The Phytochemistry and Anticarcinogenic Activity of Noni Juice †
Abstract
1. Introduction
2. Methods
2.1. Noni Juice Samples
2.2. Sample Preparation
2.3. Extraction Protocol and Measurement of TPC and FRAP
2.4. Anticarcinogenic Activity
2.5. Data Analysis
3. Results and Discussion
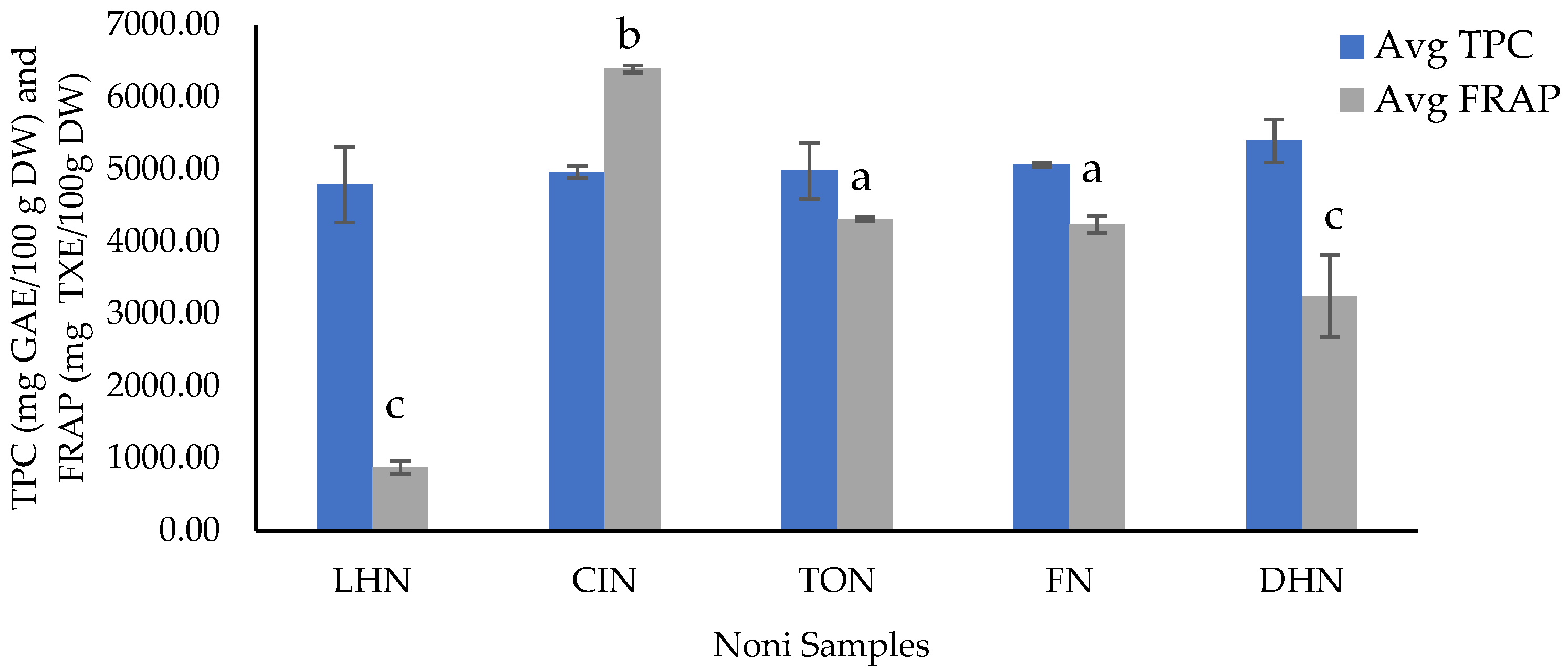
3.1. Total Phenolic Content and Antioxidant Activity
3.2. Anticarcinogenic Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akihisa, T.; Matsumoto, K.; Tokuda, H.; Yasukawa, K.; Seino, K.I.; Nakamoto, K.; Kuninaga, H.; Suzuki, T.; Kimura, Y. Anti-inflammatory and potential cancer chemopreventive constituents of the fruits of Morinda citrifolia (Noni). J. Nat. Prod. 2007, 70, 754–757. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.C. Anticancer activity of Morinda citrifolia (noni) fruit: A review. Phyther. Res. 2012, 26, 1427–1440. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.S. Cancer preventive Effect of Morinda citrifolia (Noni) fruit juice against the AflatoxinB1-induced genotoxicity in human peripheral lymphocytes in vitro. IOSR J. Pharm. 2012, 2, 228–234. [Google Scholar] [CrossRef]
- Pande, M.; Mills, G.; Singh, N.; Voro, T.; Dqg, S. The Kura Files: Qualitative Social Survey. Pac. Health Surveill. Response 2005, 12, 85–93. Available online: https://www.researchgate.net/profile/Mani-Naiker-4/publication/5667921_The_Kura_files_Qualitative_social_survey/links/53fdcf070cf22f21c2f84af5/The-Kura-files-Qualitative-social-survey.pdf (accessed on 14 February 2021).
- Wang, M.Y.; Peng, L.; Anderson, G.; Nowicki, D. Breast cancer prevention with Morinda citrifolia (noni) at the initiation stage. Funct. Foods Health Dis. 2013, 3, 203–222. [Google Scholar] [CrossRef]
- Boontha, S.; Kaewjaiboon, N.; Phitchayut, R.; Wanwalee, N.; Salisa Taolam, B.B.; Tasana, P. Cytotoxicity and Cell Migration Suppression by Noni Fruit Extract on Michigan Cancer Foundation-7 Human Breast Cancer Cells and Development of Topical Microemulsions. Pharm. Mag. 2019, 15, S38–S46. [Google Scholar] [CrossRef]
- Gupta, R.K.; Singh, N. Morinda citrifolia (Noni) alters oxidative stress marker and antioxidant activity in cervical cancer cell lines. Asian Pac. J. Cancer Prev. 2013, 14, 4603–4606. [Google Scholar] [CrossRef]
- Johnson, J.; Collins, T.; Walsh, K.; Naiker, M. Solvent extractions and spectrophotometric protocols for measuring the total anthocyanin, phenols and antioxidant content in plums. Chem. Pap. 2020, 74, 4481–4492. [Google Scholar] [CrossRef]
- Erenler, R.; Meral, B.; Sen, O.; Elmastas, M.; Aydin, A.; Eminagaoglu, O.; Topcu, G. Bioassay-guided isolation, identification of compounds from Origanum rotundifolium and investigation of their antiproliferative and antioxidant activities. Pharm. Biol. 2017, 55, 1646–1653. [Google Scholar] [CrossRef]
- Yang, J.; Gadi, R.; Thomson, T. Antioxidant capacity, total phenols, and ascorbic acid content of noni (Morinda citrifolia) fruits and leaves at various stages of maturity. Micronesica 2011, 41, 167–176. [Google Scholar]
- Samarasiri, M.H.; Chandrasiri, T.A.; Wijesinghe, D.B.; Gunawardena, S.P. Antioxidant Capacity and Total Phenolic Content Variations against Morinda citrifolia L. Fruit Juice Production Methods. ETP Int. J. Food Eng. 2019, 5, 293–299. [Google Scholar] [CrossRef]
- Pal, R.; Girhepunje, K.; Shrivastava, N.; Hussain, M.M.; Thirumoorthy, N. Antioxidant and free radical scavenging activity of ethanolic extract of Morinda citrifolia. Res. J. Pharm. Technol. 2011, 4, 1224–1226. [Google Scholar]
- Haslaniza, H.; Wan Yaacob, W.A.; Osman, H.; Maskat, M.Y. Interaction of antioxidants and organic acid from noni (Morinda citrifolia L.) juice with ion exchange resins during deodorization via deacidification. Der. Pharma Chem. 2015, 7, 9–21. [Google Scholar]
- Fidrianny, I.; Octaviani, G.D.; Kusmardiyani, S. Study of antioxidant profile and phytochemical content of different organs extracts of Morinda citrifolia L. J. Pharm. Sci. Res. 2018, 10, 2102–2105. [Google Scholar]
- Bhardwaj, R.; Yadav, A.; Sharma, R. Phytochemicals and antioxidant activity in Boerhavia diffusa. Int. J. Pharm. Pharm. Sci. 2014, 6, 344–348. [Google Scholar]
- Johnson, J.; Collins, T.; Power, A.; Chandra, S.; Skylas, D.; Portman, D.; Panozzo, J.; Blanchard, C.; Naiker, M. Antioxidative properties and macrochemical composition of five commercial mungbean varieties in Australia. Legum Sci. 2020, 2, e27. [Google Scholar] [CrossRef]
- Johnson, J.B.; Collins, T.; Mani, J.S.; Naiker, M. Nutritional Quality and Bioactive Constituents of Six Australian Plum Varieties. Int. J. Fruit Sci. 2021, 21, 115–132. [Google Scholar] [CrossRef]
- Sakulnarmrat, K.; Konczak, I. Composition of native Australian herbs polyphenolic-rich fractions and in vitro inhibitory activities against key enzymes relevant to metabolic syndrome. Food Chem. 2012, 134, 1011–1019. [Google Scholar] [CrossRef]
- Sakulnarmrat, K.; Fenech, M.; Thomas, P.; Konczak, I. Cytoprotective and pro-apoptotic activities of native Australian herbs polyphenolic-rich extracts. Food Chem. 2013, 136, 9–17. [Google Scholar] [CrossRef]
- Vuong, Q.V.; Hirun, S.; Phillips, P.A.; Chuen, T.L.K.; Bowyer, M.C.; Goldsmith, C.D.; Scarlett, C.J. Fruit-derived phenolic compounds and pancreatic cancer: Perspectives from Australian native fruits. J. Ethnopharmacol. 2014, 152, 227–242. [Google Scholar] [CrossRef]
- Gupta, R.K.; Banerjee, A.; Pathak, S.; Sharma, C.; Singh, N. Induction of mitochondrial-mediated apoptosis by Morinda citrifolia (Noni) in human cervical cancer cells. Asian Pac. J. Cancer Prev. 2013, 14, 237–242. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ali, M.; Kenganora, M.; Manjula, S.N. Health benefits of morinda citrifolia (Noni): A review. Pharm. J. 2016, 8, 321–334. [Google Scholar] [CrossRef]

| Product Name | Origin (Where Fruits Were Obtained from) | Description |
|---|---|---|
| Tahitian Organic Noni (TON) | Tahiti | Never reconstituted—always fresh. No preservatives, coloring agents, or sugars. |
| Cook Island Noni Juice (CIN) | Cook Island | 100% M. citrifolia fruit extract. Nonpasturized. |
| Dynamic Health Noni (DHN) | Tahiti | Contains no added sugar, artificial color, or preservatives. Due to the pure nature ingredients in this product, taste, color, and consistency may vary. |
| Fijian Noni (FN) | Fiji | Made from pure fruits (wild collection). No additives added. Pasteurized for optimum quality. |
| Life Health Noni (LHN) | New Zealand | 100% Noni fruit juice. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mani, J.S.; Johnson, J.B.; Naiker, M. The Phytochemistry and Anticarcinogenic Activity of Noni Juice. Eng. Proc. 2021, 11, 16. https://doi.org/10.3390/ASEC2021-11154
Mani JS, Johnson JB, Naiker M. The Phytochemistry and Anticarcinogenic Activity of Noni Juice. Engineering Proceedings. 2021; 11(1):16. https://doi.org/10.3390/ASEC2021-11154
Chicago/Turabian StyleMani, Janice S., Joel B. Johnson, and Mani Naiker. 2021. "The Phytochemistry and Anticarcinogenic Activity of Noni Juice" Engineering Proceedings 11, no. 1: 16. https://doi.org/10.3390/ASEC2021-11154
APA StyleMani, J. S., Johnson, J. B., & Naiker, M. (2021). The Phytochemistry and Anticarcinogenic Activity of Noni Juice. Engineering Proceedings, 11(1), 16. https://doi.org/10.3390/ASEC2021-11154








