Abstract
The synthesis of five new bis-furanyl-pyrrolo[3,4-b]pyridin-5-ones in 30 to 40% yields through a domino sequence based on the Ugi-Zhu three-component reaction is described. Then, on the main protease MPro (PDB: 6lu7) from the SARS-CoV-2, the synthesized products and co-crystallized ligands of MPro were in silico evaluated using the docking technique, finding moderate to good binding energies and some interesting interactions, demonstrating that the ligand 8c can be considered as a drug candidate against the SARS-CoV-2-MPro due to its LE value (−5.96 kcal/mol), which is better than other synthesized and reported molecules in the literature. At the same time, hydrophobic interactions play a crucial role in the ligand target molecular couplings, demonstrated through a hydrophobicity surfaces analysis. Finally, 8a and 8b can also be considered as drug candidates. Thus, some synthesized bis-furanyl-pyrrolo[3,4-b]pyridin-5-ones may be used for further in vitro assays against the virus.
1. Introduction
With the current pandemic COVID-19, it has become increasingly necessary to develop design-synthesis strategies based on robust computational methods behind understanding the response of the SARS-CoV-2 towards new candidate polyheterocyclic compounds for further in vitro antiviral activity studies. The rational drug design is a very valuable tool that minimizes the relationship between trial and error, providing some de novo design of antiviral compounds, including the use of nanocarriers [1,2,3]. Thanks to advances in areas such as organic physical chemistry, theoretical chemistry, computational chemistry, and pharmacochemistry, amazing algorithms can be implemented behind predict the interactions between an organic ligand and its biological receptor. A widely used methodology is the molecular docking, a simulation method that is complemented with results from in vitro assays to evaluate new compounds with potential biological activity. In February 2020, the main protease from SARS-CoV-2 Mpro (PDB code: 6lu7) was published [4], which has an important role in both replication and transcription of virus [5].
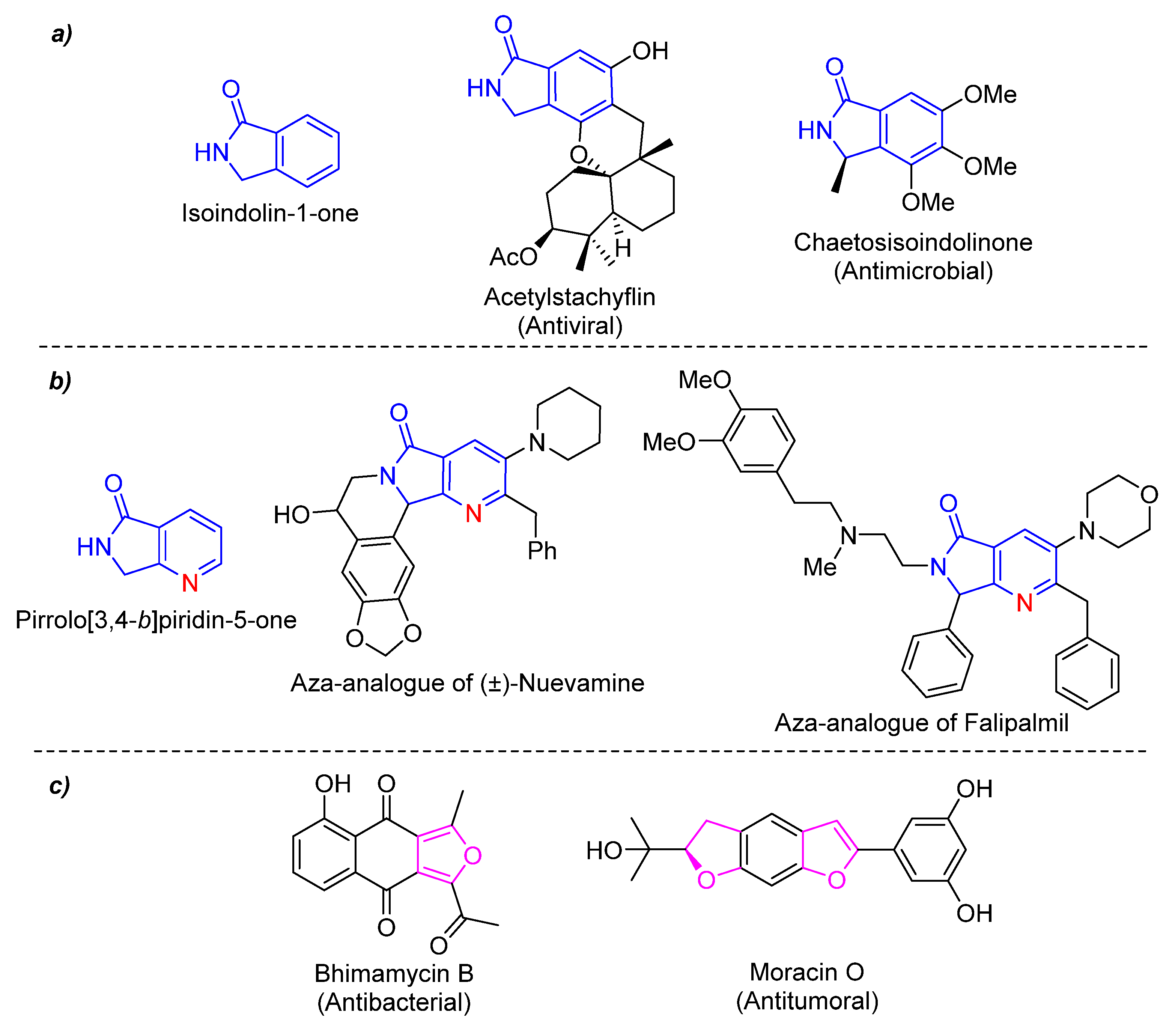
Moreover, heterocyclic chemistry is a branch of organic chemistry of special interest. Heterocycles are found in a wide variety of products such as drugs, natural products, agrochemicals, copolymers, materials, among others [6]. It is known that 90% of new drugs contain one or more heterocycles in their structure, so their importance in medicinal chemistry is crucial [7]. Isoindolin-1-one is a synthetic platform with biological properties incorporated into several products of pharmaceutical interest [8] (Figure 1a). An aza-analogue of this heterocyclic moiety is the pyrrolo[3,4-b]pyridin-5-one, found in many biological active compounds [9], analogues of natural products [10], and in some anticancer agents [11] (Figure 1b). Also, furan ring is contained in antibacterial, enzymatic inhibitors, antibiotics, antifungals, and so on [12,13] (Figure 1c).
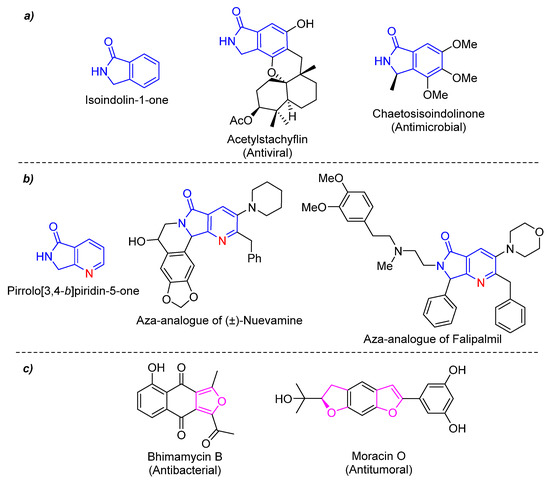
Figure 1.
(a) Isoindolin-1-one and related natural products, (b) Pyrrolo[3,4-b]pyridin-5-ones and related bioactive compounds, and (c) Furan-based drugs.
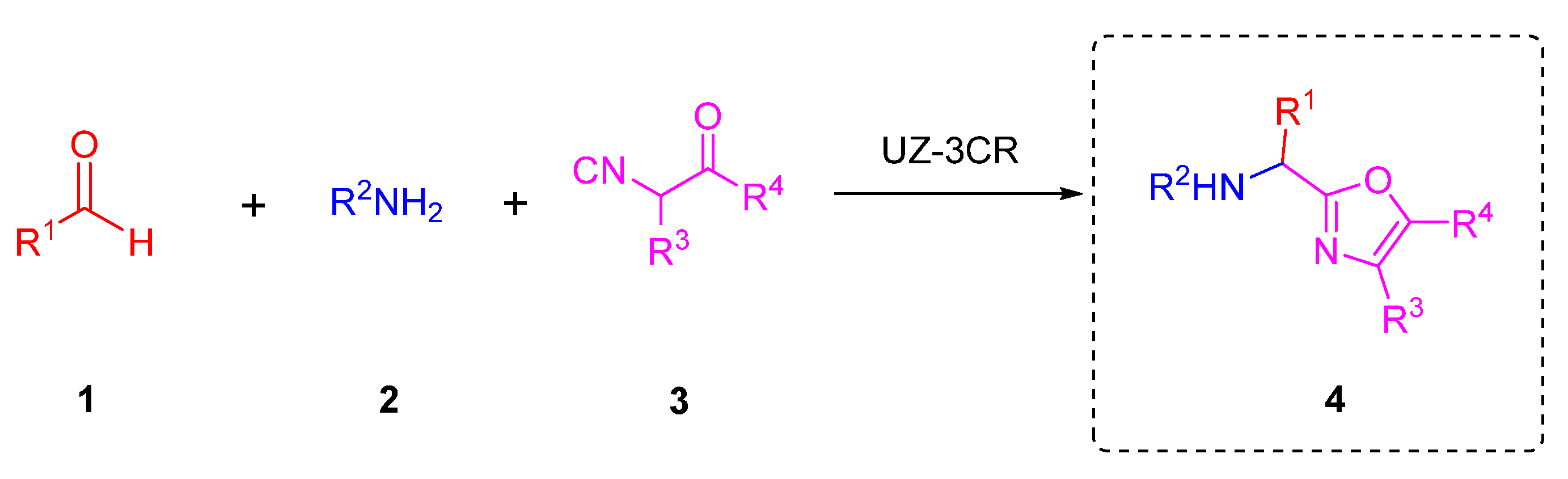
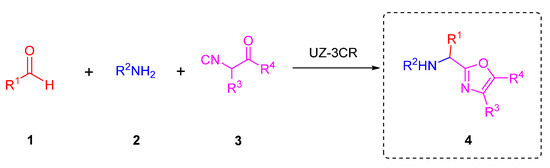
Multicomponent reactions (MCRs) are powerful tools to synthesize quickly and efficiently novel polyheterocyclic compounds. MCRs belong to one-pot process and can be based on the use of isocyanides as principal reagents due to their duality as nucleophiles and electrophiles. MCRs are carried out directly or coupled to further transformations, for instance, ionic, pericyclic, radical-mediated, or metal-catalyzed [14]. Isocyanide-based multicomponent reactions let afford a wide variety of complexes polyheterocyclic whom applied in drug discovery [15]. A classic example that incorporates isocyanides in MCRs is three and four component-Ugi reaction [16]. Within the Ugi-type reactions, it has been recently published a variant of the Ugi reaction that has been studied in our research group called the Ugi-Zhu three-component reaction (UZ-3CR), useful mainly to synthesize 5-aminooxazoles that can act as synthetic platforms toward more complex polyheterocycles. The UZ-3CR involves the combination of aldehydes 1, amines 2, and aminoacid-derived isocyanides 3 in protic or aprotic solvents to assemble just the oxazole core 4. This MCR are eventually promoted by Lewis or BrØnsted acids [17] (Scheme 1).
Scheme 1.
The Ugi-Zhu three-component reaction.
2. Results and Discussion
2.1. Chemistry
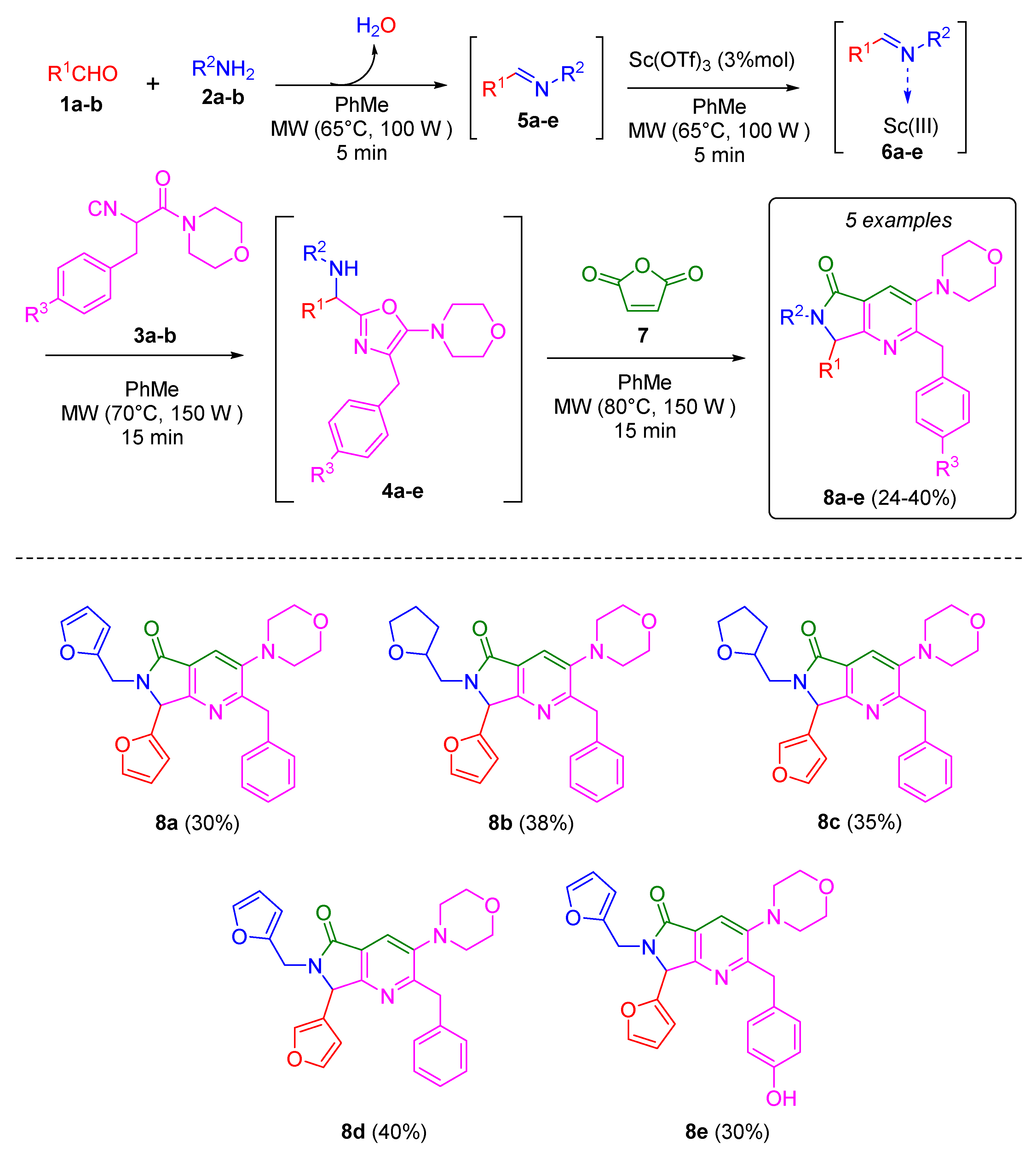
To synthesize the desired bis-furanyl-pyrrolo[3,4-b]pyridin-5-ones 8a-e, the UZ-3CR was coupled to a cascade process: aza-Diels-Alder/N-acylation/aromatization (decarboxylation/dehydration) into a one pot process. The first attempt was carried out under the Zhu’s classic conditions [18], and then optimized in our research group [19,20]. It was found that initially dichloromethane as solvent and scandium triflate as Lewis acid catalyst worked well. However, the solvent used to synthesize all products was toluene due to better solubility observed for all reagents. It was necessary first to synthesize the isocyanides 3 from their corresponding aminoacids in three steps as reported by Zhu and co-workers [18]. With the isocyanide in hands, according to the Ugi-Zhu mechanism, a condensation occurs between aldehydes 1a-b and amines 2a-b to access to imines 5a-e, which become in Lewis’ iminium ions 6a-e after reacting with catalytic amount of scandium(III) triflate. The later intermediates are nucleophilically attacked by the isocyanides 3a-b to give the 5-aminooxazoles 4a-e via a non-prototropic chain-ring tautomerization. Subsequently, after adding maleic anhydride (7), an aza-Diels-Alder cycloaddition coupled into a cascade process N-acylation/aromatization (decarboxylation/dehydration) takes place to generate the corresponding pyrrolo[3,4-b]pyridin-5-ones 8a-e in 24 to 40% yields (Scheme 2). It is worth highlighting the structural complexity of each product, as well as the formation of several new C-C and C-N bonds while leaving only one molecule of water and one of carbon dioxide, which denotes a high atomic economy and a green approach of this synthetic methodology. Also, the use of furan-substituted starting materials for the structural decoration of the pyrrolo [3,4-b] pyridin-5-one nucleus had not been reported yet in the literature.
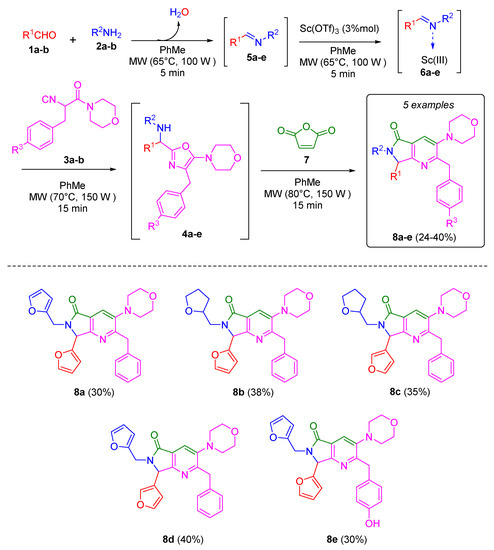
Scheme 2.
Synthesis of pyrrolo[3,4-b]pyridin-5-ones via a cascade process (Ugi-Zhu/aza-Diels-Alder/N-acylation/aromatization).
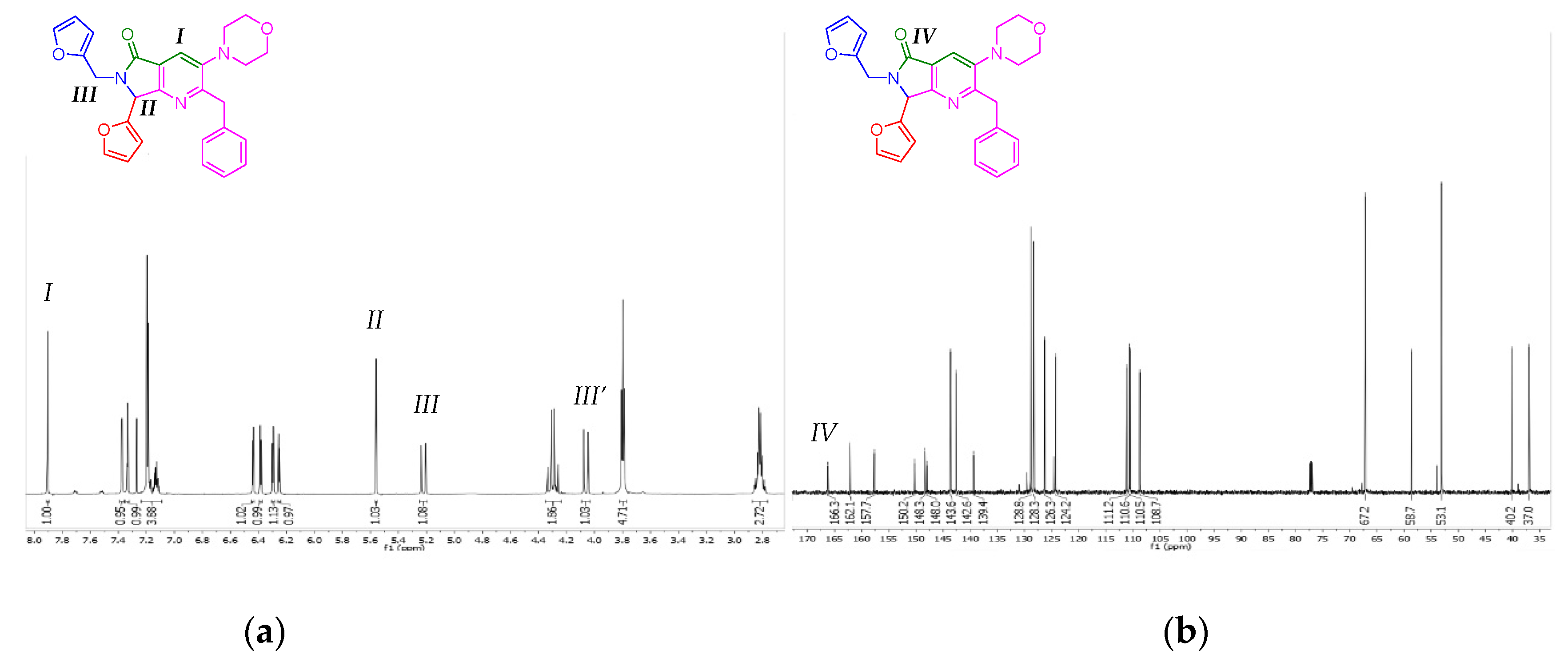
The products 8a-e were characterized by 1H and 13C NMR (see the experimental part for further details). The 2-benzyl-7-(furan-2-yl)-6-(furan-2-ylmethyl)-3-morpholino-6,7-dihydro-5H-pyrrolo[3,4-b]pyridin-5-one (8a) was selected to show its spectrums on behalf of the series 8a-e. With respect to the 1H NMR spectrum, the key signals of pyrrolo[3,4-b]pyridin-5-one nucleus are two singlets, at 7.91 and 5.56 ppm, respectively, corresponding to position 4 of pyridine (I), and methyne (II). The diasterotopic methylene (III) emits also key signals, consisting of two doublets, at 5.22 and 4.06 ppm, respectively (Figure 2a). The difference in their chemical shifts is due to an influence from nitrogen lone pair, and to the stereogenic center (II). Thus, with respect to the 13C NMR spectrum, the key peak is attributed to the carbonyl group (IV) at 166.3 ppm (Figure 2b).
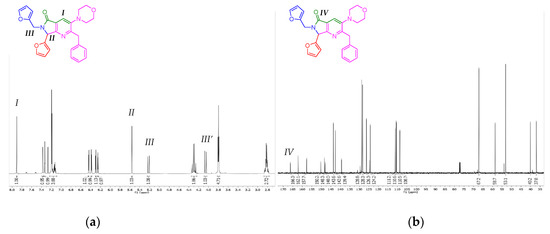
Figure 2.
(a) 1H NMR spectrum of the compound 1a, (b) 13C NMR spectrum of the compound 8a.
2.2. In Silico Study
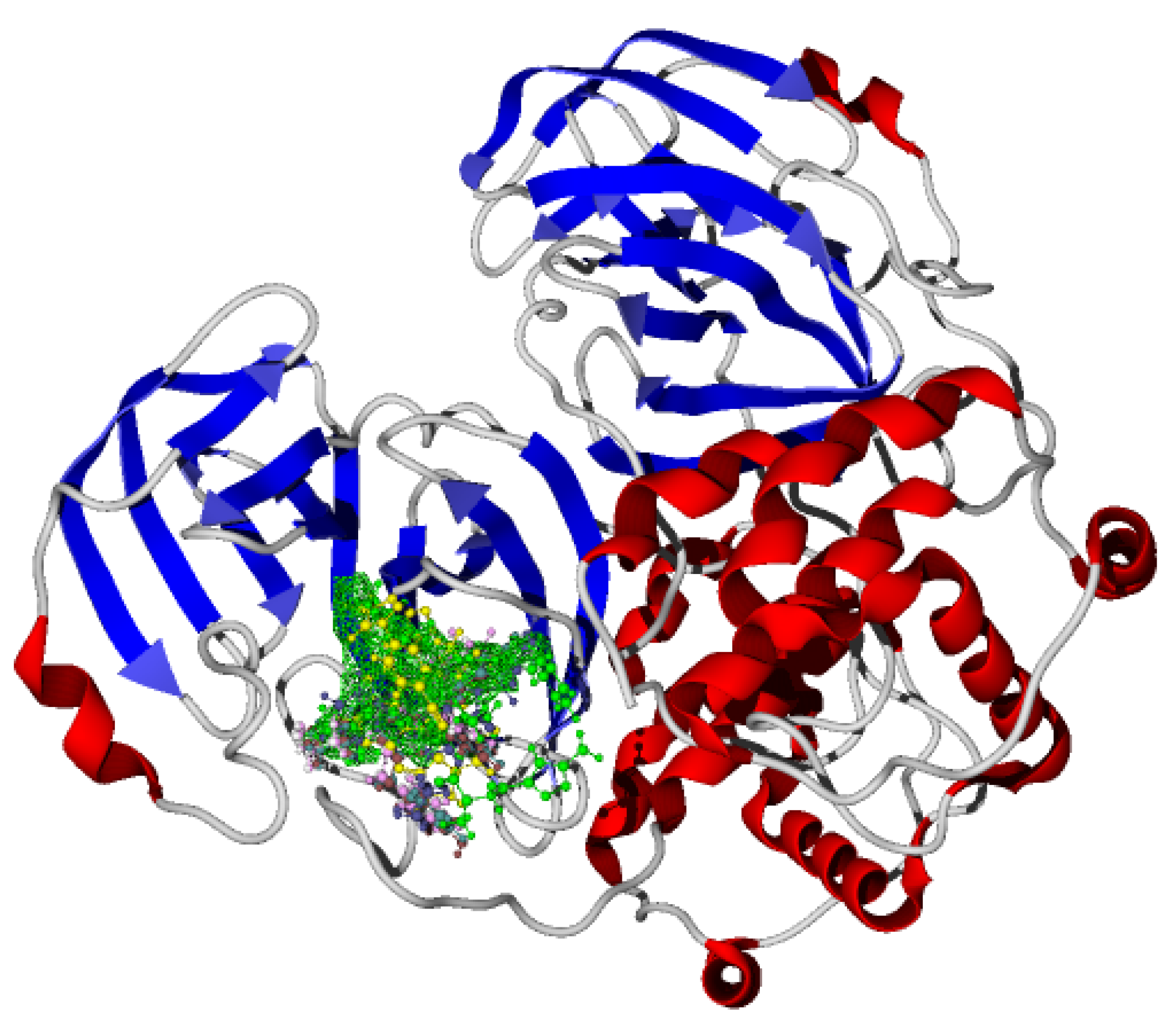
Once molecular docking was performed on the active site of the SARS-CoV-2-MPro, which has been reported for our research group [1,2,3], the studied molecules were positioned as Figure 3 shows.
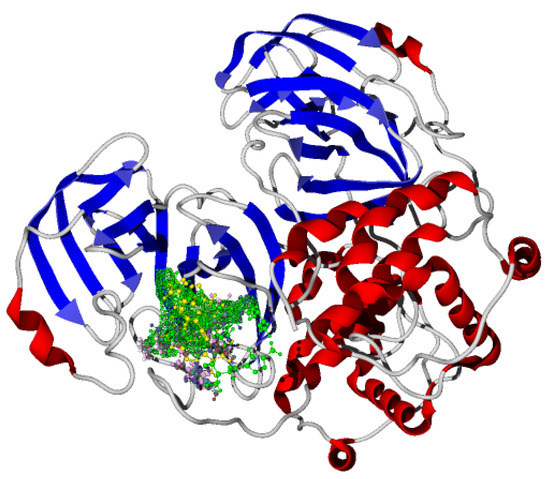
Figure 3.
Molecular docking of the synthesized novel bis-furanyl-pyrrolo[3,4-b]pyridin-5-ones and two reference molecules.
The docking results show that all of the synthesized molecules in the current work present exergonic interactions with the SARS-CoV-2-MPro (Table 1). Analyzing the results and considering that the more negative values indicate, the more stable ligand-target formed system, it is clear that 8c is the molecule with a higher interaction with the SARS-CoV-2-MPro, with a ligand efficiency (LE) value of -5.96 kcal/mol. Note that P8 is a designed molecule by Díaz-Cervantes and co-workers, with a high ligand-target interaction against the SARS-CoV-2, and co-crystallized molecules are ligand experimental tested. Although the above molecules present high interactions, 8c shows a better LE than the studied molecules depicted in Table 1.

Table 1.
Main interaction energies between the studied molecules and the SARS-CoV-2-MPro *.
8b and 8a show a LE of −5.90 and −5.89 kcal/mol, respectively, which are almost degenerate with 8c. This result denotes that the other synthesized molecules can also be considered potential drugs against the COVID-19.
The Table 1 shows that smallest size of 8c is a determinant factor to promotes a ligan-target interaction, due to H bond, electro and VdW energies are not the best for 8c compared with the other studied ligands.
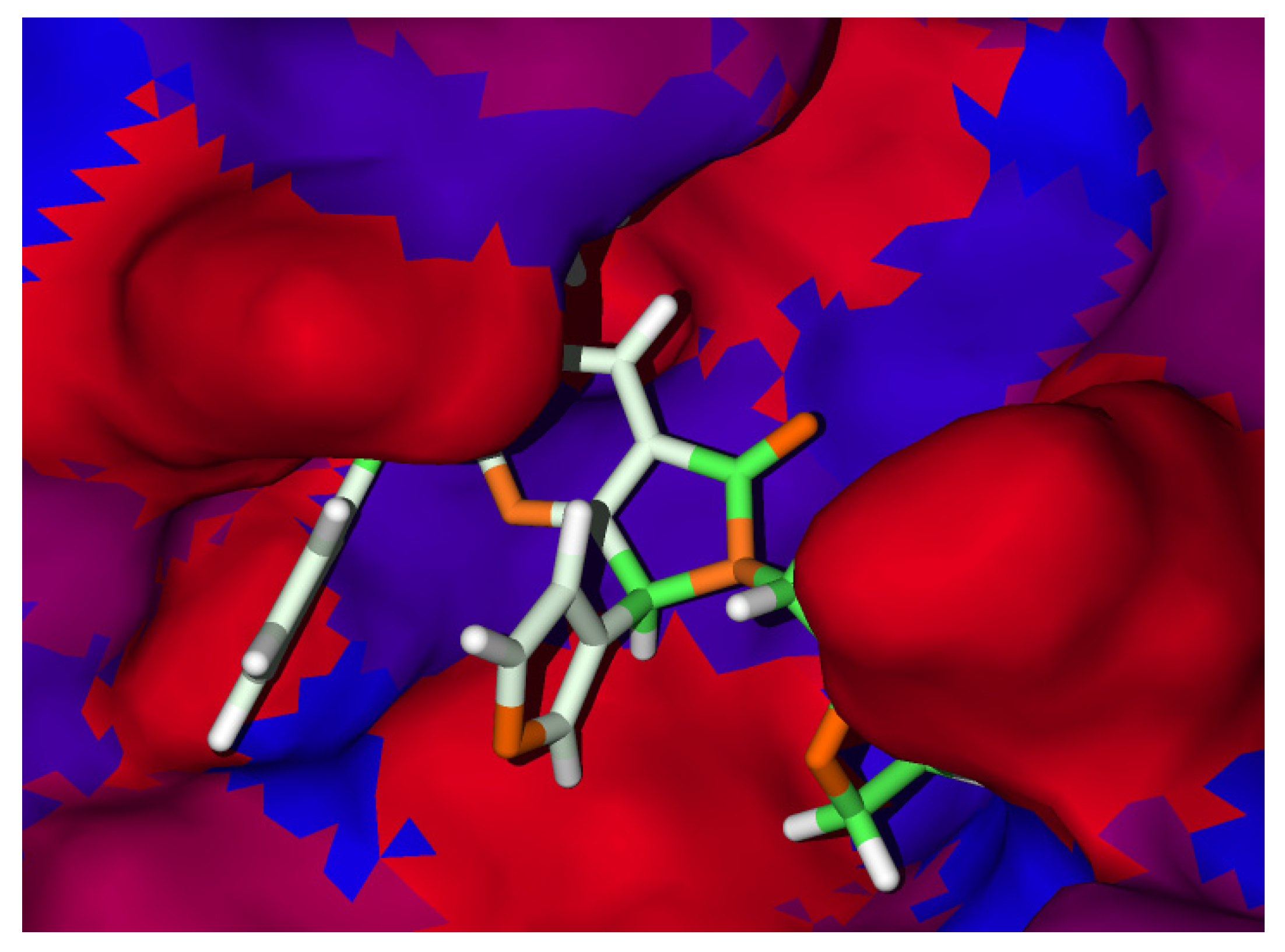
In this order, the hydrophilic interactions have been analyzed; see the Figure 4, which demonstrates that the hydrophobicity then promotes one essential interaction in this kind of target. The hydrophobic side of the target confers a higher site in the center of the cavity, which promotes better interactions by 8c.
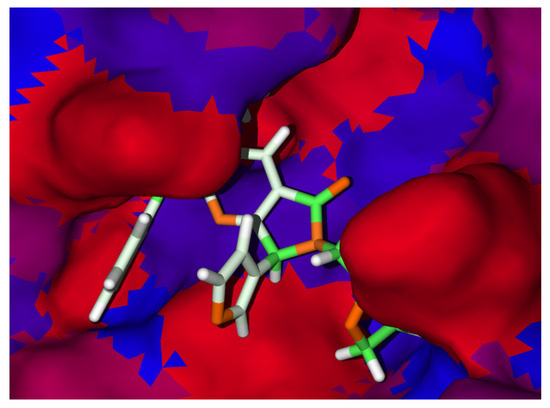
Figure 4.
Hydrophobicity surfaces for 8c. Red and blue surfaces represent the hydrophilic and hydrophobic surfaces of the SARS-CoV-2-MPro, respectively. Green and orange sticks are the hydrophilic and hydrophobic moieties of 8c.
3. Conclusions
Considering the docking assay, the present studies demonstrate that 8c can be considered as a drug candidate against the SARS-CoV-2-MPro due to its LE value (−5.96 kcal/mol), which is better than other synthesized and reported molecules. At the same time, the hydrophobic interaction plays a key role in the ligand targe molecular couplings, demonstrated through a hydrophobicity surfaces analysis. Finally, 8a and 8b can also be considered as drug candidates.
4. Experimental Section
4.1. General Information, Instrumentation and Chemicals
1H and 13C NMR spectra were acquired on a Bruker Advance III (500 MHz) spectrometer. The solvent was deuterated chloroform (CDCl3). Chemical shifts are reported in parts per million (δ/ppm). Internal reference for NMR spectra is in respect to tetramethyl silane (TMS) at 0.0 ppm. Coupling constants are reported in Hertz (J/Hz). Multiplicities of the signals are reported using the standard abbreviations: singlet (s), doublet (d), triplet (t), quartet (q), and multiplet (m). NMR data were treated using the MestReNova software (12.0.0–20080). Microwave-assisted reactions were performed in closed-vessel mode on a CEM Discover MW-reactor (Matthews, North Carolina, CA, USA). Reaction progress was monitored by thin-layer chromatography (TLC) and the spots were visualized under ultraviolet (UV) light (254 or 365 nm). Flash columns packed with silica-gel 60 and glass preparative plates (20 × 20 cm) coated with silica-gel 60 doped with UV indicator (F254) were used to purify the products. Mixtures of hexanes (Hex) and ethyl acetate (EtOAc) in 1:1 or 1:2 (v/v) proportion were used to run TLC, silica-gel columns, preparative plates, and to measure the retention factor (Rf) values (using the same mobile phase for all of the experiments). All starting reagents and solvents were used as received (without further purification, distillation, nor dehydration). Chemical structures were drawn using the ChemDraw Professional software (Ver. 15.0.0.106, Perkin Elmer Informatics, Cambridge, MA, USA). The purity for all synthesized products (>95%) was assessed by NMR.
4.2. Synthesis and Characterization of the bis-furanyl-pyrrolo[3,4-b]pyridine-5-ones 8a-e
General Procedure (GP): The corresponding aldehydes 1a-b (1.0 equiv.) and the amines 2a-b (0.1 mmol, 1.0 equiv.) were placed in a sealed CEM Discover microwave reaction tube (10 mL) and diluted in toluene [1.0 mL]. Then, the mixture was stirred and heated using microwave irradiation (65 °C, 100 W) for 5 min, and scandium (III) triflate (0.03 equiv.) was added. The mixture was stirred and heated using microwave irradiation (65 °C, 100 W) for 5 min, and then the corresponding isocyanides 3a-b (1.2 equiv.) were added. The new mixture was stirred and again heated using microwave irradiation (70 °C, 150 W) for 15 min, and then maleic anhydride (7) (1.4 equiv.) was added. Finally, the reaction mixture was stirred and heated using microwave irradiation (80 °C, 150 W) for 15 min. Then, the solvent was removed to dryness under vacuum. The crude was extracted using dichloromethane (3 × 25.0 mL) and Na2CO3 (aq.) (3 × 25 mL), and then washed with brine (3 × 25 mL). The organic layer was dried using anhydrous Na2SO4, filtered, and concentrated to dryness under vacuum. The new crude was purified by silica-gel column chromatography followed by preparative TLC using mixtures of hexanes (Hex) and ethyl acetate (EtOAc) in 1:1 or 1:2 (v/v) proportions as mobile phase to isolate the corresponding bis-furanyl-pyrrolo[3,4-b]7yridine-5-ones 8a-e as racemic mixtures.
2-benzyl-7-(furan-2-yl)-6-(furan-2-ylmethyl)-3-morpholino-6,7-dihydro-5H-pyrrolo[3,4-b]7yridine-5-one (8a)
According to the GP, 2-furaldehyde (86 µL), 2-aminomethylfuran (92 µL), scandium (III) triflate (1.5 mg), 2-isocyano-1-morpholino-3-phenylpropan-1-one (305 mg), and maleic anhydride (143 mg) were reacted together in toluene (1.0 mL) to afford 8a (30 mg, 30%) as a yellow oil; Rf = 0.38 (Hex–AcOEt = 1:1, v/v); 1H NMR (500 MHz, CDCl3): δ 7.91 (s, 1H), 7.37 (dd, J = 1.9, 0.9 Hz, 1H), 7.33 (dd, J = 1.9, 0.9 Hz, 1H), 7.24–7.09 (m, 4H), 6.44 (ddd, J = 3.3, 0.9, 0.3 Hz, 1H), 6.39 (dd, J = 3.3, 1.9 Hz, 1H), 6.30 (dd, J = 3.2, 1.9 Hz, 1H), 6.25 (dd, J = 3.2, 0.8 Hz, 1H), 5.56 (s, 1H), 5.22 (d, J = 15.7 Hz, 1H), 4.35–4.25 (m, 2H), 4.06 (d, J = 15.7 Hz, 1H), 3.80 (t, J = 4.6 Hz, 4H), 2.86–2.78 (m, 4H) ppm; 13C NMR (125 MHz, CDCl3): δ 166.3, 162.1, 157.7, 150.2, 148.3, 148.0, 143.6, 142.6, 139.4, 128.8, 128.3, 126.3, 124.2, 111.2, 110.6, 110.5, 108.7, 67.2, 58.7, 53.1, 40.2, 37.0 ppm.
2-benzyl-7-(furan-2-yl)-3-morpholino-6-((tetrahydrofuran-2-yl)methyl)-6,7-dihydro-5H-pyrrolo[3,4-b]7yridine-5-one (8b)
According to the GP, 2-furaldehyde (86 µL), 2-(aminomethyl)tetrahydrofuran (107 µL), scandium (III) triflate (1.5 mg), 2-isocyano-1-morpholino-3-phenylpropan-1-one (305 mg), and maleic anhydride (143 mg) were reacted together in toluene (1.0 mL) to afford 8b (39 mg, 38%) as a yellow oil; Rf = 0.35 (Hex–AcOEt = 1:2, v/v); 1H NMR (500 MHz, CDCl3): δ 7.91 (s, 1H), 7.36 (ddd, J = 3.8, 1.9, 0.9 Hz, 1H), 7.22–7.11 (m, 5H), 6.47 (dd, J = 4.4, 3.3, Hz, 1H), 6.38 (dd, J = 3.3, 1.9 Hz, 1H), 5.82 (s, 1H), 4.37–4.27 (m, 2H), 4.06 (m, 2H), 3.91–3.83 (m, 1H), 3.80 (m, 4H), 2.82 (m, 4H), 1.87 (dd, J = 7.9, 6.8 Hz, 2H) ppm; 13C NMR (125 MHz, CDCl3): δ 167.1, 161.9, 158.0, 148.2, 143.5, 139.4, 128.9, 128.3, 126.3, 124.4, 111.4, 110.7, 67.3, 60.4, 53.2, 44.6, 43.2, 40.1, 29.4.
2-benzyl-7-(furan-3-yl)-3-morpholino-6-((tetrahydrofuran-2-yl)methyl)-6,7-dihydro-5H-pyrrolo[3,4-b]8yridine-5-one (8c)
According to the GP, 3-furancarboxaldehyde (90 µL), 2-(aminomethyl)tetrahydrofuran (107 µL), scandium (III) triflate (1.5 mg), 2-isocyano-1-morpholino-3-phenylpropan-1-one (305 mg), and maleic anhydride (143 mg) were reacted together in toluene (1.0 mL) to afford 8c (37 mg, 35%) as a yellow oil; Rf = 0.20 (Hex–AcOEt = 1:1, v/v); 1H NMR (500 MHz, CDCl3): δ 7.89 (s, 1H), 7.62 (dd, J = 9.5, 1.6, Hz, 1H), 7.39–7.37 (m, 1H), 7.22–7.11 (m, 5H), 5.97 (dd, J = 1.9, 0.6 Hz, 1H), 5.72 (s, 1H), 4.38–4.25 (m, 2H), 4.12 (d, J = 7.2 Hz, 2H), 3.85 (m, 1H), 3.81 (dt, J = 4.3, 1.6 Hz, 4H), 2.82 (td, J = 5.9, 4.9, 2.6 Hz, 4H), 1.27–1.24 (m, 2H) ppm; 13C NMR (125 MHz, CDCl3): δ 167.2, 162.0, 160.0, 148.1, 143.9, 142.8, 139.4, 128.9, 128.3, 126.3, 124.5, 124.3, 120.2, 108.7, 68.0, 67.3, 58.2, 53.2, 44.3, 42.4, 40.2, 29.5. ppm.
2-benzyl-6-(furan-2-ylmethyl)-7-(furan-3-yl)-3-morpholino-6,7-dihydro-5H-pyrrolo[3,4-b]8yridine-5-one (8d)
According to the GP, 3-furancarboxaldehyde (90 µL), 2-aminomethylfuran (93 µL), scandium (III) triflate (1.5 mg), 2-isocyano-1-morpholino-3-phenylpropan-1-one (305 mg), and maleic anhydride (143 mg) were reacted together in toluene (1.0 mL) to afford 8d (40.4 mg, 40%) as a yellow oil; Rf = 0.43 (Hex–AcOEt = 1:1, v/v); 1H NMR (500 MHz, CDCl3): δ 7.91 (s, 1H), 7.61 (ddd, J = 1.4, 0.9, 0.4 Hz, 1H), 7.41 (ddd, J = 2.0, 1.5, 0.5 Hz, 1H), 7.35 (dd, J = 1.9, 0.9 Hz, 1H), 7.22–7.11 (m, 5H), 6.31 (dd, J = 3.2, 1.9 Hz, 1H), 6.26 (d, J = 0.7 Hz, 1H), 6.03 (ddd, J = 1.9, 0.9, 0.4 Hz, 1H), 5.45 (s, 1H), 5.29 (s, 1H), 4.30 (d, J = 13.6 Hz, 2H), 4.03 (dt, J = 15.6, 0.5 Hz, 1H), 3.81 (t, J = 4.6 Hz, 4H), 2.82 (d, J = 5.3 Hz, 4H) ppm; 13C NMR (125 MHz, CDCl3) δ 166.2, 162.2, 159.5, 150.3, 148.1, 144.1, 142.6, 139.3, 128.8, 128.3, 126.3, 124.3, 120.0, 110.5, 108.7, 67.2, 56.9, 53.1, 40.1, 36.5 ppm.
7-(furan-2-yl)-6-(furan-2-ylmethyl)-2-(4-hydroxybenzyl)-3-morpholino-6,7-dihydro-5H-pyrrolo[3,4-b]8yridine-5-one (8e)
According to the GP, 2-furaldehyde (86 µL), 2-aminomethylfuran (93 µL), scandium (III) triflate (1.5 mg), 3-(3-hydroxyphenyl)-2-isocyano-1-morpholinopropan-1-one (325 mg), and maleic anhydride (143 mg) were reacted together in toluene (1.0 mL) to afford 1e (30.3 mg, 30%) as a yellow oil; Rf = 0.29 (Hex–AcOEt = 1:2, v/v); 1H NMR (500 MHz, CDCl3): δ 7.90 (s, 1H), 7.38-7.37 (m, 1H), 7.34–7.33 (m, 1H), 7.21 (d, J = 8.8 Hz, 2H), 7.01 (d, J = 8.8 Hz, 2H), 6.44–6.43 (m, 1H), 6.39–6.38 (m, 1H), 6.31–6.29 (m, 1H), 6.26–6.24 (m, 1H), 5.55 (s, 1H), 5.22 (d, J = 16.1 Hz, 1H), 4.28 (d, J = 7.1 Hz, 2H), 4.06 (d, J = 15.7 Hz, 1H), 3.81 (s, 4H), 2.84 (d, J = 5.7 Hz, 4H) ppm; 13C NMR (125 MHz, CDCl3): δ 166.3, 161.8, 157.9, 153.8, 150.3, 149.7, 148.3, 148.0, 143.7, 142.7, 137.1, 129.9, 124.4, 120.9, 111.3, 110.7, 110.6, 108.8, 67.3, 58.7, 53.2, 39.5, 37.1 ppm.
4.3. Docking
The in-silico assay was carried out at molecular mechanics level, using the MolDock [21] scoring function through the Molegro virtual docker package [22] to perform the docking between the ligands and the SARS-CoV-2-MPRO. Previously, the whole molecules were modeled and optimized using the Avogadro software [23].
Author Contributions
I.M.-S., synthesis, and characterization; S.L.C.-A. and D.C.-G. data analysis; E.D.-C. docking, and writing–original draft preparation; E.G.-Z. supervision, and writing–original draft preparation; A.I.-J. funding acquisition, conceptualization, and writing–review and editing. All authors have read and agreed to the published version of the manuscript.
Funding
A.I.-J. acknowledges “Proyecto Apoyado por el Fondo Sectorial de Investigación para la Educación CONACyT-SEP CB-2017-2018 (A1-S-32582)” for financial support.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
I.M.-S (947606) thanks CONACyT-México for her scholarship.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Cortés-García, C.J.; Chacón-García, L.; Mejía-Benavides, J.E.; Díaz-Cervantes, E. Tackling the SARS-CoV-2 main protease using hybrid derivatives of 1,5-disubstituted tetrazole-1,2,3-triazoles: An in silico assay. PeerJ Phys. Chem. 2020, 2, e10. [Google Scholar] [CrossRef]
- García-Ramírez, V.G.; Suarez-Castro, A.; Villa-Lopez, M.G.; Díaz-Cervantes, E.; Chacón-García, L.; Cortes-García, C.J. Synthesis of Novel Acylhydrazone-Oxazole Hybrids and Docking Studies of SARS-CoV-2 Main Protease. Chem. Proc. 2021, 3, 1. [Google Scholar] [CrossRef]
- Díaz-Cervantes, E.; Zenteno-Zúñiga, C.; Rodríguez-González, V.; Aguilera-Granja, F. Design of ZnO-Drug Nanocarriers against the Main Protease of SARS-CoV 2 (COVID-19): An In Silico Assay. Appl. Nano 2021, 2, 257–266. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, B.; Jin, Z.; Yang, H.; Rao, Z. The crystal structure of COVID-19 main protease in complex with an inhibitor N3. Nature 2020, 582, 289–293. [Google Scholar] [CrossRef] [Green Version]
- Badavath, V.N.; Kumar, A.; Samanta, P.K.; Maji, S.; Das, A.; Blum, G.; Jha, A.; Sen, A. Determination of potential inhibitors based on isatin derivatives against SARS-CoV-2 main protease (Mpro): A molecular docking, molecular dynamics and structure-activity relationship studies. J. Biomol. Struct. Dyn. 2020, 1–19. [Google Scholar] [CrossRef]
- Arora, P.; Arora, V.; Lamba, H.S.; Wadhwa, D. Importance of heterocyclic chemistry: A review. Int. J. Pharm. Res. Sci. 2012, 3, 947–955. [Google Scholar]
- Saini, M.S.; Kumar, A.; Dwivedi, J.; Singh, R. A review: Biological significances of heterocyclic compounds. Int. J. Pharm. Sci. Res. 2013, 4, 66–77. [Google Scholar]
- Upadhyay, S.P.; Thapa, P.; Sharma, R.; Sharma, M. 1-Isoindolinone scaffold-based natural products with a promising diverse bioactivity. Fitoterapia 2020, 146, 104722. [Google Scholar] [CrossRef]
- Sun, X.; Janvier, P.; Zhao, G.; Bienaymé, H.; Zhu, J. A novel multicomponent synthesis of polysubstituted 5-aminooxazole and its new scaffold-generating reaction to pyrrolo[3,4-b]pyridine. Org. Lett. 2001, 3, 877–880. [Google Scholar] [CrossRef]
- Vázquez-Vera, O.; Sánchez-Badillo, J.S.; Islas-Jácome, A.; Rentería-Gómez, M.A.; Pharande, S.G.; Cortes-García, C.J.; Rincón-Guevara, M.A.; Ibarra, I.A.; Gámez-Montaño, R.; González-Zamora, E. An efficient Ugi-3CR/aza Diels–Alder/Pomeranz– Fritsch protocol towards novel aza-analogues of (±)-nuevamine, (±)-lennoxamine and magallanesine: A diversity oriented synthesis approach. Org. Biomol. Chem. 2017, 15, 2363–2369. [Google Scholar] [CrossRef]
- Segura-Olvera, D.; García-González, A.N.; Morales-Salazar, I.; Islas-Jácome, A.; Rojas-Aguirre, Y.; Ibarra, I.A.; Díaz-Cervantes, E.; Alcaraz-Estrada, S.L.; González-Zamora, E. Synthesis of pyrrolo[3,4-b]pyridin-5-ones via multicomponent reactions and in vitro–in silico studies against SiHa, HeLa, and CaSki human cervical carcinoma cell lines. Molecules 2019, 24, 2648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boto, A.; Alvarez, L. Furan and its derivatives. In Heterocycles in Natural Product Synthesis, 1st ed.; Majumdar, K.C., Chattopadhyay, S.K., Eds.; Wiley-VCH Verlag: Weinheim, Germany, 2011; pp. 99–107. [Google Scholar]
- Chandrashekarachar, D.; Kesagudu, D. Importance of Furan Based Compounds and their Biomedical Applications: An Overview. Indo Am. J. Pharm. Res. 2017, 7, 7541–7549. [Google Scholar]
- Ibarra, I.A.; Islas-Jácome, A.; González-Zamora, E. Synthesis of polyheterocycles via multicomponent reactions. Org. Biomol. Chem. 2018, 16, 1402–1418. [Google Scholar] [CrossRef]
- Váradi, A.; Palmer, T.C.; Dardashti, R.N.; Majumdar, S. Isocyanide-based multicomponent reactions for the synthesis of heterocycles. Molecules 2016, 21, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, J.; Meng, J.-P.; Tang, D.-Y.; Frett, B.; Chen, Z.-Z.; Xu, Z.-G. Recent advances in the development of polycyclic skeletons via Ugi reaction cascades. Mol. Divers. 2018, 22, 503–516. [Google Scholar] [CrossRef] [PubMed]
- Flores-Reyes, J.C.; Islas-Jácome, A.; González-Zamora, E. The Ugi three-component reaction and its variants. Org. Chem. Front. 2021, 8, 5460–5515. [Google Scholar] [CrossRef]
- Fayol, A.; Housseman, C.; Sun, X.; Janvier, P.; Bienaymé, H.; Zhu, J. Synthesis of α-Isocyano-α-alkyl(aryl)acetamides and their use in the multicomponent synthesis of 5-aminooxazole, pyrrolo[3,4-b]pyridin-5-one and 4,5,6,7-tetrahydrofuro[2,3-c]pyridine. Synthesis 2005, 1, 161–165. [Google Scholar] [CrossRef]
- Islas-Jácome, A.; Cárdenas-Galindo, L.E.; Jerezano, A.V.; Tamariz, J.; González-Zamora, E.; Gámez-Montaño, R. Synthesis of nuevamine aza-analogues by a sequence: I-MCR–Aza-Diels–Alder–Pictet–Spengler. Synlett 2012, 23, 2951–2956. [Google Scholar] [CrossRef]
- Islas-Jácome, A.; Gutiérrez-Carrillo, A.; García-Garibay, M.A.; González-Zamora, E. One-pot synthesis of nuevamine aza-analogues by combined use of an oxidative Ugi type reaction and aza-Diels–Alder cycloaddition. Synlett 2014, 25, 0403–0406. [Google Scholar] [CrossRef]
- Thomsen, R.; Christensen, M.H. MolDock: A new technique for high-accuracy molecular docking. J. Med. Chem. 2006, 49, 3315–3321. [Google Scholar] [CrossRef]
- Yang, J.; Chen, C. Gemdock: A generic evolutionary method for molecular docking. Proteins 2004, 55, 288–304. [Google Scholar] [CrossRef] [PubMed]
- Hanwell, M.; Curtis, D.; Lonie, D.; Vandermeersch, T.; Zurek, E.; Hutchison, G. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).