Anion Colorimetric Chemosensor Based on a Benzimidazole-Functionalized BODIPY Derivative †
Abstract
:1. Introduction
2. Methods and Materials
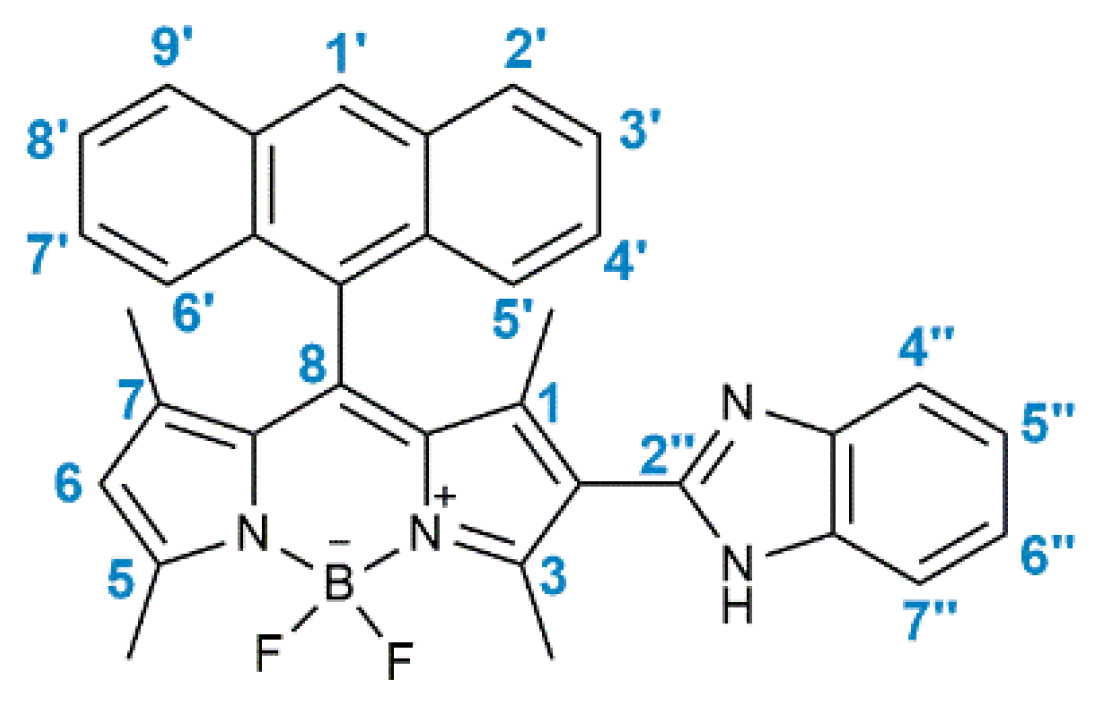
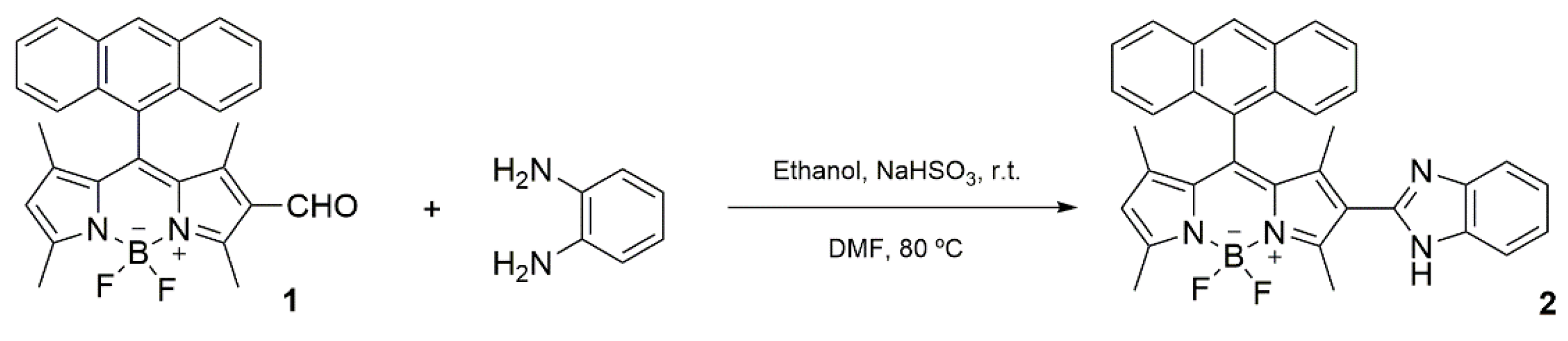
2.1. Synthesis of BODIPY Derivative 2
2.2. Chemosensing Studies of BODIPY Derivative 2 in Aqueous Media
3. Results and Discussion
3.1. Synthesis of BODIPY Derivative 2
3.2. Photophysical Characterization of BODIPY Derivative 2
3.3. Chemosensing Studies of BODIPY Derivative 2 in Aqueous Media
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goshisht, M.K.; Tripathi, N. Fluorescence-Based Sensors as an Emerging Tool for Anion Detection: Mechanism, Sensory Materials and Applications. J. Mater. Chem. C 2021, 9, 9820–9850. [Google Scholar] [CrossRef]
- Patil, A.; Salunke-Gawali, S. Overview of The Chemosensor Ligands used for Selective Detection of Anions and Metal Ions (Zn2+, Cu2+, Ni2+, Co2+, Fe2+, Hg2+). Inorg. Chim. Acta 2018, 482, 99–112. [Google Scholar] [CrossRef]
- Wu, D.; Sedgwick, A.C.; Gunnlaugsson, T.; Akkaya, E.U.; Yoon, J.; James, T.D. Fluorescent Chemosensors: The Past, Present and Future. Chem. Soc. Rev. 2017, 46, 7105–7123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, B.; Kaur, N.; Kumar, S. Colorimetric Metal Ion Sensors–A Comprehensive Review of the Years 2011–2016. Coord. Chem. Rev. 2018, 358, 13–69. [Google Scholar] [CrossRef]
- Loudet, A.; Burgess, K. BODIPY Dyes and their Derivatives: Syntheses and Spectroscopic Properties. Chem. Rev. 2007, 107, 4891–4932. [Google Scholar] [CrossRef]
- Boens, N.; Leen, V.; Dehaen, W. Fluorescent Indicators based on BODIPY. Chem. Soc. Rev. 2012, 41, 1130–1172. [Google Scholar] [CrossRef]
- Bañuelos, J. BODIPY Dye, The Most Versatile Fluorophore Ever? Chem. Rec. 2016, 16, 335–348. [Google Scholar] [CrossRef]
- Lu, H.; Shen, Z. BODIPYs and Their Derivatives: The Past, Present and Future. Front. Chem. 2020, 8, 290. [Google Scholar] [CrossRef]
- Gonçalves, R.; Pina, J.; Costa, S.P.G.; Raposo, M.M.M. Synthesis and Characterization of Aryl-substituted BODIPY Dyes Displaying Distinct Solvatochromic Singlet Oxygen Photosensitization Efficiencies. Dyes Pigm. 2021, 196, 109784. [Google Scholar] [CrossRef]
- Presti, M.L.; Martínez-Máñez, R.; Ros-Lis, J.V.; Batista, R.M.F.; Costa, S.P.G.; Raposo, M.M.M.; Sancenón, F. A Dual Channel Sulphur-containing Macrocycle Functionalised BODIPY Probe for the Detection of Hg(II) in Mixed Aqueous Solution. New J. Chem. 2018, 42, 7863–7868. [Google Scholar] [CrossRef] [Green Version]
- Collado, D.; Casado, J.; González, S.R.; Navarrete, J.T.L.; Suau, R.; Perez-Inestrosa, E.; Pappenfus, T.M.; Raposo, M.M.M. Enhanced Functionality for Donor-Acceptor Oligothiophenes by means of Inclusion of BODIPY: Synthesis, Electrochemistry, Photophysics and Model Chemistry. Chem. Eur. J. 2011, 17, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, R.C.R.; Pinto, S.C.S.; Costa, S.P.G.; Raposo, M.M.M. Meso-Triphenylamine BODIPY Derivative for Optical Chemosensing of Metal Ions. Chem. Proc. 2021, 3, 8291. [Google Scholar] [CrossRef]
- Gonçalves, R.C.R.; Pinto, S.C.S.; Costa, S.P.G.; Raposo, M.M.M. Synthesis, Characterization and Evaluation of a Novel BODIPY Derivative as a Colorimetric Chemosensor for Fe3+ Recognition. Proceedings 2019, 41, 6625. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, R.; Nogueira, M.; Costa, S.P.G.; Raposo, M.M.M. BODIPY derivatives: Synthesis and Evaluation of their Optical Properties. Proceedings 2019, 9, 5700. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, R.; Nogueira, M.; Costa, S.P.G.; Raposo, M.M.M. Functionalized BODIPY derivatives as Potential Fluorescent labels. Proceedings 2019, 9, 5701. [Google Scholar] [CrossRef] [Green Version]
- Batista, R.M.F.; Ferreira, R.C.M.; Raposo, M.M.M.; Costa, S.P.G. Novel Optical Chemosensors for Anions and Cations based on an Amino Acid Core Functionalised with Benzimidazoles. Tetrahedron 2012, 68, 7322–7330. [Google Scholar] [CrossRef]
- Alfonso, M.; Sola, A.; Caballero, A.; Tárraga, A.; Molina, P. Heteroditopic Ligands based on Ferrocenyl Benzimidazoles Fused to an Additional Diaza Heterocyclic Ring System. Dalton Trans. 2009, 43, 9653–9658. [Google Scholar] [CrossRef]
- Moon, K.S.; Singh, N.; Lee, G.W.; Jang, D.O. Colorimetric Anion Chemosensor based on 2-Aminobenzimidazole: Naked-eye Detection of Biologically Important Anions. Tetrahedron 2007, 63, 9106–9111. [Google Scholar] [CrossRef]
- Molina, P.; Tárraga, A.; Otón, F. Imidazole Derivatives: A Comprehensive Survey of Their Recognition Properties. Org. Biomol. Chem. 2012, 10, 1711–1724. [Google Scholar] [CrossRef]
- Esteves, C.I.C.; Ferreira, R.C.M.; Raposo, M.M.M.; Costa, S.P.G. New Fluoroionophores for Metal Cations based on Benzo[d]Oxazol-5-yl-Alanine Bearing Pyrrole and Imidazole. Dyes Pigm. 2018, 151, 211–218. [Google Scholar] [CrossRef]
- Okda, H.E.; Sayed, S.E.; Ferreira, R.C.M.; Otri, I.; Costa, S.P.G.; Raposo, M.M.M.; Martínez-Máñez, R.; Sancenón, F. A Simple and Easy-to-prepare Imidazole-based Probe for the Selective Chromofluorogenic Recognition of Biothiols and Cu(II) in Aqueous Environments. Dyes Pigm. 2019, 162, 303–308. [Google Scholar] [CrossRef]
- Li, Z.; Li, L.J.; Sun, T.; Liu, L.; Xie, Z. Benzimidazole-BODIPY as Optical and Fluorimetric pH Sensor. Dyes Pigm. 2016, 128, 165–169. [Google Scholar] [CrossRef]
- Montalti, M.; Credi, A.; Prodi, L.; Gandolfi, M.T. Handbook of Photochemistry, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar]
- Demas, J.N.; Crosby, G.A. Measurement of Photoluminescence Quantum Yields. Review. J. Phys. Chem. 1971, 75, 991–1024. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, R.C.R.; Pinto, S.C.S.; Costa, S.P.G.; Raposo, M.M.M. Anion Colorimetric Chemosensor Based on a Benzimidazole-Functionalized BODIPY Derivative. Chem. Proc. 2022, 8, 90. https://doi.org/10.3390/ecsoc-25-11754
Gonçalves RCR, Pinto SCS, Costa SPG, Raposo MMM. Anion Colorimetric Chemosensor Based on a Benzimidazole-Functionalized BODIPY Derivative. Chemistry Proceedings. 2022; 8(1):90. https://doi.org/10.3390/ecsoc-25-11754
Chicago/Turabian StyleGonçalves, Raquel C. R., Sónia C. S. Pinto, Susana P. G. Costa, and Maria Manuela M. Raposo. 2022. "Anion Colorimetric Chemosensor Based on a Benzimidazole-Functionalized BODIPY Derivative" Chemistry Proceedings 8, no. 1: 90. https://doi.org/10.3390/ecsoc-25-11754
APA StyleGonçalves, R. C. R., Pinto, S. C. S., Costa, S. P. G., & Raposo, M. M. M. (2022). Anion Colorimetric Chemosensor Based on a Benzimidazole-Functionalized BODIPY Derivative. Chemistry Proceedings, 8(1), 90. https://doi.org/10.3390/ecsoc-25-11754






