Abstract
A photometric flow cell for the in-line determination of the biomass content in a microalgae Nannochloropsis sp. cultivation has been constructed, integrated into the photobioreactor and tested. This process is used for the development of third-generation biofuel technology. The results obtained have shown the linear dependence of the detected optical density on the biomass content. Therefore, the concept of the in-line determination of the biomass content in similar cultivation processes using a photometric sensor based on a single laser light source has been proved.
1. Introduction
In-line process monitoring is increasingly used in biotechnological production. Modern biotechnology is used in different areas, including the production of biofuels [1]. Microalgae are currently the most promising renewable raw materials for the biofuel. The technology of biofuel production significantly differs from the production technology of traditional fossil fuels. The chemical composition of the biomass is affected by the conditions of its cultivation process. For example, nitrogen deprivation of Nannochloropsis sp. culture, combined with increasing the brightness of illumination, leads to a 51% increase in the concentration of lipids in the biomass composition [2]. The process of the cultivation of microorganisms consists of the accumulation of their biomass and metabolic products under the conditions of nutrient medium [3,4,5]. For the successful cultivation of photoautotrophic microalgae cells, the necessary amount of light energy and carbon dioxide, the most favorable temperature and acidic conditions are important. The optimization of the cultivation processes requires an in-line analytical control of its main parameters [6].
One of the critical indicators of biotechnological processes is the biomass content in the reactor. In photosynthesis processes, the biomass is represented by microalgae or bacteria. The stages of photosynthesis take from 10−5 to 103 s to occur, and cell division stages and biomass accumulation are counted in minutes [7]. Therefore, traditional biomass determination methods, such as cell counting chambers (e.g., Goryaev chamber, Fuchs–Rosenthal chamber), are too time-consuming and labor-intensive and hardly suitable for in-line control. Existing in-line methods for the determination of biomass in solution use turbidity sensors [8], which can be inaccurate because of the presence of suspended particles, gas bubbles and turbulence in the medium, affecting the observed signal [9]. Turbidity sensors are typically equipped with fiber probes, which should be immersed in a reactor and require regular maintenance [10].
The present proof-of-concept study is aimed at testing the possibility of the in-line determination of the biomass in the cultivation of the microalgae Nannochloropsis sp. using a photometric flow cell based on a semiconductor laser operating at a specially chosen wavelength. Microalgae Nannochloropsis sp., unlike the majority of cultivated microalgae, contains only chlorophyll a in its photosystem, which allowed us to minimize the influence of other chlorophylls and carotenes on the measurement results [11].
2. Materials and Methods
The photobioreactor was constructed, and the biomass of the microalgae Nannochloropsis sp. was cultivated in the culture medium Guillard f/2. The tracking of culture growth stages was carried out under visual control on a MIKMED-6 microscope using a Levenhuk C1400NG camera. Cell concentration in the culture medium solution was counted in a Goryaev cell-counting chamber model 851.
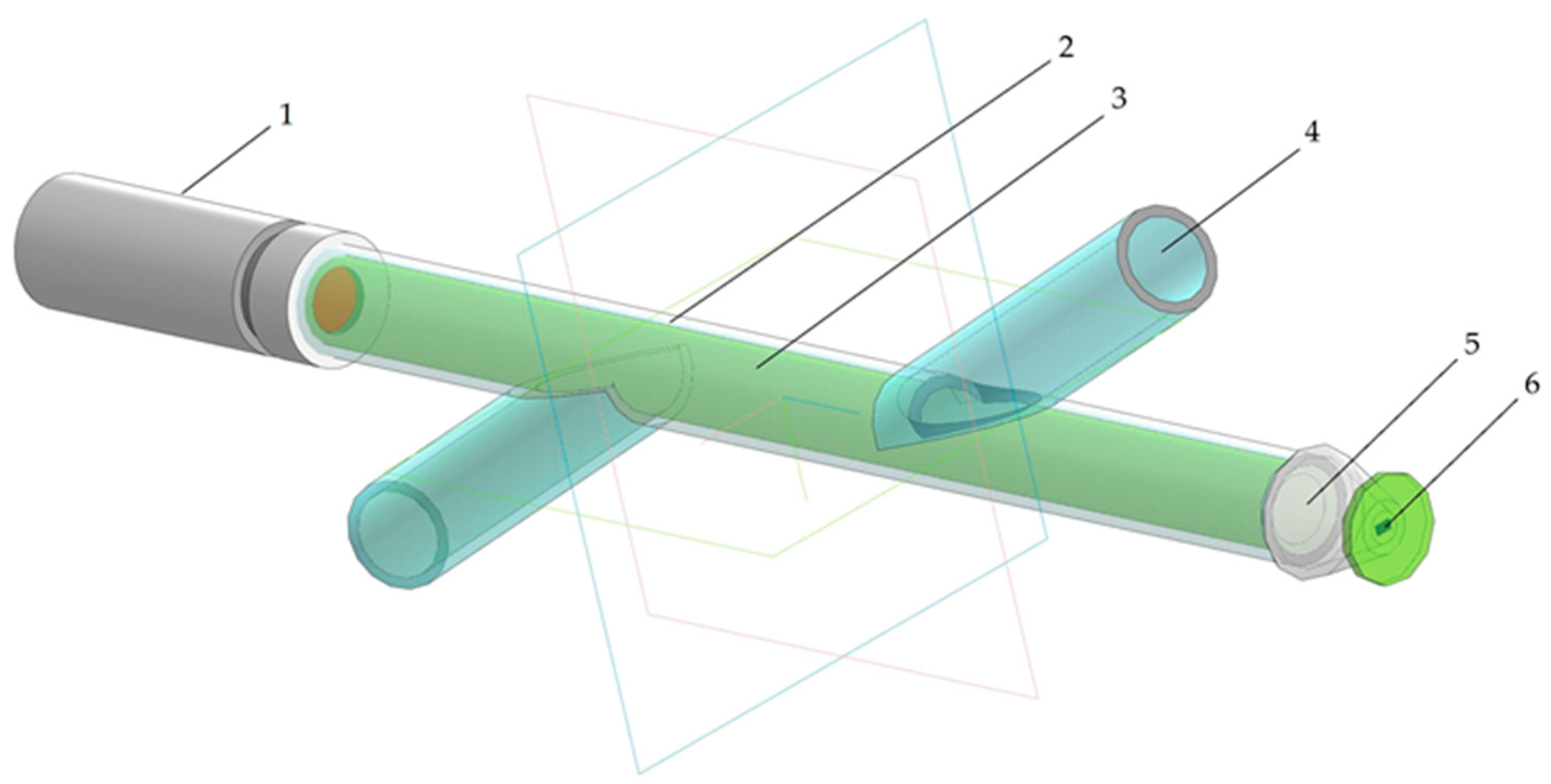
Taking into account the above-described problems of the optical determination of biomass concentration, an external photometric flow cell has been constructed (Figure 1). The flow cell was implemented in a transparent glass tube with two intersecting lateral bends. One side of the tube contained a source of polarized coherent light based on a semiconductor laser. The laser wavelength was chosen at 650 nm, close to the maximum of the absorption peak of chlorophyll a, situated at 665 nm. A photodiode for measuring the intensity of light that passed through the solution layer was placed on the opposite side of the cell. The use of a monochromatic light source reduces the influence of absorbing components and impurities contained in the solution. The electrical signal from the photodiode was transformed and processed via the analog-to-digital (AD) converter and recorded to a memory disk using an Arduino Uno microcontroller [12,13]. The detected light intensity was measured in millivolts (mV).
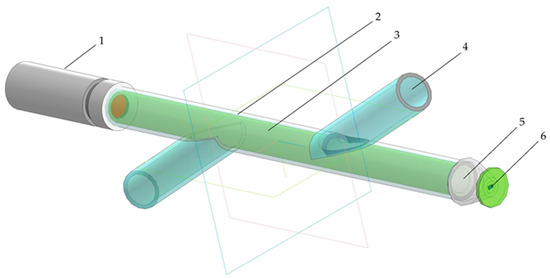
Figure 1.
The photometric flow cell: 1—laser diode module; 2—flow cell; 3—sample solution; 4—inlet/outlet tube; 5—focal lens; 6—photosensitive element.
3. Results and Discussion
A series of diluted biomass solutions was prepared for the experiment. The culture medium was used as a reference sample for the calibration of the reference signal to the AD converter. The solutions were measured in the flow cell, and light transmittance and absorbance at 650 nm has been calculated [14]. The same series of solutions was examined for biomass cell content using a Goryaev cell counting chamber. The results of the measurements are presented in Table 1.

Table 1.
Results of measurements for culture medium and diluted solutions.
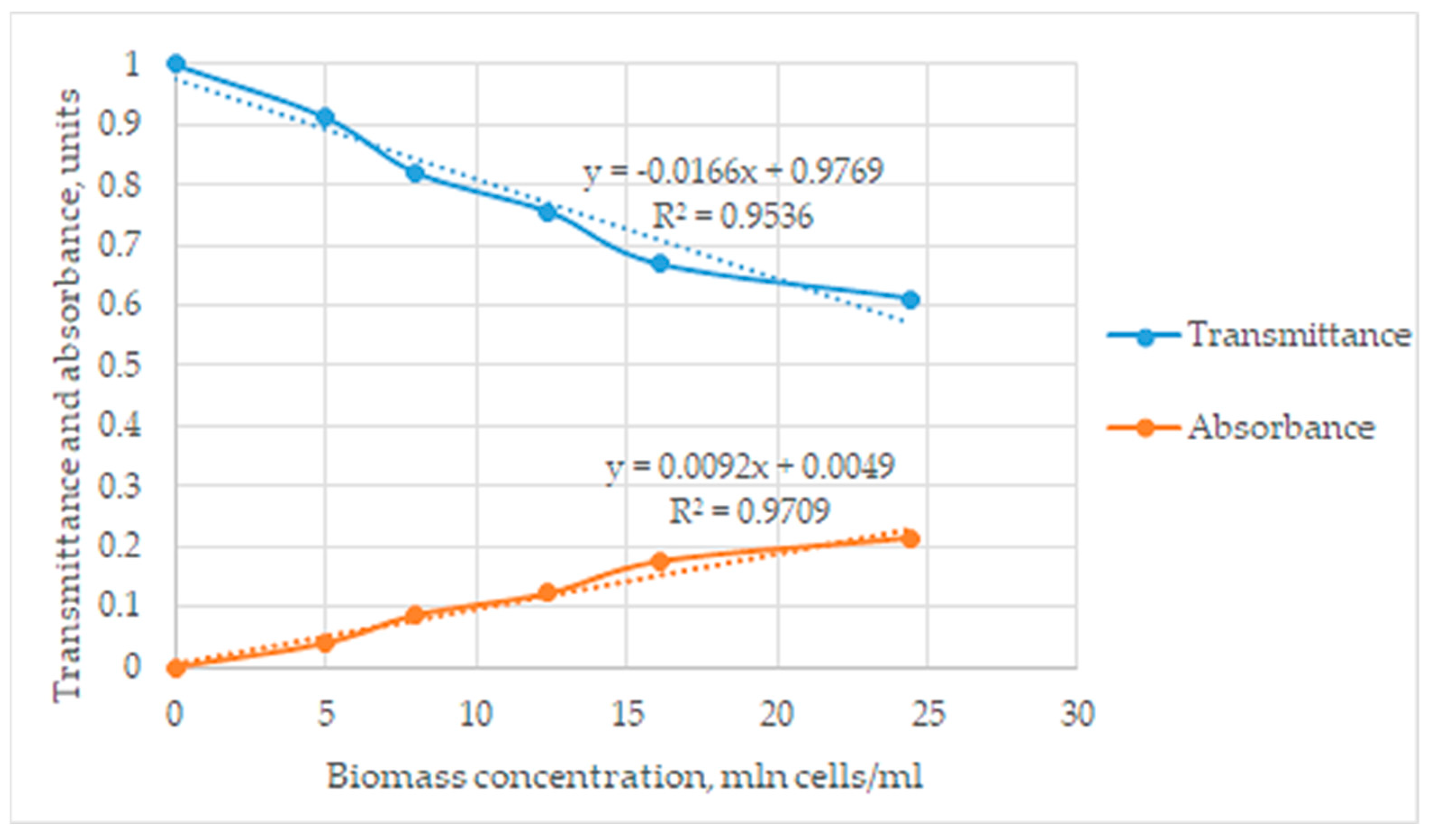
The observed signal in absorbance units was directly proportional to the biomass content. Based on the obtained data, graphs of transmittance and absorbance dependences on the concentrations of cells in the studied solutions were plotted, and mathematical dependencies were established according to the obtained graphs at Figure 2.
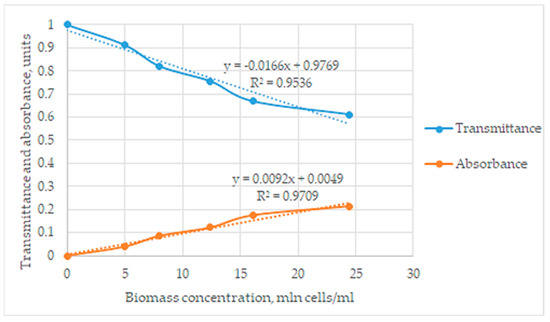
Figure 2.
The graphical dependences between transmittance and absorbance at biomass concentration.
The biomass content in photometric measurements does not generally obey Beer’s law, and biomass cells are able not only to absorb but also to scatter the light radiation. Nevertheless, the observed coefficient of determination (R2 = 0.97) shows the linear dependence of absorbance on the biomass content. This result can be explained by an assumption that light scattering effects observed by the flow cell also have a linear character in the considered concentration range.
4. Conclusions
The possibility of the in-line quantification of the microalgae Nannochloropsis sp. biomass content using a photometric flow cell was proved A simple calibration model has been obtained, which allow the determining of the concentration of microalgae cells in the medium based on the detected light intensity in absorbance units. The obtained data allow us to conclude that the application of optical sensors for the control and optimization of biotechnological processes of microalgae cultivation is promising. To improve the accuracy of measurements, it is necessary to evaluate the contribution of light scattering to the measurement results and improve the photometric flow cell construction accordingly [15].
Author Contributions
Conceptualization, A.B.; methodology, E.B.; formal analysis, E.B.; investigation, E.B. and A.B.; writing—original draft preparation, E.B.; writing—review and editing, A.B.; supervision, A.B.; project administration, A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available on request.
Conflicts of Interest
The authors declare that they have no known competing financial interest or personal relationship that could have appeared to influence the work reported in this paper.
References
- Muhammad, U.; Shamsuddin, I.; Danjuma, A.; Ru, S.M.; Dembo, U. Biofuels as the starring substitute to fossil fuels. Petrol. Sci. Eng. 2018, 2, 44–49. [Google Scholar] [CrossRef]
- Mishra, N.; Mishra, P.; Gupta, E.; Singh, P. Synergistic effects of nitrogen deprivation and high irradiance to enhance biomass and lipid production in Nannochloropsis. J. Microbiol. Biotech. Food Sci. 2023, 12, e3632. [Google Scholar] [CrossRef]
- Gojkovic, Z.; Lu, Y.; Ferro, L.; Toffolo, A.; Funk, C. Modeling biomass production during progressive nitrogen starvation by North Swedish green microalgae. Algal. Res. 2020, 47, 101835. [Google Scholar] [CrossRef]
- Feng, P.; Xu, Z.; Qin, L.; Alam, A.; Wang, Z.; Zhu, S. Effects of different nitrogen sources and light paths of flat plate photobioreactors on the growth and lipid accumulation of Chlorella sp. GN1 outdoors. Bioresour. Technol. 2020, 301, 122762. [Google Scholar] [CrossRef] [PubMed]
- Khoo, K.; Ahmad, I.; Chew, K.; Iwamoto, K.; Bhatnagar, A.; Show, P. Enhanced microalgal lipid production for biofuel using different strategies including genetic modification of microalgae: A review. Prog. Energy Combust. Sci. 2023, 96, 101071. [Google Scholar] [CrossRef]
- Benner, P.; Effenberger, S.; Franzgrote, L.; Kurzrock-Wolf, T.; Kress, K.; Weuster-Botz, D. Contact-free infrared OD measurement for online monitoring of parallel stirred-tank bioreactors up to high cell densities. Biochem. Eng. J. 2020, 164, 107749. [Google Scholar] [CrossRef]
- Dong, L.-Q.; Niu, K.; Cong, S.-L. Theoretical study of vibrational relaxation and internal conversion dynamics of chlorophyll-a in ethyl acetate solvent in femtosecond laser fields. Chem. Phys. Lett. 2006, 432, 286–290. [Google Scholar] [CrossRef]
- Nguyen, B.; Rittmann, B. Low-cost optical sensor to automatically monitor and control biomass concentration in microalgal cultivation. Algal Res. 2018, 32, 101–106. [Google Scholar] [CrossRef]
- Cáceres, I.; Alsina, J.; Zanden, J.; Ribberink, D.; Sánchez-Areilla, A. The effect of air bubbles on optical backscatter sensor measurements under plunging breaking waves. Coast. Eng. 2020, 159, 103721. [Google Scholar] [CrossRef]
- Zhou, Y.; Fu, X.; Ying, Y.; Fang, Z. An integrated fiber-optic probe combined with support vector regression for fast estimation of optical properties of turbid media. Anal. Chem. Acta 2015, 880, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Basso, S.; Simionato, D.; Gerotto, C.; Segalla, A.; Giacometti, G.; Morosinotto, T. Characterization of the photosynthetic apparatus of the Eustigmatophycean Nannochloropsis gaditana: Evidence of convergent evolution in the supramolecular organization of photosystem I. Biocim. Boiphys. Acta 2014, 1837, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Wankhede, S.; Kale, V.; Shaligram, A.; Patil, A.; Halwar, D. IoT based dielectric constant measurement system for solid or semi-liquid materials using Arduino WeMos D1R1. Mater. Today Proc. 2023, 73, 474–480. [Google Scholar] [CrossRef]
- Itterheimová, P.; Foret, F.; Kubáň, P. High-resolution Arduino-based data acquisition devices for microscale separation systems. Anal. Chim. Acta 2021, 1153, 338294. [Google Scholar] [CrossRef] [PubMed]
- Larkum, A.W.D.; Douglas, S.E.; Raven, J.A. Photosynthesis in Algae. Advances in Photosynthesis and Respiration; Kluwer: London, UK, 2003; pp. 34–35. [Google Scholar]
- Gonzalez-Fernandez, F.; DeSa, R. Obtaining absorbance spectra from turbid retinal cell and tissue suspensions—Beating the light-scatter problem. Exp. Eye Res. 2023, 230, 109434. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).