ZnO-Incorporated ZSM-5 for Photocatalytic CO2 Reduction into Solar Fuels under UV–Visible Light †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. ZnO/ZSM-5 Synthesis
2.3. ZnO/ZSM-5 Characterization
2.4. Photocatalytic Activity Assessment
3. Results and Discussion
3.1. Structural Analysis
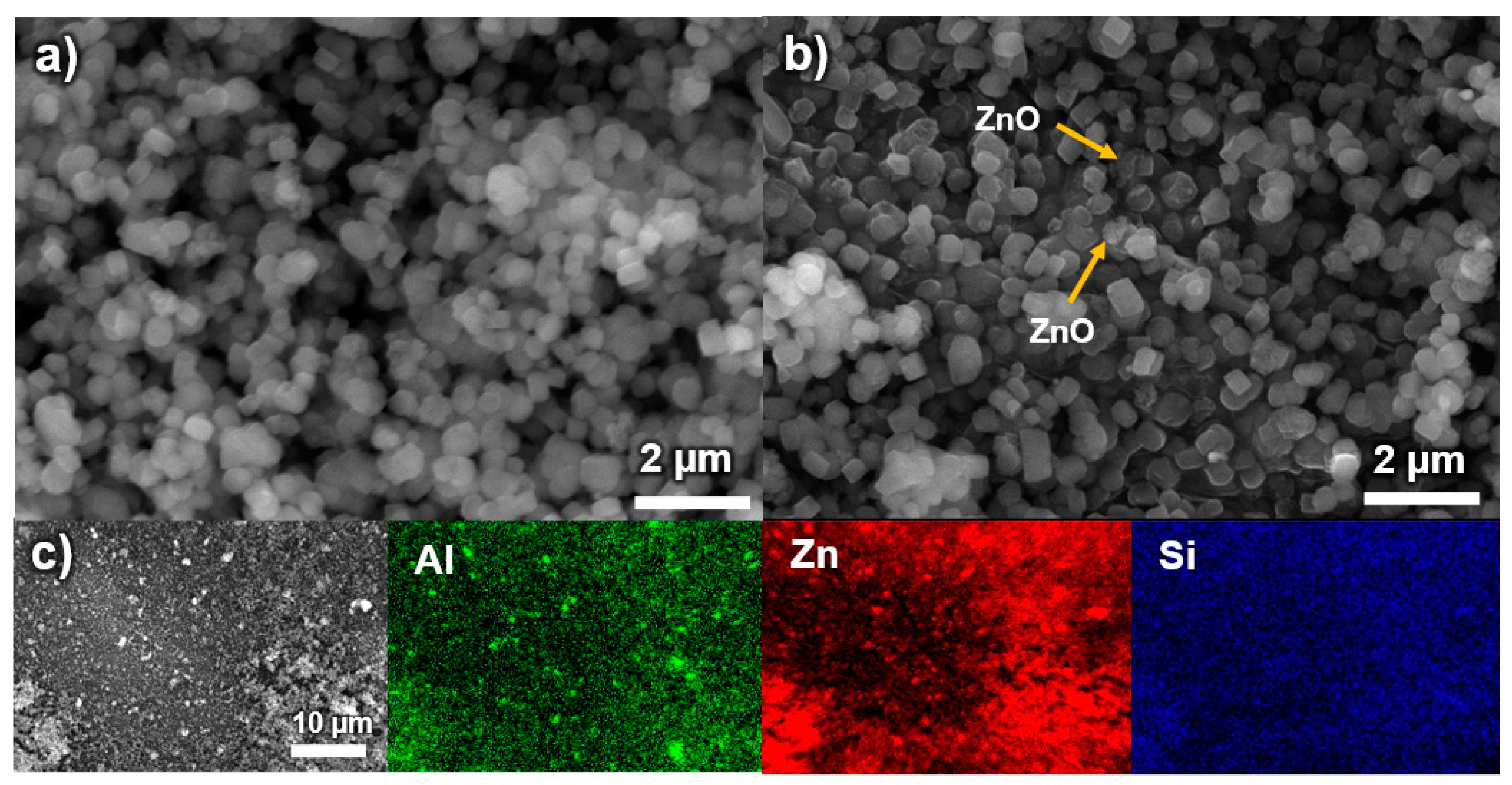
3.2. Morphological Analysis
3.3. Optical Analysis
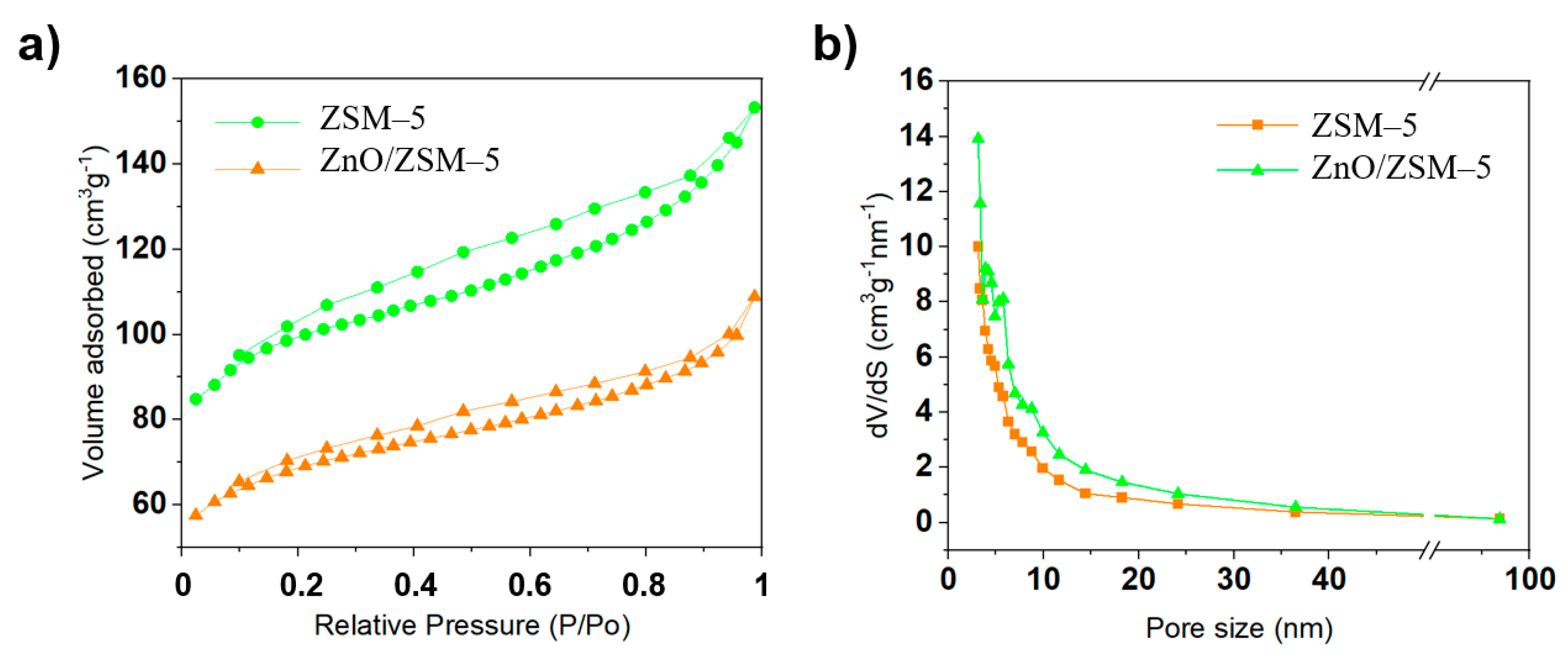
3.4. Textural Analysis
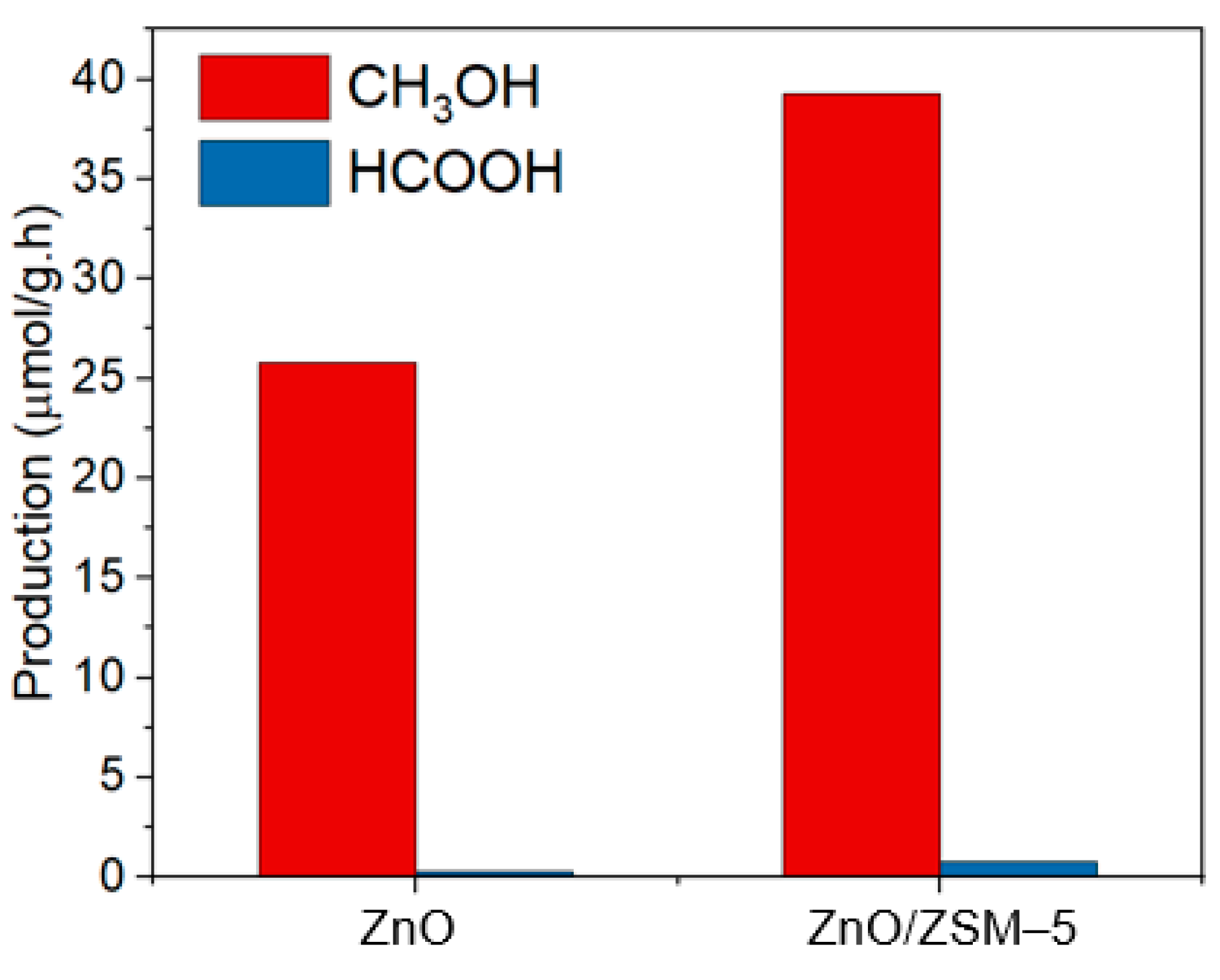
3.5. Photocatalytic Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodriguez, J.; Puzenat, E.; Thivel, P.-X. From solar photocatalysis to fuel-cell: A hydrogen supply chain. J. Environ. Chem. Eng. 2016, 4, 3001–3005. [Google Scholar] [CrossRef]
- Mustafa, A.; Lougou, B.G.; Shuai, Y.; Wang, Z.; Tan, H. Current technology development for CO2 utilization into solar fuels and chemicals: A review. J. Energy Chem. 2020, 49, 96–123. [Google Scholar] [CrossRef]
- Ma, S.; Wang, W. Preparation and photocatalytic hydrogen evolution of g-C3N4/ZnO composite. E3S Web Conf. 2020, 165, 05007. [Google Scholar] [CrossRef]
- Salazar, H.; Martins, P.M.; Santos, B.; Fernandes, M.M.; Reizabal, A.; Sebastián, V.; Botelho, G.; Tavares, C.J.; Vilas-Vilela, J.L.; Lanceros-Mendez, S. Photocatalytic and antimicrobial multifunctional nanocomposite membranes for emerging pollutants water treatment applications. Chemosphere 2020, 250, 126299. [Google Scholar] [CrossRef]
- Theerthagiri, J.; Salla, S.; Senthil, R.A.; Nithyadharseni, P.; Madankumar, A.; Arunachalam, P.; Maiyalagan, T.; Kim, H.-S. A review on ZnO nanostructured materials: Energy, environmental and biological applications. Nanotechnology 2019, 30, 392001. [Google Scholar] [CrossRef] [PubMed]
- Kumaresan, N.; Ramamurthi, K.; Ramesh Babu, R.; Sethuraman, K.; Moorthy Babu, S. Hydrothermally grown ZnO nanoparticles for effective photocatalytic activity. Appl. Surf. Sci. 2017, 418, 138–146. [Google Scholar] [CrossRef]
- Rai, S.; Bhujel, R.; Khadka, M.; Chetry, R.L.; Swain, B.P.; Biswas, J. Synthesis, characterizations, and electrochemical studies of ZnO/reduced graphene oxide nanohybrids for supercapacitor application. Mater. Today Chem. 2021, 20, 100472. [Google Scholar] [CrossRef]
- Paul, R.; Kumbhakar, P.; Mitra, A.K. Blue–green luminescence by SWCNT/ZnO hybrid nanostructure synthesized by a simple chemical route. Phys. E Low-Dimens. Syst. Nanostruct. 2010, 43, 279–284. [Google Scholar] [CrossRef]
- Zhao, L.; Ji, Y.; Sun, P.; Li, R.; Xiang, F.; Wang, H.; Ruiz-Martinez, J.; Yang, Y. Effects of individual and complex ciprofloxacin, fullerene C60, and ZnO nanoparticles on sludge digestion: Methane production, metabolism, and microbial community. Bioresour. Technol. 2018, 267, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Kumari, V.; Yadav, S.; Mittal, A.; Kumari, K.; Mari, B.; Kumar, N. Surface Plasmon response of Pd deposited ZnO/CuO nanostructures with enhanced photocatalytic efficacy towards the degradation of organic pollutants. Inorg. Chem. Commun. 2020, 121, 108241. [Google Scholar] [CrossRef]
- Wang, C.; Shi, H.; Li, Y. Synthesis and characteristics of natural zeolite supported Fe3+-TiO2 photocatalysts. Appl. Surf. Sci. 2011, 257, 6873–6877. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Mkhalid, I.A.; Abdel Salam, M.; Barakat, M.A. Zeolite Y from rice husk ash encapsulated with Ag-TiO2: Characterization and applications for photocatalytic degradation catalysts. Desalination Water Treat. 2013, 51, 7562–7569. [Google Scholar] [CrossRef]
- Batistela, V.R.; Fogaça, L.Z.; Fávaro, S.L.; Caetano, W.; Fernandes-Machado, N.R.C.; Hioka, N. ZnO supported on zeolites: Photocatalyst design, microporosity and properties. Colloids Surf. A Physicochem. Eng. Asp. 2017, 513, 20–27. [Google Scholar] [CrossRef]
- Šuligoj, A.; Pavlović, J.; Arčon, I.; Rajić, N.; Novak Tušar, N. SnO2-Containing Clinoptilolite as a Composite Photocatalyst for Dyes Removal from Wastewater under Solar Light. Catalysts 2020, 10, 253. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Wang, K.; Yu, Y.; He, H. TiO2/HZSM-5 nano-composite photocatalyst: HCl treatment of NaZSM-5 promotes photocatalytic degradation of methyl orange. Chem. Eng. J. 2010, 163, 62–67. [Google Scholar] [CrossRef]
- Takeuchi, M.; Kimura, T.; Hidaka, M.; Rakhmawaty, D.; Anpo, M. Photocatalytic oxidation of acetaldehyde with oxygen on TiO2/ZSM-5 photocatalysts: Effect of hydrophobicity of zeolites. J. Catal. 2007, 246, 235–240. [Google Scholar] [CrossRef]
- Noorjahan, M. A novel and efficient photocatalyst: TiO2-HZSM-5 combinate thin film. Appl. Catal. B Environ. 2004, 47, 209–213. [Google Scholar] [CrossRef]
- Haghighi, M.; Rahmani, F.; Dehghani, R.; Tehrani, A.M.; Bagher Miranzadeh, M. Photocatalytic reduction of Cr (VI) in aqueous solution over ZnO/ HZSM-5 nanocomposite: Optimization of ZnO loading and process conditions. Desalination Water Treat. 2017, 58, 168–180. [Google Scholar] [CrossRef]
- Khoshbin, R.; Haghighi, M. Direct syngas to DME as a clean fuel: The beneficial use of ultrasound for the preparation of CuO–ZnO–Al2O3/HZSM-5 nanocatalyst. Chem. Eng. Res. Des. 2013, 91, 1111–1122. [Google Scholar] [CrossRef]
- Korkuna, O.; Leboda, R.; Skubiszewska-Zieba, J.; Vrublevska, T.; Gunko, V.M.; Ryczkowski, J. Structural and physicochemical properties of natural zeolites: Clinoptilolite and mordenite. Microporous Mesoporous Mater. 2006, 87, 243–254. [Google Scholar] [CrossRef]
- Khatamian, M.; Divband, B.; Jodaei, A. Degradation of 4-nitrophenol (4-NP) using ZnO nanoparticles supported on zeolites and modeling of experimental results by artificial neural networks. Mater. Chem. Phys. 2012, 134, 31–37. [Google Scholar] [CrossRef]
- Flanigen, E.M. Zeolites and Molecular Sieves: An Historical Perspective. In Studies in Surface Science and Catalysis; Elsevier: Amsterdam, The Netherlands, 2001; Volume 173, Chapter 2; pp. 11–35. [Google Scholar]
- Seijger, G.B.; Oudshoorn, O.; van Kooten, W.E.; Jansen, J.; van Bekkum, H.; van den Bleek, C.; Calis, H.P. In situ synthesis of binderless ZSM-5 zeolitic coatings on ceramic foam supports. Microporous Mesoporous Mater. 2000, 39, 195–204. [Google Scholar] [CrossRef]
- Song, C.; Li, X.; Zhu, X.; Liu, S.; Chen, F.; Liu, F.; Xu, L. Influence of the state of Zn species over Zn-ZSM-5/ZSM-11 on the coupling effects of cofeeding n-butane with methanol. Appl. Catal. A Gen. 2016, 519, 48–55. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Y.; Diao, J.; Qiang, M.; Chen, Z. Synthesis and Photocatalytic Activity of Hierarchical Zn-ZSM-5 Structures. Catalysts 2021, 11, 797. [Google Scholar] [CrossRef]
- Wang, L.; Jin, P.; Duan, S.; She, H.; Huang, J.; Wang, Q. In-situ incorporation of Copper(II) porphyrin functionalized zirconium MOF and TiO2 for efficient photocatalytic CO2 reduction. Sci. Bull. 2019, 64, 926–933. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Chen, J.; Feng, Z.; Chen, T.; Wang, X.; Ying, P.; Li, C. Time-Resolved Photoluminescence Characteristics of Subnanometer ZnO Clusters Confined in the Micropores of Zeolites. J. Phys. Chem. B 2006, 110, 25612–25618. [Google Scholar] [CrossRef] [PubMed]
- Flores, G.; Carrillo, J.; Luna, J.A.; Martínez, R.; Sierra-Fernandez, A.; Milosevic, O.; Rabanal, M.E. Synthesis, characterization and photocatalytic properties of nanostructured ZnO particles obtained by low temperature air-assisted-USP. Adv. Powder Technol. 2014, 25, 1435–1441. [Google Scholar] [CrossRef]
- Yaripour, F.; Shariatinia, Z.; Sahebdelfar, S.; Irandoukht, A. Conventional hydrothermal synthesis of nanostructured H-ZSM-5 catalysts using various templates for light olefins production from methanol. J. Nat. Gas Sci. Eng. 2015, 22, 260–269. [Google Scholar] [CrossRef]
- Luévano-Hipólito, E.; Torres-Martínez, L.M.; Fernández-Trujillo, A. Ternary ZnO/CuO/Zeolite composite obtained from volcanic ash for photocatalytic CO2 reduction and H2O decomposition. J. Phys. Chem. Solids 2021, 151, 109917. [Google Scholar] [CrossRef]
- Huerta-Flores, A.M.; Luévano-Hipólito, E.; Torres-Martínez, L.M.; Torres-Sánchez, A. Photocatalytic H2 production and CO2 reduction on Cu, Ni-doped ZnO: Effect of metal doping and oxygen vacancies. J. Mater. Sci. Mater. Electron. 2019, 30, 18506–18518. [Google Scholar] [CrossRef]


| Samples | Crystallite Size (nm) | Relative Crystallinity (%) |
|---|---|---|
| ZnO | 26.37 | - |
| ZSM-5 | 11.53 | 87.53 |
| ZnO/ZSM-5 | 9.94 | 68.33 |
| Samples | ZnO (%-wt) | Al2O3 (%-wt) | SiO2 (%) | Si/Al Ratio |
|---|---|---|---|---|
| ZnO | 86.12 | - | - | - |
| ZSM-5 | - | 0.92 | 46.62 | 50.67 |
| ZnO/ZSM-5 | 4.92 | 0.86 | 35.11 | 40.82 |
| Samples | SBET (m2/g) | Pore Size (nm) | Pore Volume (cm3/g) |
|---|---|---|---|
| ZnO | 8.43 | 11.34 | 0.023 |
| ZSM-5 | 314.68 | 3.05 | 0.178 |
| ZnO/ZSM-5 | 220.49 | 3.02 | 0.237 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Satriyatama, A.; Budi, I.D.M.; Iman, H.N.; Susilo, H.; Saputera, W.H. ZnO-Incorporated ZSM-5 for Photocatalytic CO2 Reduction into Solar Fuels under UV–Visible Light. Chem. Proc. 2022, 6, 1. https://doi.org/10.3390/ECCS2021-11205
Satriyatama A, Budi IDM, Iman HN, Susilo H, Saputera WH. ZnO-Incorporated ZSM-5 for Photocatalytic CO2 Reduction into Solar Fuels under UV–Visible Light. Chemistry Proceedings. 2022; 6(1):1. https://doi.org/10.3390/ECCS2021-11205
Chicago/Turabian StyleSatriyatama, Adhi, Ignatius Dozy Mahatmanto Budi, Hilya Nadhira Iman, Henry Susilo, and Wibawa Hendra Saputera. 2022. "ZnO-Incorporated ZSM-5 for Photocatalytic CO2 Reduction into Solar Fuels under UV–Visible Light" Chemistry Proceedings 6, no. 1: 1. https://doi.org/10.3390/ECCS2021-11205
APA StyleSatriyatama, A., Budi, I. D. M., Iman, H. N., Susilo, H., & Saputera, W. H. (2022). ZnO-Incorporated ZSM-5 for Photocatalytic CO2 Reduction into Solar Fuels under UV–Visible Light. Chemistry Proceedings, 6(1), 1. https://doi.org/10.3390/ECCS2021-11205







