Comparative Investigation of Membrane Systems for Crystallization and Spherical Agglomeration †
Abstract
:1. Introduction
2. Methods
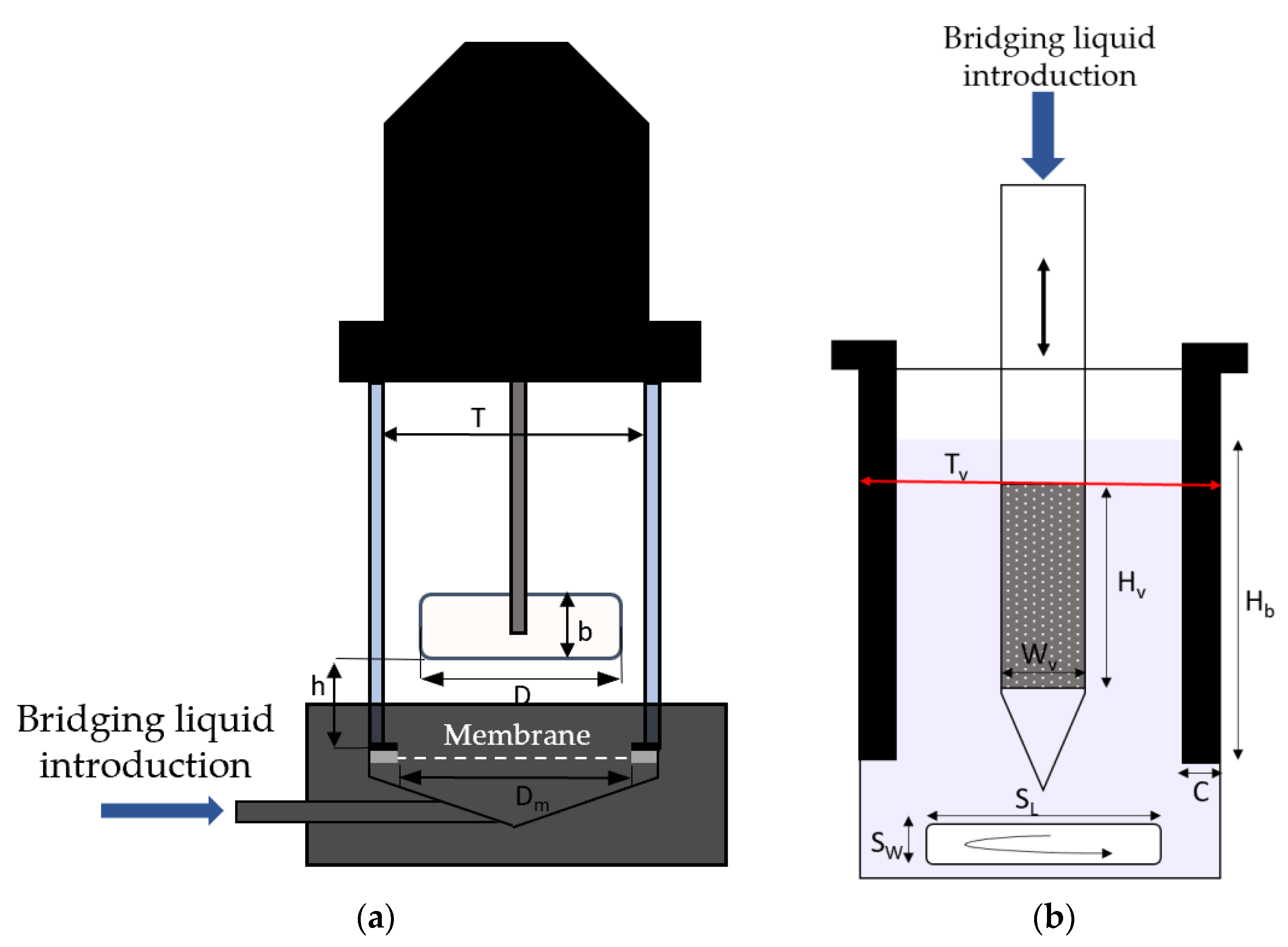
2.1. Dispersion Cell
2.2. Oscillating Module
2.3. Membrane
2.4. Materials
2.5. Procedure
3. Results and Discussion
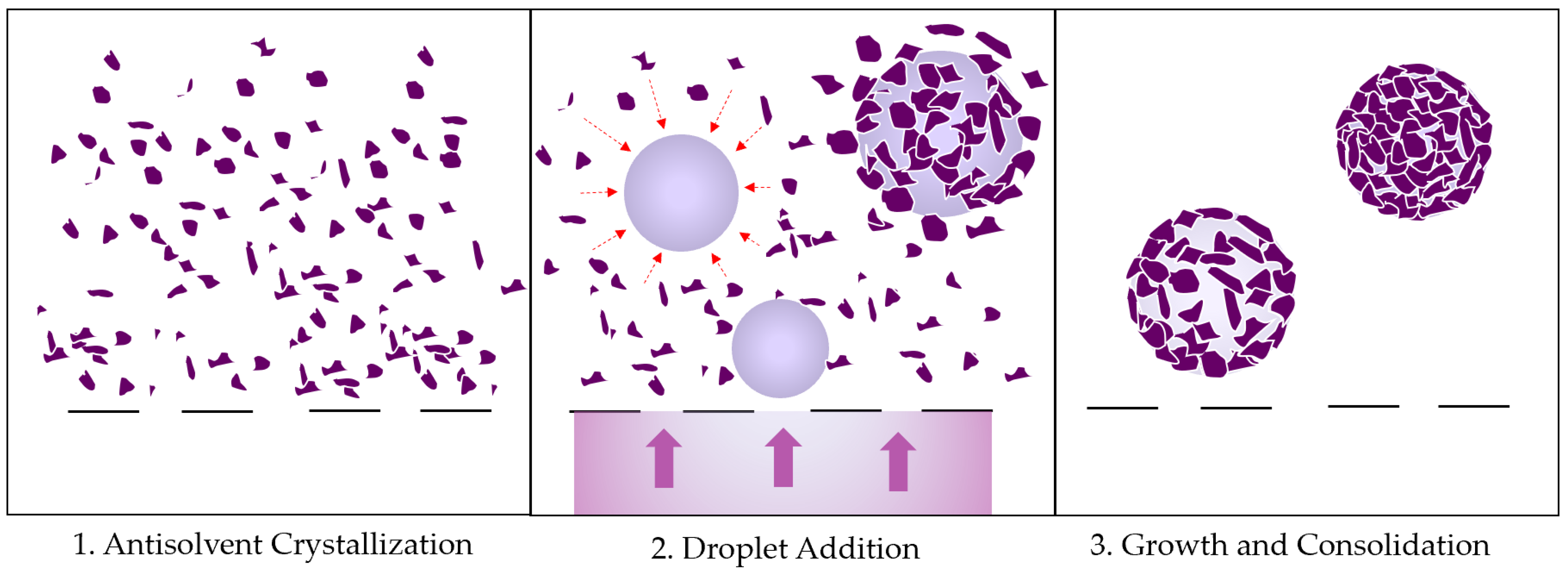
3.1. Agglomerate Formation Mechanism
3.2. Optical Microscopy Results
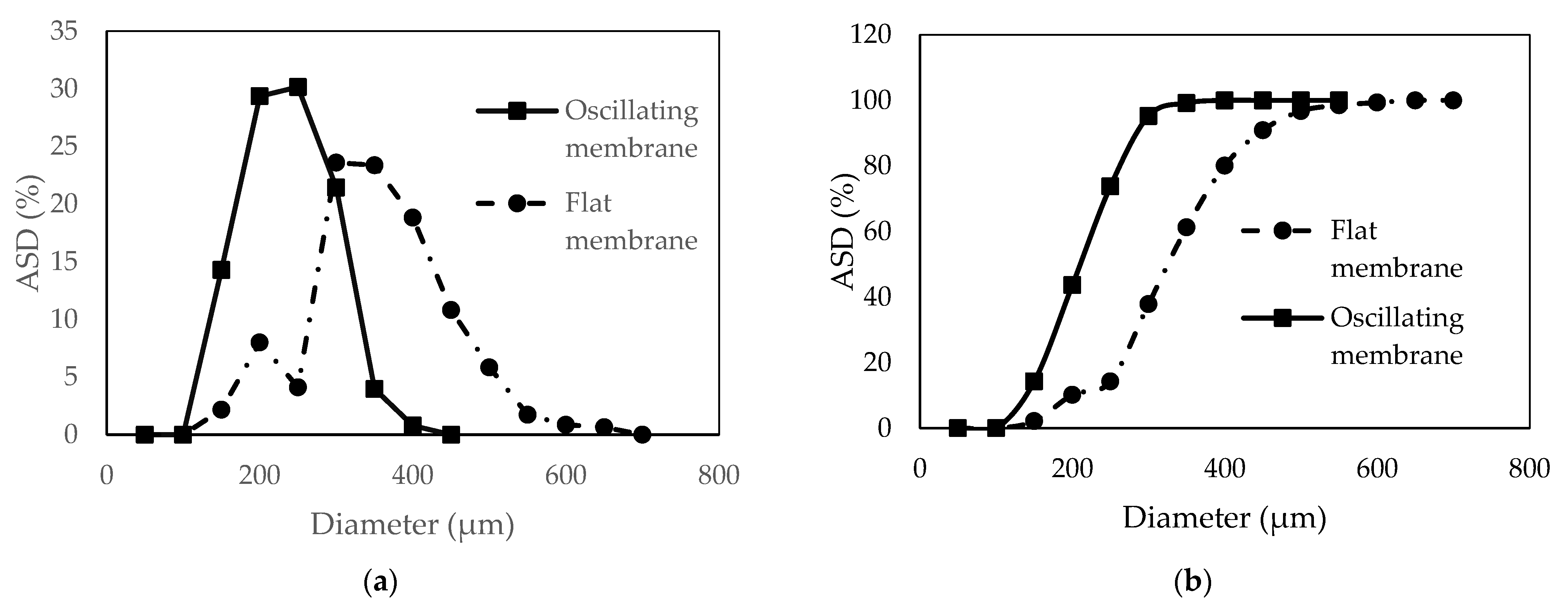
3.3. Agglomerate Size Distribution
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hatcher, L.; Li, W.; Payne, P.; Benyahia, B.; Rielly, C.D.; Wilson, C.C. Tuning Morphology in Active Pharmaceutical Ingredients: Controlling the Crystal Habit of Lovastatin through Solvent Choice and Non-Size-Matched Polymer Additives. Cryst. Growth Des. 2020, 20, 5854–5862. [Google Scholar] [CrossRef]
- Zhou, L.; Su, M.; Benyahia, B.; Singh, A.; Barton, P.I.; Trout, B.L.; Myerson, A.S.; Braatz, R.D. Mathematical modeling and design of layer crystallization in a concentric annulus with and without recirculation. AIChE J. 2013, 59, 1308–1321. [Google Scholar] [CrossRef] [Green Version]
- Fysikopoulos, D.; Benyahia, B.; Borsos, A.; Nagy, Z.K.; Rielly, C.D. A Framework for Model Reliability and Estimability Analysis of Crystallization Processes with Multi-Impurity Multi-Dimensional Population Balance Models. Comput. Chem. Eng. 2019, 122, 275–292. [Google Scholar] [CrossRef]
- Chen, C.W.; Lee, T. Round Granules of Dimethyl Fumarate by Three-in-One Intensified Process of Reaction, Crystallization, and Spherical Agglomeration in a Common Stirred Tank. Org. Process Res. Dev. 2017, 21, 1326–1339. [Google Scholar] [CrossRef]
- Peña, R.; Oliva, J.A.; Burcham, C.L.; Jarmer, D.J.; Nagy, Z.K. Process Intensification through Continuous Spherical Crystallization Using an Oscillatory Flow Baffled Crystallizer. Cryst. Growth Des. 2017, 17, 4776–4784. [Google Scholar] [CrossRef]
- Yeap, E.W.Q.; Ng, D.Z.L.; Lai, D.; Ertl, D.J.; Sharpe, S.A.; Khan, S.A. Continuous Flow Droplet-Based Crystallization Platform for Producing Spherical Drug Microparticles. Org. Process Res. Dev. 2019, 23, 93–101. [Google Scholar] [CrossRef]
- Peña, R.; Nagy, Z.K. Process Intensification through Continuous Spherical Crystallization Using a Two-Stage Mixed Suspension Mixed Product Removal (MSMPR) System. Cryst. Growth Des. 2015, 15, 4225–4236. [Google Scholar] [CrossRef]
- Yeap, E.W.Q.; Acevedo, A.J.; Khan, S.A. Microfluidic Extractive Crystallization for Spherical Drug/Drug-Excipient Microparticle Production. Org. Process Res. Dev. 2019, 23, 375–381. [Google Scholar] [CrossRef]
- Thati, J.; Rasmuson, Å.C. Particle engineering of benzoic acid by spherical agglomeration. Eur. J. Pharm. Sci. 2012, 45, 657–667. [Google Scholar] [CrossRef]
- Katta, J.; Rasmuson, Å.C. Spherical crystallization of benzoic acid. Int. J. Pharm. 2008, 348, 61–69. [Google Scholar] [CrossRef]
- Orlewski, P.M.; Ahn, B.; Mazzotti, M. Tuning the particle sizes in spherical agglomeration. Cryst. Growth Des. 2018, 18, 6257–6265. [Google Scholar] [CrossRef]
- Thati, J.; Rasmuson, Å.C. On the mechanisms of formation of spherical agglomerates. Eur. J. Pharm. Sci. 2011, 42, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Arjmandi-Tash, O.; Tew, J.D.; Pitt, K.; Smith, R.; Litster, J.D. A new mathematical model for nucleation of spherical agglomerates by the immersion mechanism. Chem. Eng. Sci. X 2019, 4, 100048. [Google Scholar] [CrossRef]
- Pitt, K.; Peña, R.; Tew, J.D.; Pal, K.; Smith, R.; Nagy, Z.K.; Litster, J.D. Particle design via spherical agglomeration: A critical review of controlling parameters, rate processes and modelling. Powder Technol. 2018, 326, 327–343. [Google Scholar] [CrossRef]
- Lackowska, I.; Dragosavac, M.; Benyahia, B. Spherical Agglomeration of Benzoic Acid Using Membrane Emulsification. In Proceedings of the 4th International Symposium on Pharmaceutical Engineering Research (SPhERe Proceedings 2021), Braunschweig, Germany, 15–17 September 2021. [Google Scholar] [CrossRef]
- Chen, K.; Hou, B.; Wu, H.; Huang, X.; Li, F.; Xiao, Y.; Li, J.; Bao, Y.; Hao, H. Hollow and solid spherical azithromycin particles prepared by different spherical crystallization technologies for direct tableting. Processes 2019, 7, 276. [Google Scholar] [CrossRef] [Green Version]
- Dragosavac, M.; Sovilj, M.N.; Kosvintsev, S.R.; Holdich, R.G.; Vladisavljević, G.T. Controlled production of oil-in-water emulsions containing unrefined pumpkin seed oil using stirred cell membrane emulsification. J. Memb. Sci. 2008, 322, 178–188. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Qin, L.; Su, J.; Sun, Y.; Zhang, L.; Li, J.; Beck-Broichsitter, M.; Muenster, U.; Chen, L.; Mao, S. Engineering large porous microparticles with tailored porosity and sustained drug release behavior for inhalation. Eur. J. Pharm. Biopharm. 2020, 155, 139–146. [Google Scholar] [CrossRef]
- Imbrogno, A.; Dragosavac, M.; Piacentini, E.; Vladisavljević, G.; Holdich, R.; Giorno, L. Polycaprolactone multicore-matrix particle for the simultaneous encapsulation of hydrophilic and hydrophobic compounds produced by membrane emulsification and solvent diffusion processes. Colloids Surf. B Biointerfaces 2015, 135, 116–125. [Google Scholar] [CrossRef] [Green Version]
- Gehrmann, S.; Bunjes, H. Influence of membrane material on the production of colloidal emulsions by premix membrane emulsification. Eur. J. Pharm. Biopharm. 2018, 126, 140–148. [Google Scholar] [CrossRef]
- Joseph, S.; Bunjes, H. Evaluation of Shirasu Porous Glass (SPG) membrane emulsification for the preparation of colloidal lipid drug carrier dispersions. Eur. J. Pharm. Biopharm. 2014, 87, 178–186. [Google Scholar] [CrossRef]
- Vladisavljević, G.T.; Wang, B.; Dragosavac, M.M.; Holdich, R.G. Production of food-grade multiple emulsions with high encapsulation yield using oscillating membrane emulsification. Colloids Surf. A Physicochem. Eng. Asp. 2014, 458, 78–84. [Google Scholar] [CrossRef] [Green Version]
- Holdich, R.G.; Dragosavac, M.M.; Vladisavljević, G.T.; Kosvintsev, S.R. Membrane Emulsification with Oscillating and Stationary Membranes. Ind. Eng. Chem. Res. 2010, 49, 3810–3817. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lackowska, I.; Dragosavac, M.; Benyahia, B. Comparative Investigation of Membrane Systems for Crystallization and Spherical Agglomeration. Chem. Proc. 2022, 9, 2. https://doi.org/10.3390/IOCC_2022-12162
Lackowska I, Dragosavac M, Benyahia B. Comparative Investigation of Membrane Systems for Crystallization and Spherical Agglomeration. Chemistry Proceedings. 2022; 9(1):2. https://doi.org/10.3390/IOCC_2022-12162
Chicago/Turabian StyleLackowska, Izabela, Marijana Dragosavac, and Brahim Benyahia. 2022. "Comparative Investigation of Membrane Systems for Crystallization and Spherical Agglomeration" Chemistry Proceedings 9, no. 1: 2. https://doi.org/10.3390/IOCC_2022-12162







