Dysprosium(III)-Mediated Carboxylate Formation from a Schiff Base †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and General Methods
2.2. Syntheses
3. Results and Discussion
3.1. Synthesis and Spectroscopic Characterization
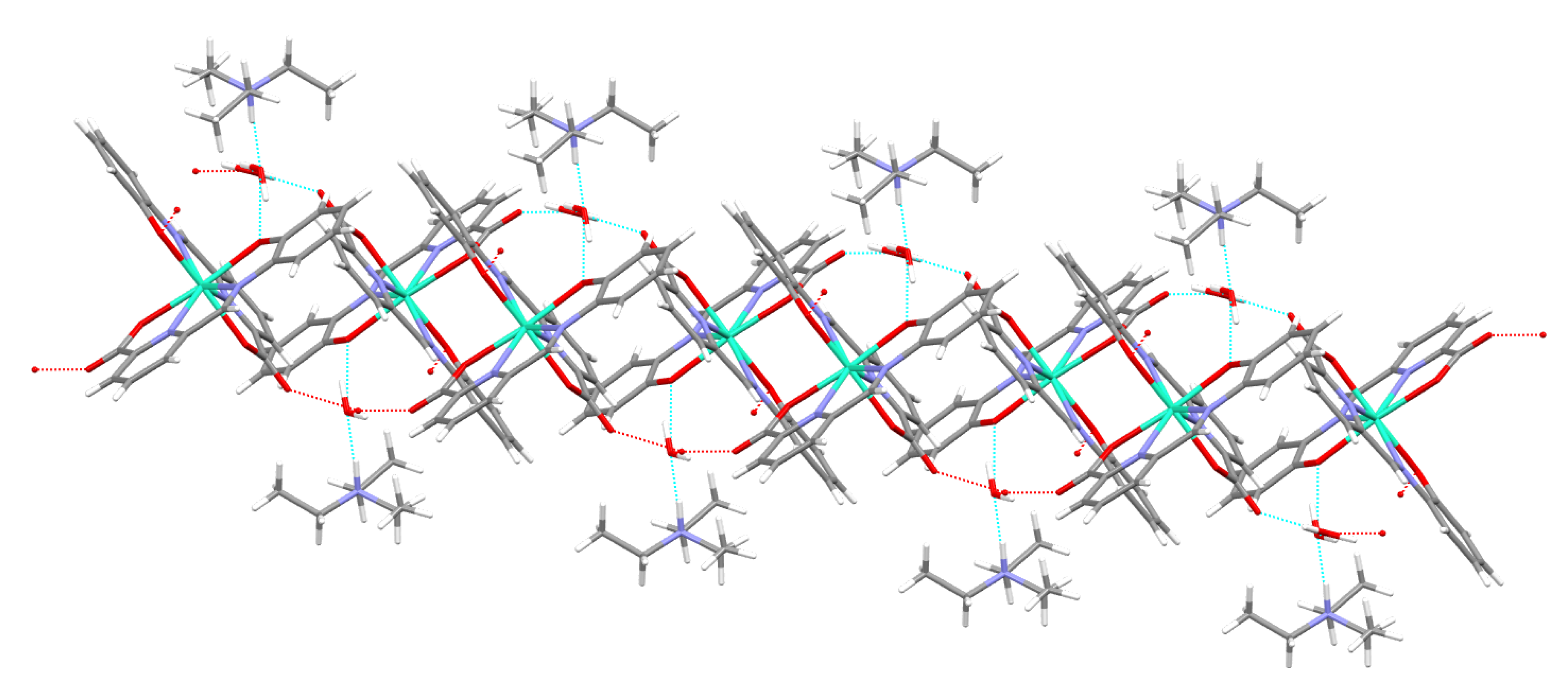
3.2. Single X-ray Diffraction Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Reeves, L.R. On the Mechanism, substituent effects, and intramolecular catalysis in Schiff base hydrolysis. J. Org. Chem. 1965, 9, 3129–3135. [Google Scholar] [CrossRef]
- Fenton, D.E.; Westwood, G.P.; Bashall, A.; McPartlin, M.; Scowen, I.J. Partial hydrolysis of a Schiff-base tripodal ligand induced by copper(II) salts: Crystal structure of the product from reaction with copper(II) perchlorate. J. Chem. Soc. Dalton Trans. 1994, 14, 2213–2214. [Google Scholar] [CrossRef]
- Czaun, M.; Nelana, S.M.; Guzei, I.A.; Hasselgren, C.; Håkansson, M.; Jagner, S.; Lisensky, G.; Darkwa, J.; Nordlander, E. An investigation of Cu(II) and Ni(II)-catalysed hydrolysis of (di)imines. Inorg. Chim. Acta 2010, 363, 3102–3112. [Google Scholar] [CrossRef]
- Barma, A.; Bhattacharjee, A.; Roy, P. Dinuclear copper(II) complexes with N,O donor ligands: Partial ligand hydrolysis and alcohol oxidation catalysis. Eur. J. Inorg. Chem. 2021, 2021, 2284–2292. [Google Scholar] [CrossRef]
- Fondo, M.; Corredoira-Vázquez, J.; García-Deibe, A.M.; Gómez-Coca, S.; Ruiz, E.; Sanmartín-Matalobos, J. Dysprosium-based complexes with a flat pentadentate donor: A magnetic and ab initio study. Dalton Trans. 2020, 49, 8389–8401. [Google Scholar] [CrossRef] [PubMed]
- Khatana, A.K.; Singh, V.; Gupta, M.K.; Tiwari, B. A highly efficient NHC-catalyzed aerobic oxidation of aldehydes to carboxylic acids. Synthesis 2018, 50, 4290–4294. [Google Scholar] [CrossRef]
- Dai, P.-F.; Qu, J.-P.; Kang, Y.-B. Organocatalyzed aerobic oxidation of aldehydes to acids. Org. Lett. 2019, 21, 1393–1396. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Wu, C.; Zhou, L.; Zhu, L.; Yao, X. Aerobic oxidation of aldehydes to carboxylic acids catalyzed by recyclable Ag/C3N4 catalyst. Lett. Org. Chem. 2021, 18, 167–175. [Google Scholar] [CrossRef]
- Geissman, T.A. The Cannizzaro reaction. Org. React. 1944, 2, 94–113. [Google Scholar] [CrossRef]
- Siemens Industrial Automation, Inc. SADABS: Area-Detector Absorption Correction; Siemens Industrial Automation, Inc.: Madison, WI, USA, 1996. [Google Scholar]
- Sheldrick, G.M. SHELXT-Integrated Space-Group and Crystal-Structure Determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar] [CrossRef]
- Fecteau, K.M.; Gould, I.R.; Glein, C.R.; Williams, L.B.; Hartnett, H.E.; Shock, E.L. Production of carboxylic acids from aldehydes under hydrothermal conditions: A kinetics study of benzaldehyde. ACS Earth Space Chem. 2019, 3, 170–191. [Google Scholar] [CrossRef]
- Llunell, M.; Casanova, D.; Cirera, J.; Bofill, J.M.; Alemany, P.; Alvarez, S.; Pinsky, M.; Avnir, D. SHAPE v1.1b; University of Barcelona: Barcelona, Spain, 2005. [Google Scholar]
- Ruiz-Martínez, A.; Casanova, D.; Alvarez, S. Polyhedral Structures with an Odd Number of Vertices: Nine-Coordinate Metal Compounds. Chem. Eur. J. 2008, 14, 1291–1303. [Google Scholar] [CrossRef] [PubMed]
- Llunell, M.; Casanova, D.; Cirera, J.; Alemany, P.; Alvarez, S. SHAPE: Program for the Stereochemical Analysis of Molecular Fragments by Means of Continuous Shape Measures and Associated Tools; University of Barcelona: Barcelona, Spain, 2010. [Google Scholar]


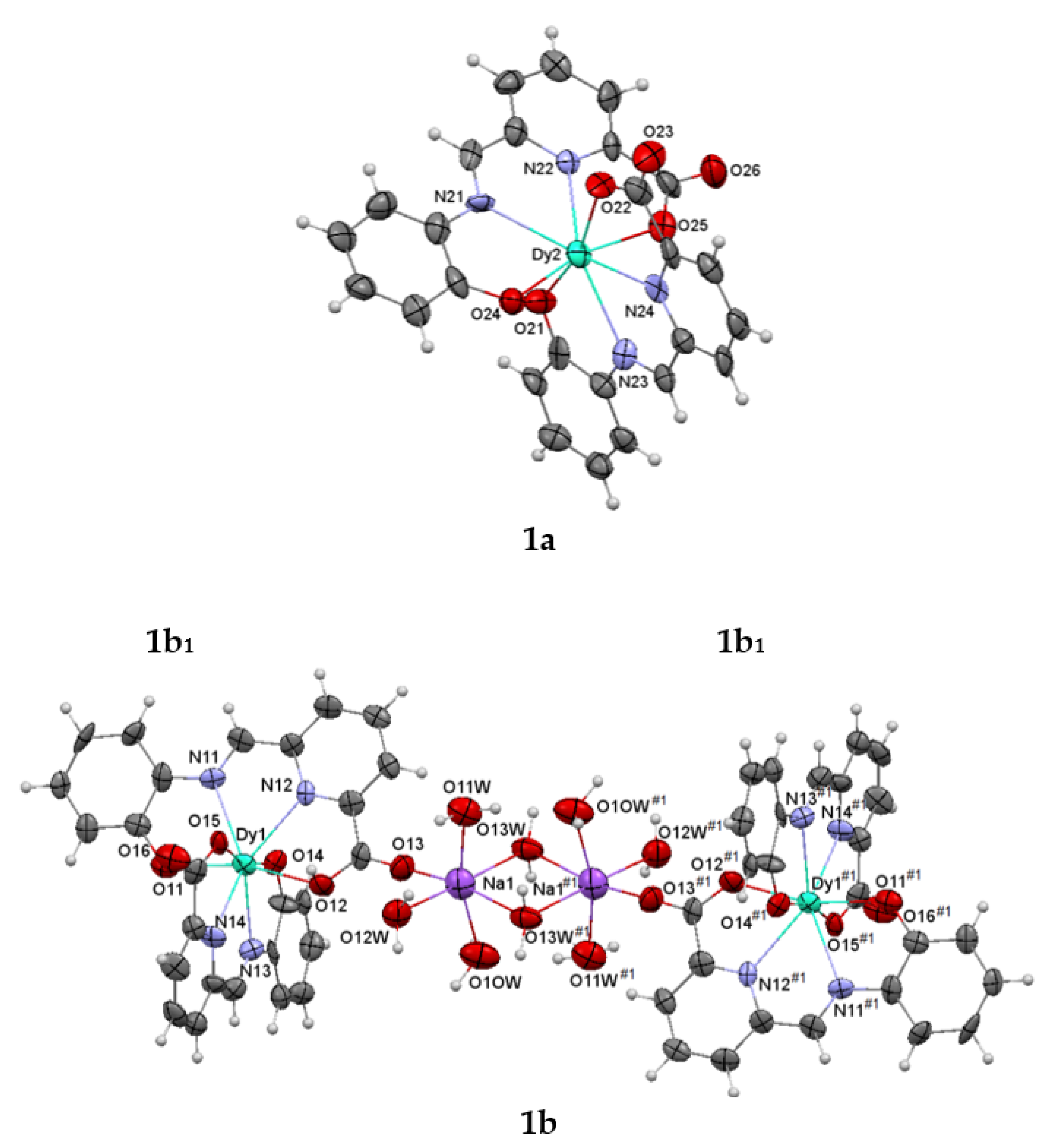
| Dy1-O11 | 2.216(15) | Dy2-O21 | 2.285(13) |
| Dy1-O12 | 2.354(14) | Dy2-O22 | 2.388(12) |
| Dy1-O14 | 2.297(12) | Dy2-O24 | 2.263(13) |
| Dy1-O15 | 2.349(13) | Dy2-O25 | 2.317(13) |
| Dy1-N11 | 2.502(14) | Dy2-N23 | 2.514(16) |
| Dy1-N12 | 2.494(15) | Dy2-N24 | 2.502(14) |
| Dy1-N13 | 2.475(15) | Dy2-N21 | 2.501(16) |
| Dy1-N14 | 2.496(14) | Dy2-N22 | 2.508(15) |
| Na1-O1OW | 2.34(2) | Na1-O13 | 2.347(18) |
| Na1-O11W | 2.392(19) | Na1-O13W | 2.614(18) |
| Na1-O12W | 2.383(17) | Na1-O13W #1 | 2.436(17) |
| N12-Dy1-N11 | 63.5(5) | N24-Dy2-N23 | 63.1(5) |
| O14-Dy1-O15 | 163.7(5) | O24-Dy2-O25 | 165.2(5) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Corredoira-Vázquez, J.; González-Barreira, C.; Deibe, A.M.G.; Sanmartín-Matalobos, J.; Fondo, M. Dysprosium(III)-Mediated Carboxylate Formation from a Schiff Base. Chem. Proc. 2022, 8, 36. https://doi.org/10.3390/ecsoc-25-11740
Corredoira-Vázquez J, González-Barreira C, Deibe AMG, Sanmartín-Matalobos J, Fondo M. Dysprosium(III)-Mediated Carboxylate Formation from a Schiff Base. Chemistry Proceedings. 2022; 8(1):36. https://doi.org/10.3390/ecsoc-25-11740
Chicago/Turabian StyleCorredoira-Vázquez, Julio, Cristina González-Barreira, Ana M. García Deibe, Jesús Sanmartín-Matalobos, and Matilde Fondo. 2022. "Dysprosium(III)-Mediated Carboxylate Formation from a Schiff Base" Chemistry Proceedings 8, no. 1: 36. https://doi.org/10.3390/ecsoc-25-11740
APA StyleCorredoira-Vázquez, J., González-Barreira, C., Deibe, A. M. G., Sanmartín-Matalobos, J., & Fondo, M. (2022). Dysprosium(III)-Mediated Carboxylate Formation from a Schiff Base. Chemistry Proceedings, 8(1), 36. https://doi.org/10.3390/ecsoc-25-11740










