Optical Investigation of PVA/PbTiO3 Composite for UV-Protective Approach Applications †
Abstract
:1. Introduction
2. Experimental
3. Results and Discussion
3.1. ATR-FTIR
3.2. Optical Properties


4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bailey, E.J.; Winey, K.I. Dynamics of polymer segments, polymer chains, and nanoparticles in polymer nanocomposite melts: A review. Prog. Polym. Sci. 2020, 105, 101242. [Google Scholar] [CrossRef]
- Ananth, A.N.; Umapathy, S.; Sophia, J.; Mathavan, T.; Mangalaraj, D. On the optical and thermal properties of in situ/ex situ reduced Ag NP’s/PVA composites and its role as a simple SPR-based protein sensor. Appl. Nanosci. 2011, 1, 87–96. [Google Scholar] [CrossRef] [Green Version]
- Ghanipour, M.; Dorranian, D. Effect of ag-nanoparticles doped in polyvinyl alcohol on the structural and optical properties of PVA films. J. Nanomater. 2013, 2013, 897043. [Google Scholar] [CrossRef]
- Sebastian, M.T. Polymer–Ceramic Composites of 0–3 Connectivity for Circuits in Electronics: A Review. Int. J. Appl. Ceram. Technol. 2010, 7, 415–434. [Google Scholar] [CrossRef]
- Uma, S.; Philip, J. Induction of electro-activity in polyvinyl alcohol with addition of nanocrystalline PZT ceramic. Int. J. Indian J. Pure Appl. Phys. 2013, 5, 717–723. [Google Scholar]
- Mir, M.; de Paula, C.C.; Garcia, D.; Kiminami, R.H.G.A.; Eiras, J.A.; Mascarenhas, Y.P. Microstructural characterization using the Rietveld method in lead lanthanum titanate ceramics. J. Eur. Ceram. 2007, 27, 3719–3721. [Google Scholar] [CrossRef]
- Kinadjian, N.; Achard, M.-F.; Julián-López, B.; Maugey, M.; Poulin, P.; Prouzet, E.; Backov, R. ZnO/PVA macroscopic fibers bearing anisotropic photonic properties. Adv. Funct. Mater 2012, 22, 3994–4003. [Google Scholar] [CrossRef]
- Taha, E.O.; Nasr, G.M. Dielectric and dynamic mechanical properties of Rochelle salt/PVA composites. In IOP Conference Series Materials Science and Engineering; IOP Publishing: Hurghada, Egypt, 2021; p. 12008. [Google Scholar]
- Shehap, A.M.; Akil, D.S. Structural and optical properties of TiO2 nanoparticles/PVA for different composites thin films. Int. J. Nanoelectron. Mater. 2016, 9, 17–36. [Google Scholar]
- Atta, M.M.; Taha, E.O.; Abdelreheem, A.M. Nitrogen plasma effect on the structural, thermal, and dynamic mechanical properties of PVA/starch/graphene oxide nanocomposite. Appl. Phys. A 2021, 127, 532. [Google Scholar] [CrossRef]
- Afzal, H.M.; Shehzad, F.; Zubair, M.; Bakather, O.Y.; Al-Harthi, M.A. Influence of microwave irradiation on thermal properties of PVA and PVA/graphene nanocomposites. J. Therm. Anal. Calorim. 2020, 139, 353–365. [Google Scholar] [CrossRef]
- Hassaan, M.Y.; Osman, H.M.; Hassan, H.H.; El-Deeb, A.S.; Al-Helal, M.A. Optical and electrical studies of borosilicate glass containing vanadium and cobalt ions for smart windows applications. Ceram. Int. 2017, 43, 1795–1801. [Google Scholar] [CrossRef]
- Aziz, S.B.; Rasheed, M.A.; Ahmed, H.M. Synthesis of polymer nanocomposites based on [methyl cellulose](1−x):(CuS)x (0.02 M ≤ x ≤ 0.08 M) with desired optical band gaps. Polymers 2017, 9, 194. [Google Scholar] [CrossRef] [PubMed]

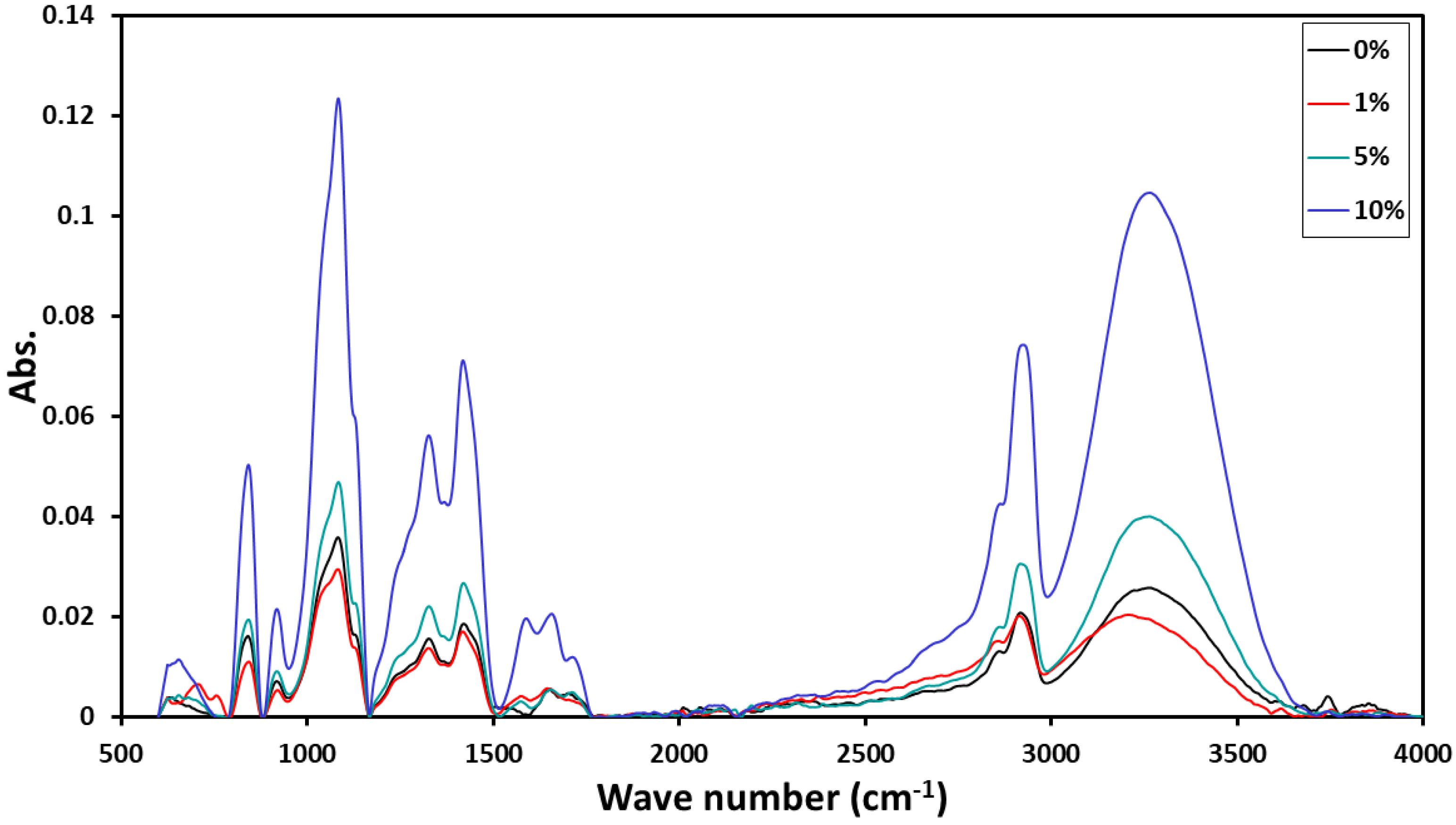
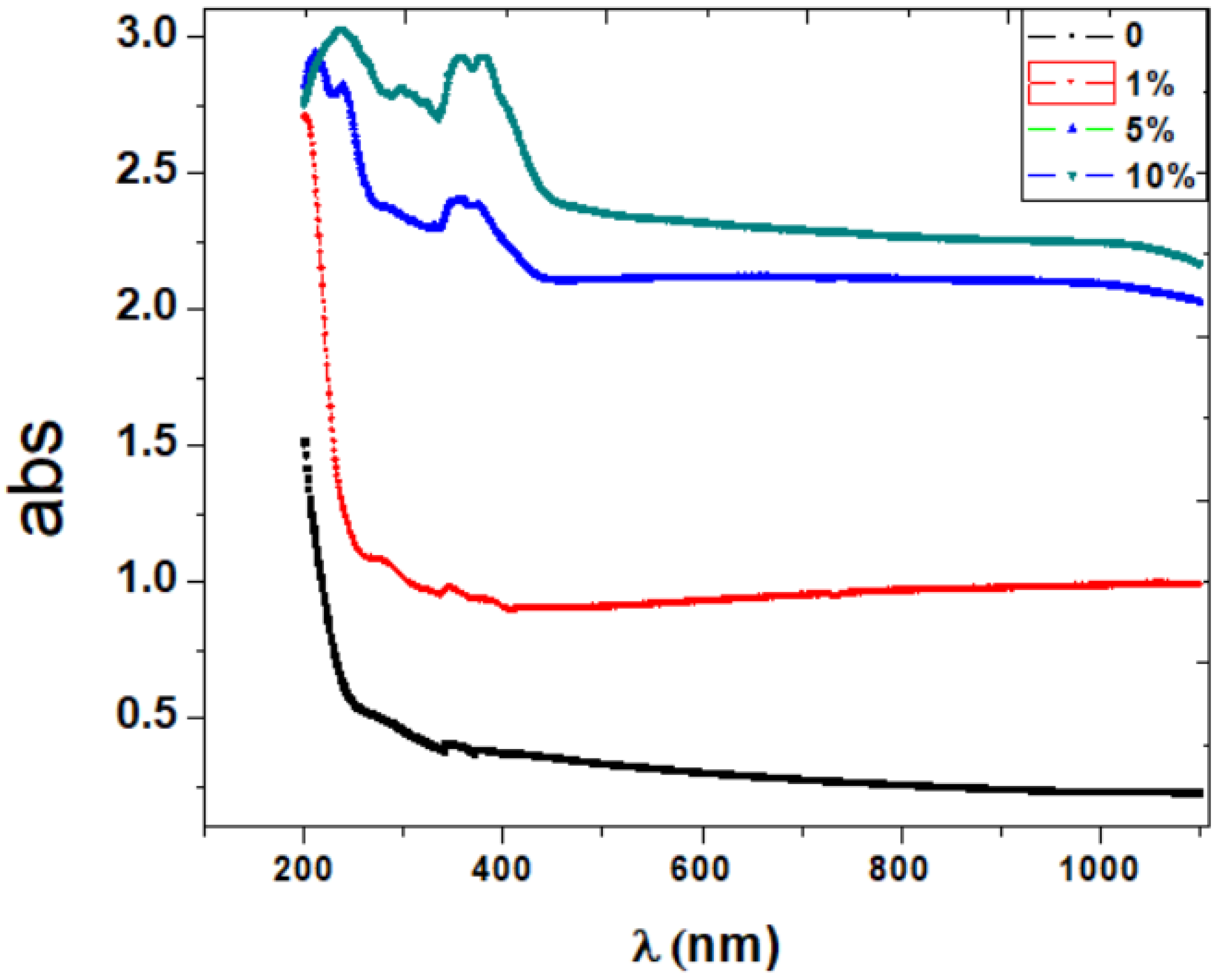

| PVA/PbTiO3 | Absorption Edge (eV) | Band Tail Eu (eV) | Energy Gap (eV) | |
|---|---|---|---|---|
| Ei | Ed | |||
| 0% | 4.6 | 1.12 | 5.3 | 5.2 |
| 1% | 4.46 | 2 | 5.8 | 4.8 |
| 5% | 1.36 | 2.34 | 2.8 | 2.7 |
| 10% | 0.56 | 2.74 | 2.3 | 2.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Deeb, A.S.; Kader, M.M.A.; Nasr, G.M.; Ahmed, M.A.; Taha, E.O. Optical Investigation of PVA/PbTiO3 Composite for UV-Protective Approach Applications. Eng. Proc. 2021, 11, 4. https://doi.org/10.3390/ASEC2021-11172
El-Deeb AS, Kader MMA, Nasr GM, Ahmed MA, Taha EO. Optical Investigation of PVA/PbTiO3 Composite for UV-Protective Approach Applications. Engineering Proceedings. 2021; 11(1):4. https://doi.org/10.3390/ASEC2021-11172
Chicago/Turabian StyleEl-Deeb, Asmaa. S., Marwa. M. Abdel Kader, Gamal. M. Nasr, Mona. A. Ahmed, and Eman O. Taha. 2021. "Optical Investigation of PVA/PbTiO3 Composite for UV-Protective Approach Applications" Engineering Proceedings 11, no. 1: 4. https://doi.org/10.3390/ASEC2021-11172
APA StyleEl-Deeb, A. S., Kader, M. M. A., Nasr, G. M., Ahmed, M. A., & Taha, E. O. (2021). Optical Investigation of PVA/PbTiO3 Composite for UV-Protective Approach Applications. Engineering Proceedings, 11(1), 4. https://doi.org/10.3390/ASEC2021-11172







