar] [CrossRef] [Green Version]
- Oyewunmi, O.D.; Safiabadi-Tali, S.H.; Jahanshahi-Anbuhi, S. Dual-Modal Assay Kit for the Qualitative and Quantitative Determination of the Total Water Hardness Using a Permanent Marker Fabricated Microfluidic Paper-Based Analytical Device. Chemosensors 2020, 8, 97. [Google Scholar] [CrossRef]
- Heidari-Bafroui, H.; Charbaji, A.; Anagnostopoulos, C.; Faghri, M. A Colorimetric Dip Strip Assay for Detection of Low Concentrations of Phosphate in Seawater. Sensors 2021, 21, 3125. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Hyung, J.; Noh, H. Rationalization of In-Situ Synthesized Plasmonic Paper for Colorimetric Detection of Glucose in Ocular Fluids. Chemosensors 2020, 8, 81. [Google Scholar] [CrossRef]
- Islam, N.; Ahmed, I.; Anik, M.I.; Ferdous, S.; Khan, M.S. Developing Paper Based Diagnostic Technique to Detect Uric Acid in Urine. Front. Chem. 2018, 6, 496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deroco, P.; Junior, D.W.; Kubota, L. Silver Inkjet-Printed Electrode on Paper for Electrochemical Sensing of Paraquat. Chemosensors 2021, 9, 61. [Google Scholar] [CrossRef]
- Migliorini, F.L.; Dos Santos, D.M.; Soares, A.C.; Mattoso, L.H.C.; Oliveira, J.O.N.; Correa, D.S. Design of A Low-Cost and Disposable Paper-Based Immunosensor for the Rapid and Sensitive Detection of Aflatoxin B1. Chemosensors 2020, 8, 87. [Google Scholar] [CrossRef]
- Teepoo, S.; Arsawiset, S.; Chanayota, P. One-Step Polylactic Acid Screen-Printing Microfluidic Paper-Based Analytical Device: Application for Simultaneous Detection of Nitrite and Nitrate in Food Samples. Chemosensors 2019, 7, 44. [Google Scholar] [CrossRef] [Green Version]
- Rosati, G.; Cunego, A.; Fracchetti, F.; Del Casale, A.; Scaramuzza, M.; De Toni, A.; Torriani, S.; Paccagnella, A. Inkjet Printed Interdigitated Biosensor for Easy and Rapid Detection of Bacteriophage Contamination: A Preliminary Study for Milk Processing Control Applications. Chemosensors 2019, 7, 8. [Google Scholar] [CrossRef] [Green Version]
- Shriver-Lake, L.C.; Zabetakis, D.; Dressick, W.J.; Stenger, D.A.; Trammell, S.A. Paper-Based Electrochemical Detection of Chlorate. Sensors 2018, 18, 328. [Google Scholar] [CrossRef] [Green Version]
- Zikulnig, J.; Khalifa, M.; Rauter, L.; Lammer, H.; Kosel, J. Low-Cost Inkjet-Printed Temperature Sensors on Paper Substrate for the Integration into Natural Fiber-Reinforced Lightweight Components. Chemosensors 2021, 9, 95. [Google Scholar] [CrossRef]
- Vargas, A.; Gámez, F.; Roales, J.; Lopes-Costa, T.; Pedrosa, J. A Paper-Based Ultrasensitive Optical Sensor for the Selective Detection of H2S Vapors. Chemosensors 2021, 9, 40. [Google Scholar] [CrossRef]
- Li, Z. Nanoporous Silica-Dye Microspheres for Enhanced Colorimetric Detection of Cyclohexanone. Chemosensors 2018, 6, 34. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.T.; Lantigua, D.; Meka, A.; Taing, S.; Pandher, M.; Camci-Unal, G. Paper-Based Sensors: Emerging Themes and Applications. Sensors 2018, 18, 2838. [Google Scholar] [CrossRef] [Green Version]
- Charbaji, A.; Heidari-Bafroui, H.; Anagnostopoulos, C.; Faghri, M. Literature Review of the Use of Zinc and Zinc Compounds in Paper-Based Microfluidic Devices. J. Miner. Mater. Charact. Eng. 2021, 9, 257–270. [Google Scholar] [CrossRef]
- Charbaji, A.; Heidari-Bafroui, H.; Kumar, A.; Ragmani, N. Characterization and Modeling of Paper-based Bi-Material Actuator Cantilever; Application in Phosphate Detection. In Proceedings of the Innovations in Microfluidics and Single Cell Analysis, Boston, MA, USA, 18–19 March 2021. [Google Scholar]
- Smith, W.; Rahmani, N.; Charbaji, A.; Lemos, N.; Anagnostopoulos, C.; Fanghri, M.; Hong, C. A Fluidically Controlled Bi-Material Actuator for Automation of Paper-Based Assays. In Proceedings of the International Symposium on Thermal Effects in Gas Flows in Microscale, Ettlingen, Germany, 24–25 October 2019. [Google Scholar]
- Charbaji, A.; Heidari-Bafroui, H.; Anagnostopoulos, C.; Faghri, M. Sensitive Detection of Nitrate using a Paper-based Microfluidic Device. In Proceedings of the Innovations in Microfluidics and Single Cell Analysis, Boston, MA, USA, 17–18 August 2020. [Google Scholar]
- Xu, Y.; Liu, M.; Kong, N.; Liu, J. Lab-on-paper micro- and nano-analytical devices: Fabrication, modification, detection and emerging applications. Microchim. Acta 2016, 183, 1521–1542. [Google Scholar] [CrossRef]
- Fernandes, G.M.; Silva, W.R.; Barreto, D.N.; Lamarca, R.S.; Lima Gomes, P.C.F.; Flávio, D.S.; Petruci, J.; Batista, A.D. Novel approaches for colorimetric measurements in analytical chemistry—A review. Anal. Chim. Acta 2020, 1135, 187–203. [Google Scholar] [CrossRef]
- Fernandes, S.C.; Walz, J.A.; Wilson, D.; Brooks, J.; Mace, C.R. Beyond Wicking: Expanding the Role of Patterned Paper as the Foundation for an Analytical Platform. Anal. Chem. 2017, 89, 5654–5664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasetsirikul, S.; Shiddiky, M.; Nguyen, N.-T. Wicking in Paper Strips under Consideration of Liquid Absorption Capacity. Chemosensors 2020, 8, 65. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking—Water Quality, 4th Edition, Incorporating the 1st Addendum. 2017. Available online: https://www.who.int/publications/i/item/9789241549950 (accessed on 22 October 2020).
- US Geological Survey Nitrogen and Water. Available online: https://www.usgs.gov/special-topic/water-science-school/science/nitrogen-and-water?qt-science_center_objects=0#qt-science_center_objects (accessed on 22 October 2020).
- US EPA Nutrient Pollution. Available online: https://www.epa.gov/nutrientpollution/issue (accessed on 13 May 2021).
- Silva, C.G.; Pereira, M.F.; Órfão, J.J.M.; Faria, J.L.; Soares, S. Catalytic and Photocatalytic Nitrate Reduction Over Pd-Cu Loaded Over Hybrid Materials of Multi-Walled Carbon Nanotubes and TiO2. Front. Chem. 2018, 6, 632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.; Gupta, R.C.; Gupta, A.B.; Eskiocak, S.; Rao, P.; Puttanna, K.; Singhvi, A. Pathophysiology of Nitrate Toxicity in Human and its Mitigation Measures. Bull. Reg. Assess React. Nitrogen 2010, 20, 1–78. [Google Scholar]
- Ward, M.H.; Jones, R.R.; Brender, J.D.; De Kok, T.M.; Weyer, P.J.; Nolan, B.T.; Villanueva, C.M.; Van Breda, S.G. Drinking Water Nitrate and Human Health: An Updated Review. Int. J. Environ. Res. Public Health 2018, 15, 1557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wierzbicka, E. Novel methods of nitrate and nitrite determination—A review. J. Elem. 2020, 25, 97–106. [Google Scholar] [CrossRef]
- Kapoor, A.; Balasubramanian, S.; Muthamilselvi, P.; Vaishampayan, V.; Prabhakar, S. Lab-on-a-Chip Devices for Water Quality Monitoring. In Nanotechnology in the Life Sciences; Springer: Cham, Switzerland, 2020; pp. 455–469. [Google Scholar]
- Charbaji, A.; Heidari-Bafroui, H.; Anagnostopoulos, C.; Faghri, M. A New Paper-Based Microfluidic Device for Improved Detection of Nitrate in Water. Sensors 2020, 21, 102. [Google Scholar] [CrossRef]
- Jayawardane, B.M.; Wongwilai, W.; Grudpan, K.; Kolev, S.; Heaven, M.; Nash, D.M.; McKelvie, I. Evaluation and Application of a Paper-Based Device for the Determination of Reactive Phosphate in Soil Solution. J. Environ. Qual. 2014, 43, 1081–1085. [Google Scholar] [CrossRef] [Green Version]
- Ratnarathorn, N.; Dungchai, W. Paper-based Analytical Device (PAD) for the Determination of Borax, Salicylic Acid, Nitrite, and Nitrate by Colorimetric Methods. J. Anal. Chem. 2020, 75, 487–494. [Google Scholar] [CrossRef]
- Thongkam, T.; Hemavibool, K. An environmentally friendly microfluidic paper-based analytical device for simultaneous colorimetric detection of nitrite and nitrate in food products. Microchem. J. 2020, 159, 105412. [Google Scholar] [CrossRef]
- Ferreira, F.T.S.M.; Mesquita, R.B.R.; Rangel, A.O.S.S. Novel microfluidic paper-based analytical devices (μPADs) for the determination of nitrate and nitrite in human saliva. Talanta 2020, 219, 121183. [Google Scholar] [CrossRef]
- Wang, Q.-H.; Yu, L.-J.; Liu, Y.; Lin, L.; Lu, R.-G.; Zhu, J.-P.; He, L.; Lu, Z.-L. Methods for the detection and determination of nitrite and nitrate: A review. Talanta 2017, 165, 709–720. [Google Scholar] [CrossRef]
- Mahmud, M.A.P.; Ejeian, F.; Azadi, S.; Myers, M.; Pejcic, B.; Abbassi, R.; Razmjou, A.; Asadnia, M. Recent progress in sensing nitrate, nitrite, phosphate, and ammonium in aquatic environment. Chemosphere 2020, 259, 127492. [Google Scholar] [CrossRef]
- Alahi, M.E.E.; Mukhopadhyay, S.C. Detection methods of nitrate in water: A review. Sens. Actuators A Phys. 2018, 280, 210–221. [Google Scholar] [CrossRef]
- Jaywant, S.A.; Arif, K.M. A Comprehensive Review of Microfluidic Water Quality Monitoring Sensors. Sensors 2019, 19, 4781. [Google Scholar] [CrossRef] [Green Version]
- Ellis, P.S.; Shabani, A.M.H.; Gentle, B.S.; McKelvie, I.D. Field measurement of nitrate in marine and estuarine waters with a flow analysis system utilizing on-line zinc reduction. Talanta 2011, 84, 98–103. [Google Scholar] [CrossRef]
- Charbaji, A.; Smith, W.; Anagnostopoulos, C.; Faghri, M. Zinculose: A new fibrous material with embedded zinc particles. Eng. Sci. Technol. Int. J. 2021, 24, 571–578. [Google Scholar] [CrossRef]
- Charbaji, A.; Heidari-Bafroui, H.; Anagnostopoulos, C.; Faghri, M. A Practical System for the Quantitative Determination of Nitrate and Nitrite in the Field. In Proceedings of the 6th International Microfluidics Conference, Las Vegas, NV, USA, 26 March 2021. [Google Scholar]
- James, N.; Miller, J.C. Statistics and Chemometrics for Analytical Chemistry, 5th ed.; Pearson: London, UK, 2005. [Google Scholar]
- Weng, C.-H.; Chen, M.-Y.; Shen, C.-H.; Yang, R.-J. Colored wax-printed timers for two-dimensional and three-dimensional assays on paper-based devices. Biomicrofluidics 2014, 8, 066502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heidari-Bafroui, H.; Ribeiro, B.; Charbaji, A.; Anagnostopoulos, C.; Faghri, M. Portable infrared lightbox for improving the detection limits of paper-based phosphate devices. Meas. J. Int. Meas. Conf. 2021, 173, 108607. [Google Scholar] [CrossRef]


| Reference | Media | LOD (ppm) | LOQ (ppm) |
|---|---|---|---|
| [32] | Water | 0.533 | 1.765 |
| [33] | Water | 1.178 | 2.976 |
| [9] | Food Sample | 3.6 | 12 |
| [34] | Food Sample | 0.4 | NA 1 |
| [35] | Food Sample | 0.4 | 1.4 |
| [36] | Human Saliva | 4.96 | 16.74 |
| Nitrate Concentration (ppm) | Normalized Nitrite Concentration Calculated (ppm) 1 | Reduction Efficiency (%) |
|---|---|---|
| 0 | 0 | 0 |
| 0.5 | 0.098 | 19.54 ± 0.80 |
| 1 | 0.006 | 0.61 ± 0.23 |
| 2.5 | 0.300 | 12 ± 0. 05 |
| 5 | 0.524 | 10.48 ± 0.08 |
| 10 | 1.086 | 10.86 ± 0.01 |
| 20 | 3.296 | 16.48 ± 0.03 |
| 40 | 10.896 | 27.24 ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charbaji, A.; Heidari-Bafroui, H.; Rahmani, N.; Anagnostopoulos, C.; Faghri, M. Colorimetric Determination of Nitrate after Reduction to Nitrite in a Paper-Based Dip Strip. Chem. Proc. 2021, 5, 9. https://doi.org/10.3390/CSAC2021-10459
Charbaji A, Heidari-Bafroui H, Rahmani N, Anagnostopoulos C, Faghri M. Colorimetric Determination of Nitrate after Reduction to Nitrite in a Paper-Based Dip Strip. Chemistry Proceedings. 2021; 5(1):9. https://doi.org/10.3390/CSAC2021-10459
Chicago/Turabian StyleCharbaji, Amer, Hojat Heidari-Bafroui, Nasim Rahmani, Constantine Anagnostopoulos, and Mohammad Faghri. 2021. "Colorimetric Determination of Nitrate after Reduction to Nitrite in a Paper-Based Dip Strip" Chemistry Proceedings 5, no. 1: 9. https://doi.org/10.3390/CSAC2021-10459
APA StyleCharbaji, A., Heidari-Bafroui, H., Rahmani, N., Anagnostopoulos, C., & Faghri, M. (2021). Colorimetric Determination of Nitrate after Reduction to Nitrite in a Paper-Based Dip Strip. Chemistry Proceedings, 5(1), 9. https://doi.org/10.3390/CSAC2021-10459








