Ultrasound Assisted Synthesis of 1,5-Disubstituted Tetrazoles Containing Propargyl or 2-Azidophenyl Moieties via Ugi-Azide Reaction †
Abstract
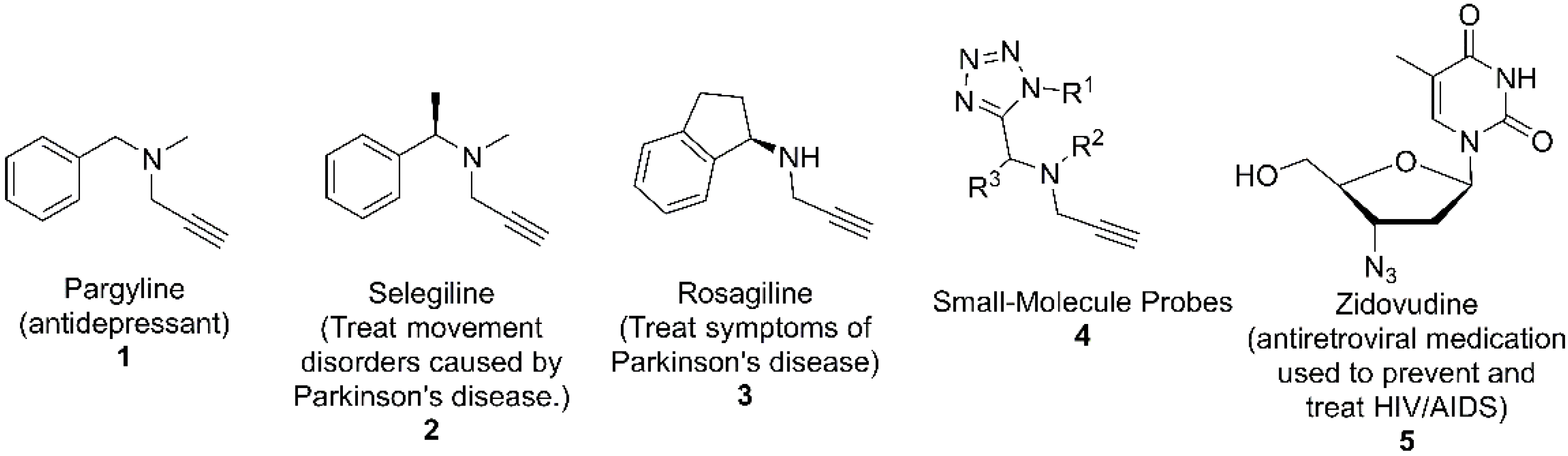
1. Introduction
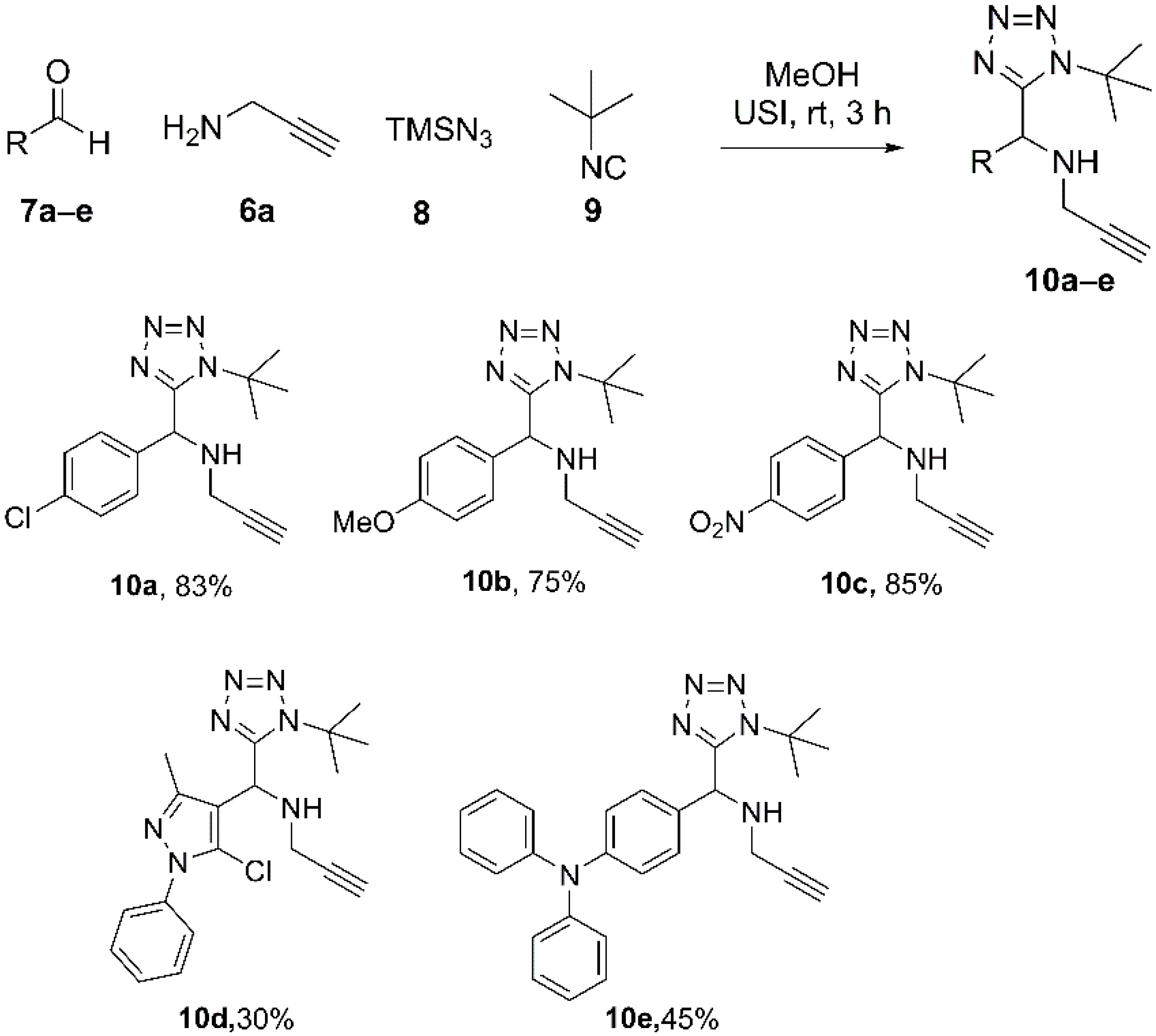
2. Results and Discussion
3. Conclusions
4. Experimental Section
4.1. General Information, Instrumentation, and Chemicals
4.2. Synthesis and Characterization of 1,5-DS-T (10a–e)
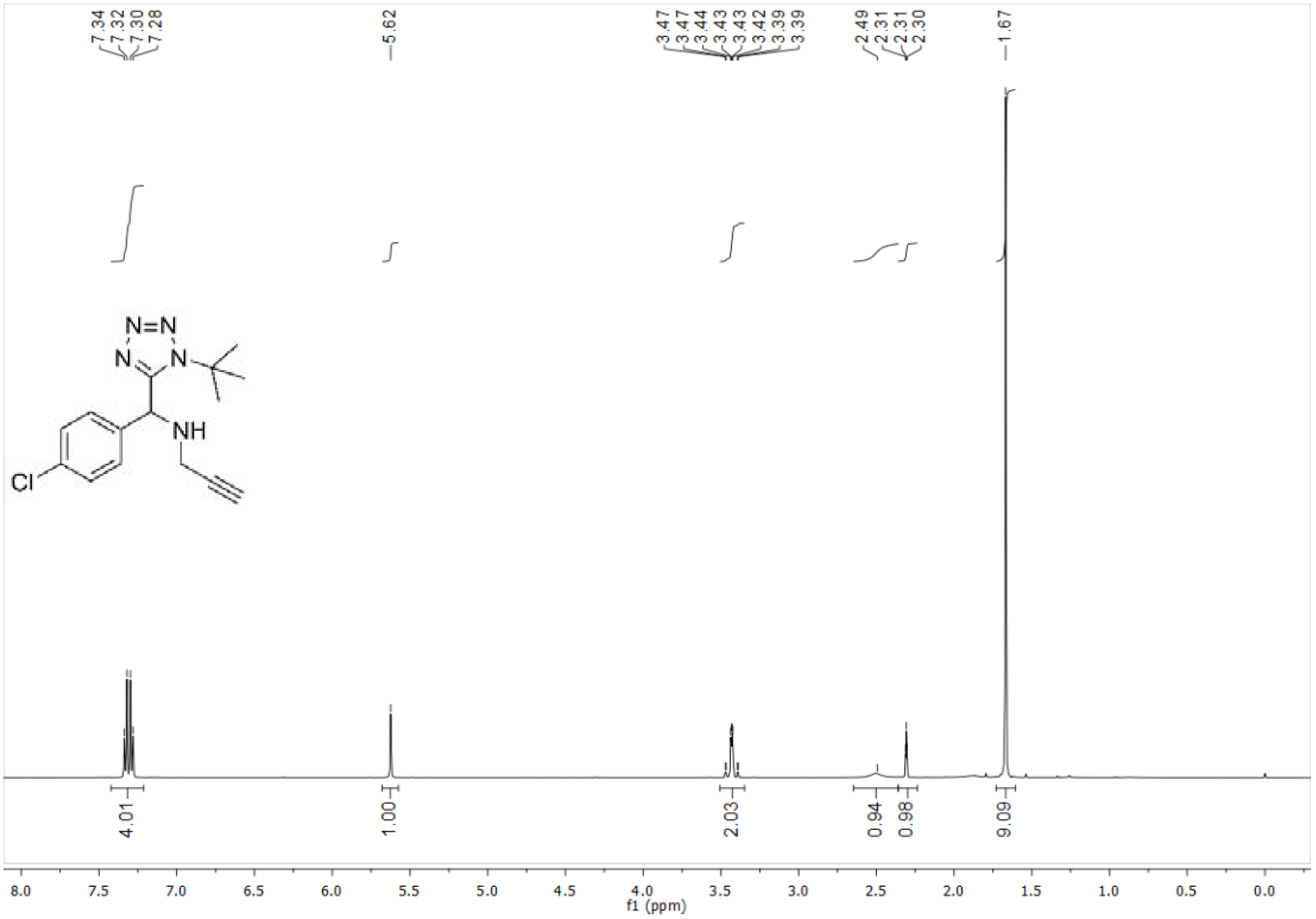
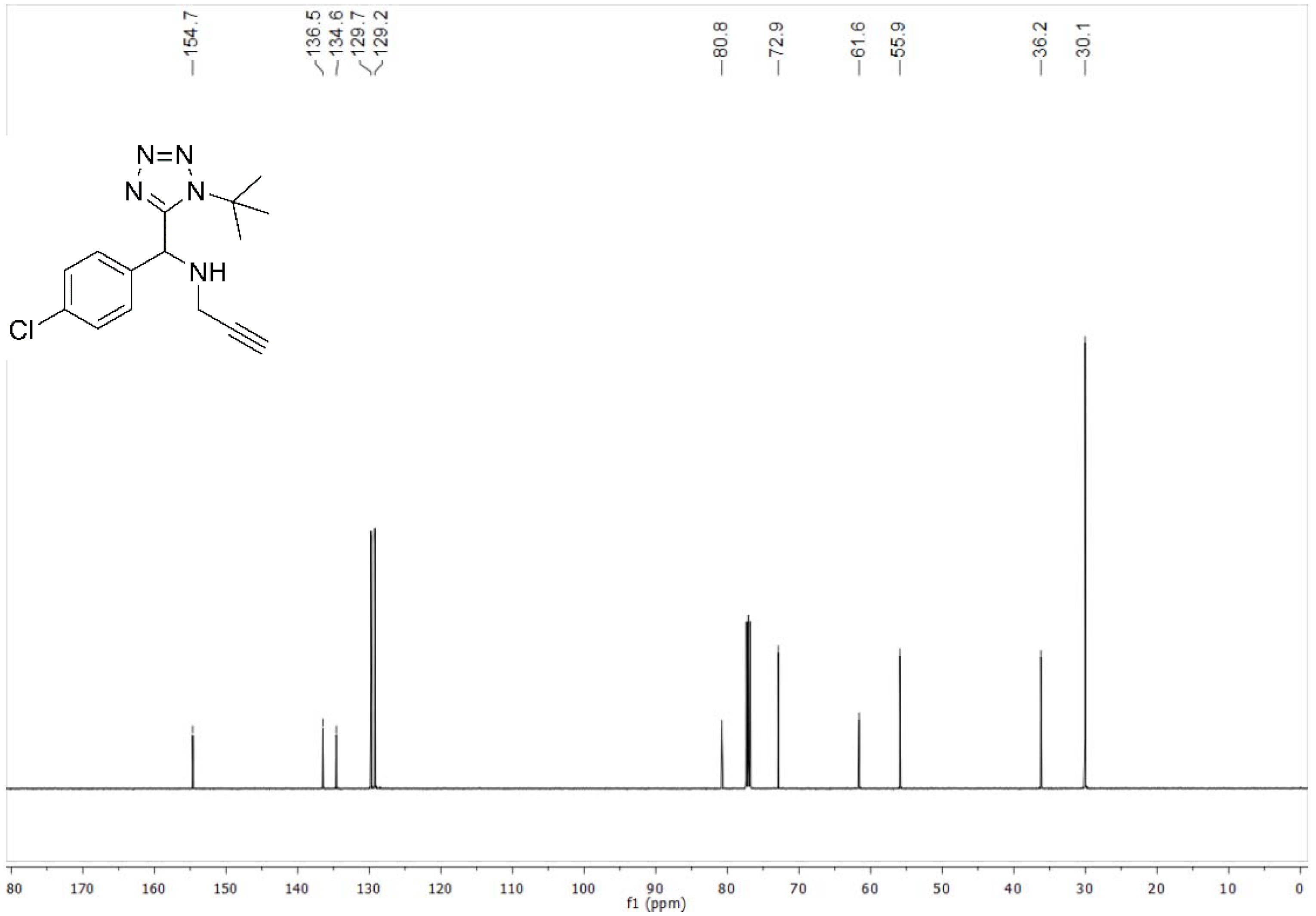
4.2.1. N-((1-(tert-Butyl)-1H-tetrazol-5-yl)(4-chlorophenyl)methyl)prop-2-yn-1-amine (10a)
4.2.2. N-((1-(tert-Butyl)-1H-tetrazol-5-yl)(4-methoxyphenyl)methyl)prop-2-yn-1-amine (10b)
4.2.3. N-((1-(tert-Butyl)-1H-tetrazol-5-yl)(4-nitrophenyl)methyl)prop-2-yn-1-amine (10c)
4.2.4. N-((1-(tert-Butyl)-1H-tetrazol-5-yl)(5-chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)methyl)prop-2-yn-1-amine (10d)
4.2.5. 4-((1-(tert-Butyl)-1H-tetrazol-5-yl)(prop-2-yn-1-ylamino)methyl)-N,N-diphenylaniline (10e)
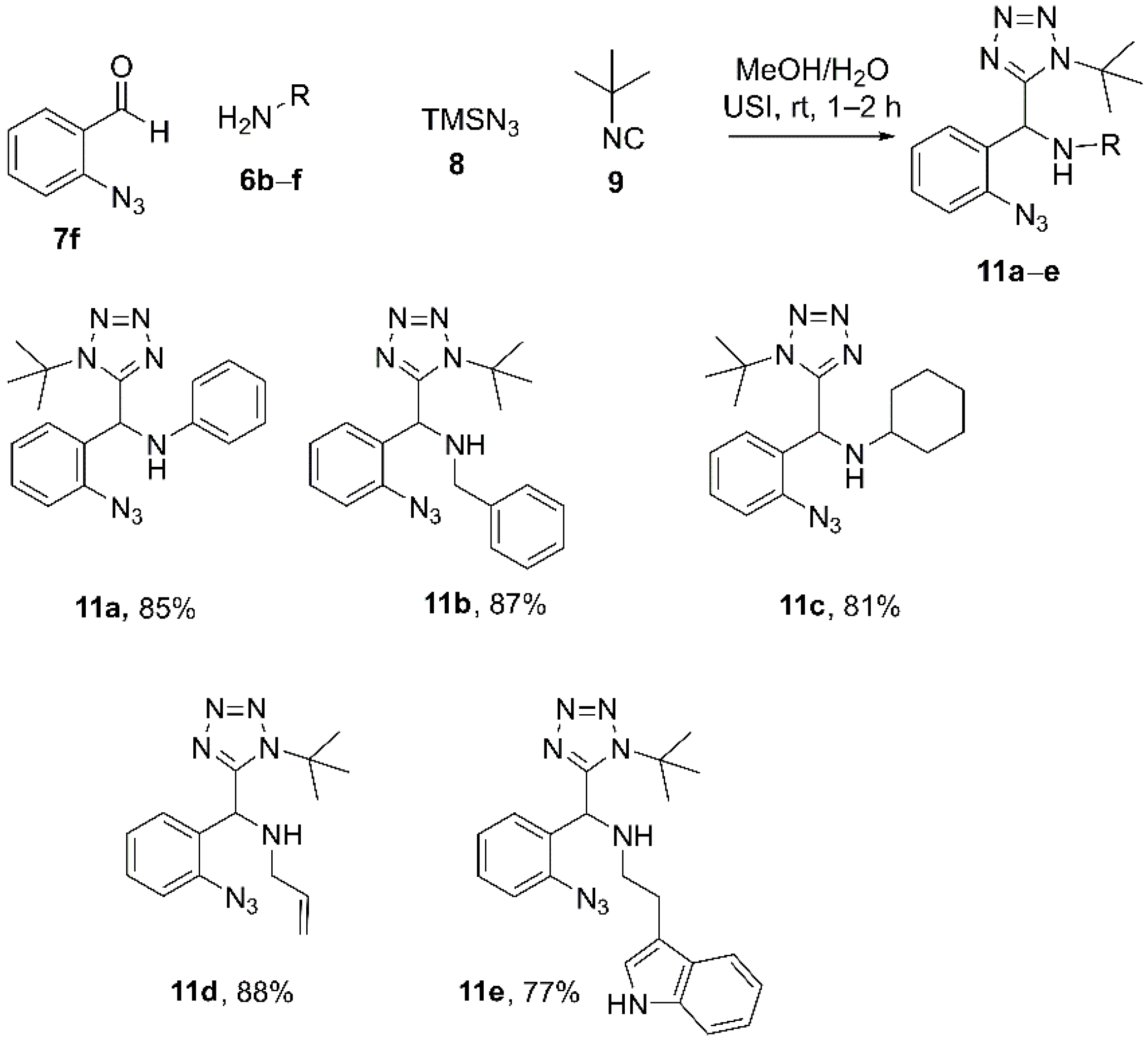
4.3. Synthesis and Characterization of 1,5-DS-T (11a–e)
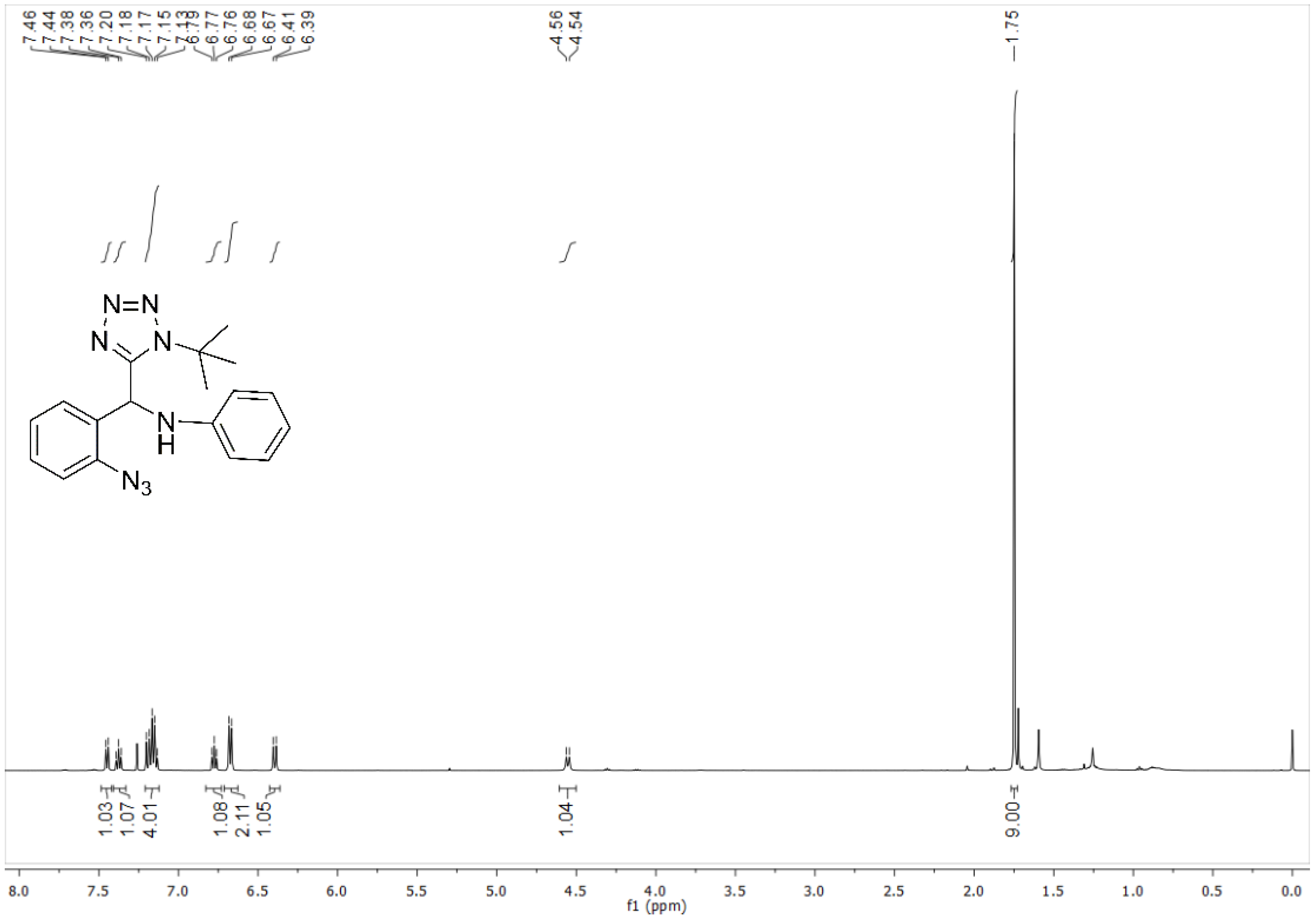
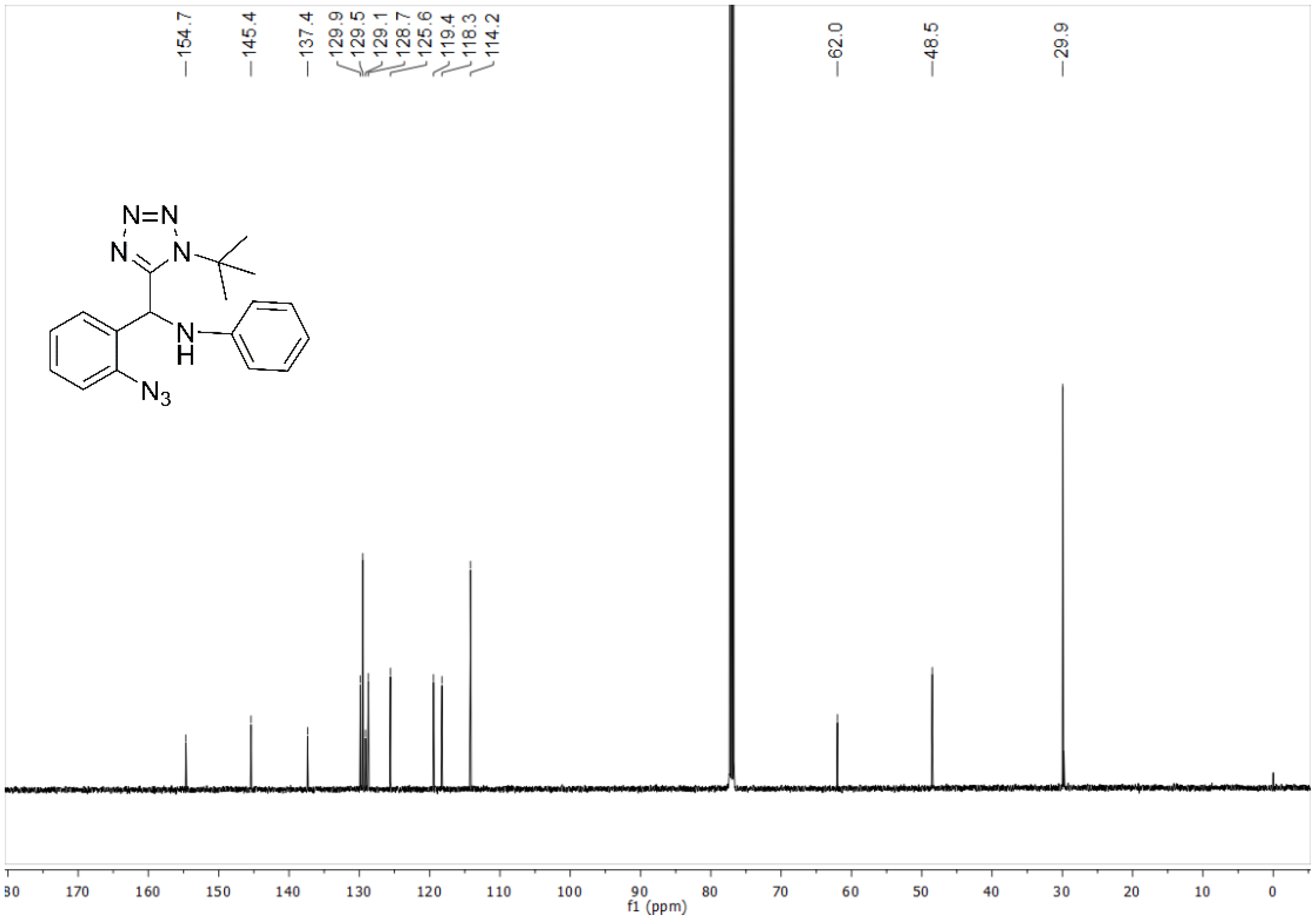
4.3.1. N-((2-Azidophenyl)(1-(tert-butyl)-1H-tetrazol-5-yl)methyl)aniline (11a)
4.3.2. 1-(2-Azidophenyl)-N-benzyl-1-(1-(tert-butyl)-1H-tetrazol-5-yl)methanamine (11b)
4.3.3. N-((2-Azidophenyl)(1-(tert-butyl)-1H-tetrazol-5-yl)methyl)cyclohexanamine (11c)
4.3.4. N-((2-Azidophenyl)(1-(tert-butyl)-1H-tetrazol-5-yl)methyl)prop-2-en-1-amine (11d)
4.3.5. N-((2-Azidophenyl)(1-(tert-butyl)-1H-tetrazol-5-yl)methyl)-2-(1H-indol-3-yl)ethan-1-amine (11e)
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dömling, A.; Wang, K.; Wang, W. Chemistry and biology of multicomponent reactions. Chem. Rev. 2012, 112, 3083–3135. [Google Scholar] [CrossRef] [PubMed]
- Neochoritis, C.G.; Zhao, T.; Dömling, A. Tetrazoles via multicomponent reactions. Chem Rev. 2019, 119, 1970–2042. [Google Scholar] [CrossRef] [PubMed]
- Ibarra, I.A.; Islas-Jácome, A.; González-Zamora, E. Synthesis of polyheterocycles via multicomponent reactions. Org. Biomol. Chem. 2018, 16, 1402–1418. [Google Scholar] [CrossRef] [PubMed]
- Lei, R.; Wu, Y.; Dong, S.; Jia, K.; Liu, S.; Hu, W. A Diastereoselective Multicomponent Reaction for Construction of Alkynylamide-Substituted α,β-Diamino Acid Derivatives To Hunt Hits. Org. Chem. 2017, 82, 2862–2869. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Liu, J.; Zhang, W. De novo biosynthesis of terminal alkyne-labeled natural products. Nat. Chem. Biol. 2014, 11, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Morales, C.M.; de Loera, D.; Contreras-Celedón, C.; Cortés-García, C.J.; Chacón-García, L. Synthesis of 1,5-disubstituted tetrazole-1,2,3 triazoles hybrids via Ugi-azide/CuAAC. Synth. Commun. 2019, 49, 2086–2095. [Google Scholar] [CrossRef]
- Zindo, F.T.; Joubert, J.; Malan, S.F. Propargylamine as functional moiety in the design of multifunctional drugs for neurodegenerative disorders: MAO inhibition and beyond. Future Med. Chem. 2015, 7, 609–629. [Google Scholar] [CrossRef] [PubMed]
- Kambe, T.; Correia, B.E.; Niphakis, M.J.; Cravatt, B.F. Mapping the protein interaction landscape for fully functionalized small-molecule probes in human cells. J. Am. Chem. Soc. 2014, 136, 10777–10782. [Google Scholar] [CrossRef] [PubMed]
- McLeod, G.X. Zidovudine: Five Years Later. Ann. Intern. Med. 1992, 117, 487–501. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Yan, G. Recent advances in reactions of azides. Adv. Synth. Catal. 2017, 359, 1600–1619. [Google Scholar] [CrossRef]
- Scriven, E.F.V.; Turnbull, K. Azides: Their preparation and synthetic uses. Chem. Rev. 1988, 88, 297–368. [Google Scholar] [CrossRef]
- Tanimoto, H.; Kakiuchi, K. Recent applications and developments of organic azides in total synthesis of natural products. Nat. Prod. Commun. 2013, 8, 1021–1034. [Google Scholar] [CrossRef] [PubMed]
- Schore, N.E. Transition metal-mediated cycloaddition reactions of alkynes in organic synthesis. Chern. Rev. 1988, 88, 1081–1119. [Google Scholar] [CrossRef]
- Suja, T.D.; Menon, R.S. Cu-Catalyzed Multicomponent Reactions. In Copper Catalysis in Organic Synthesis, 1st ed.; Anilkumar, G., Saranya, S., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2020; pp. 209–237. [Google Scholar]
- Bariwal, J.; Kaur, R.; Voskressensky, L.G.; Van der Eycken, E.V. Post-Ugi cyclization for the construction of diverse heterocyclic compounds: Recent updates. Front. Chem. 2018, 6, 557. [Google Scholar] [CrossRef] [PubMed]
- Rentería-Gómez, M.A.; Islas-Jácome, A.; Pharande, S.G.; Vosburg, D.A.; Gámez-Montaño, R. Synthesis of tris-heterocycles via a cascade IMCR/Aza Diels-Alder+ CuAAC strategy. Front. Chem. 2019, 7, 546. [Google Scholar] [CrossRef] [PubMed]
- Rentería-Gómez, M.A.; Cárdenas Galindo, L.E.; Gámez-Montaño, R. Synthesis of the 2-tetrazolylmethyl-2,3,4,9-tetrahydro-1H-β-carbolines via Ultrasound-Assisted One-Pot Ugi-azide/Pictet–Spengler Process. Proceedings 2019, 41, 67. [Google Scholar]
- Kaveti, B.; Ramírez-López, S.C.; Gámez Montaño, R. Ultrasound-assisted green one-pot synthesis of linked bis-heterocycle peptidomimetics via IMCR/post-transformation/tandem strategy. Tetrahedron Lett. 2018, 59, 4355–4358. [Google Scholar] [CrossRef]
- Kurva, M.; Pharande, S.G.; Quezada-Soto, A.; Gámez-Montaño, R. Ultrasound assisted green synthesis of bound type bis-heterocyclic carbazolyl imidazo [1, 2-a] pyridines via Groebke-Blackburn-Bienayme reaction. Tetrahedron Lett. 2018, 59, 1596–1599. [Google Scholar] [CrossRef]
- Kurva, M.; Gámez-Montaño, R. Ultrasound Assisted Green One Pot Synthesis of Bound Type bis-Heterocyclic furan-2-yl imidazo [1,2-a] Pyridines via GBBR. Proceedings 2019, 9, 46. [Google Scholar]
- Rentería-Gómez, M.A.; Morales-Salazar, I.; García-González, N.; Segura-Olvera, D.; Sánchez-Serratos, M.; Ibarra, I.A.; González-Zamora, E.; Gámez-Montaño, R.; Islas-Jácomeb, A. Ultrasound-assisted synthesis of eight novel and highly functionalized 2-aminonitrile oxazoles via Ugi-3CR. In Proceedings of the 21st International Electronic Conference on Synthetic Organic Chemistry, Online, 1–30 November 2017. [Google Scholar] [CrossRef]
- Rentería-Gómez, A.; Gámez-Montaño, R. Synthesis of 1,5-disubstituted tetrazoles containing propargyl moiety. In Proceedings of the 20th International Electronic Conference on Synthetic Organic Chemistry, Online, 1–30 November 2016. [Google Scholar] [CrossRef]
- Pharande, S.G.; Corrales Escobosa, A.R.; Gámez-Montaño, R. Endogenous water-triggered and ultrasound accelerated synthesis of 1,5-disubstituted tetrazoles via a solvent and catalyst-free Ugi-azide reaction. Green. Chem. 2017, 19, 1259–1262. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rentería-Gómez, M.A.; Solorio-Alvarado, C.R.; Gámez-Montaño, R. Ultrasound Assisted Synthesis of 1,5-Disubstituted Tetrazoles Containing Propargyl or 2-Azidophenyl Moieties via Ugi-Azide Reaction. Chem. Proc. 2022, 8, 42. https://doi.org/10.3390/ecsoc-25-11758
Rentería-Gómez MA, Solorio-Alvarado CR, Gámez-Montaño R. Ultrasound Assisted Synthesis of 1,5-Disubstituted Tetrazoles Containing Propargyl or 2-Azidophenyl Moieties via Ugi-Azide Reaction. Chemistry Proceedings. 2022; 8(1):42. https://doi.org/10.3390/ecsoc-25-11758
Chicago/Turabian StyleRentería-Gómez, Manuel A., César R. Solorio-Alvarado, and Rocío Gámez-Montaño. 2022. "Ultrasound Assisted Synthesis of 1,5-Disubstituted Tetrazoles Containing Propargyl or 2-Azidophenyl Moieties via Ugi-Azide Reaction" Chemistry Proceedings 8, no. 1: 42. https://doi.org/10.3390/ecsoc-25-11758
APA StyleRentería-Gómez, M. A., Solorio-Alvarado, C. R., & Gámez-Montaño, R. (2022). Ultrasound Assisted Synthesis of 1,5-Disubstituted Tetrazoles Containing Propargyl or 2-Azidophenyl Moieties via Ugi-Azide Reaction. Chemistry Proceedings, 8(1), 42. https://doi.org/10.3390/ecsoc-25-11758





