Innovative Multipolymer-Based Electrochemical Biosensor Built on a Sonogel–Carbon Electrode Aiming for Continuous and Real-Time Lactate Determination in Physiological Samples: A New Scenario to Exploit Additive Printing †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Sonogel–Carbon Electrode
2.2. Synthesis of Gold Sononanoparticles
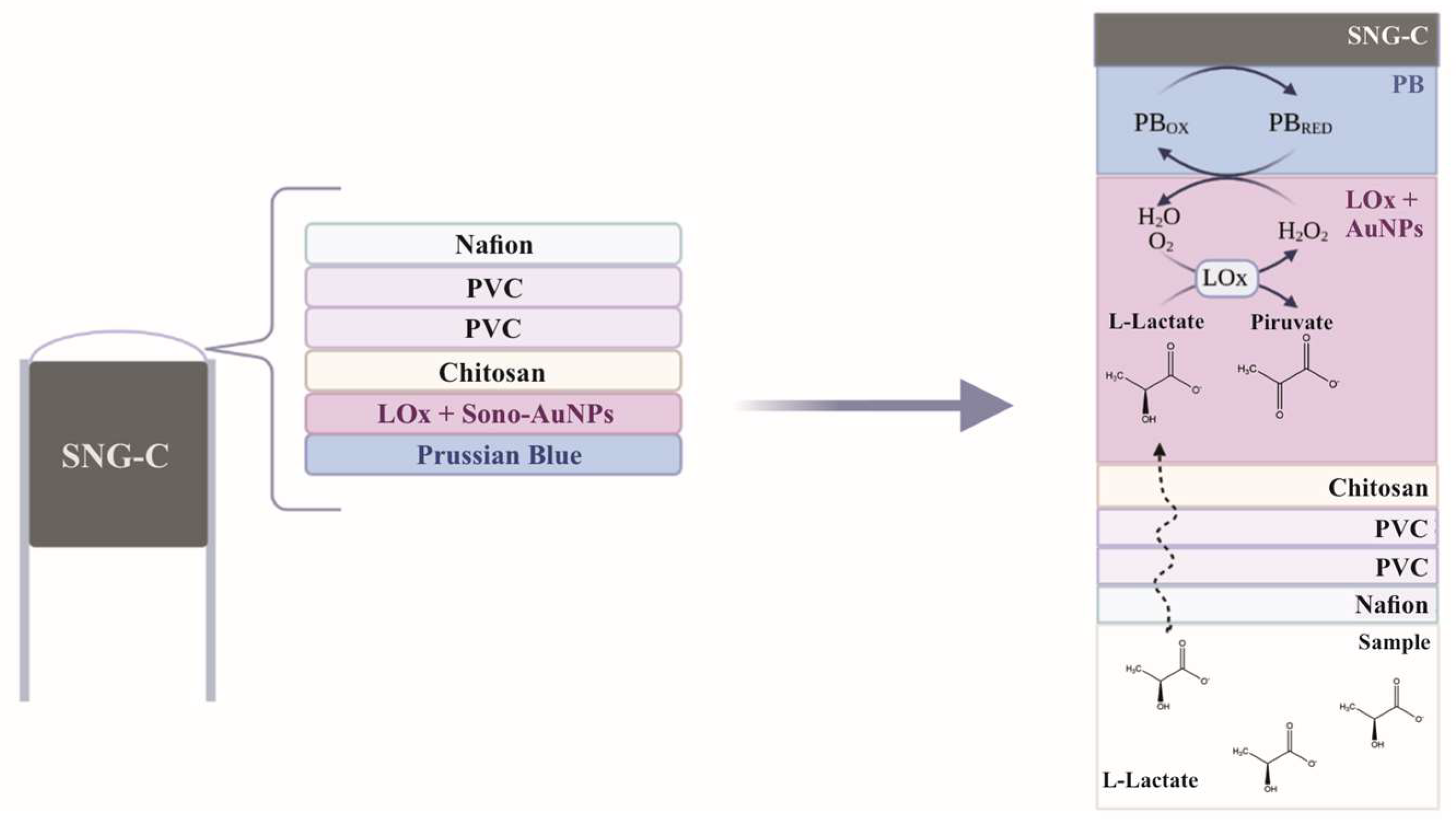
2.3. Fabrication of the Lactate Biosensor
2.4. Analytical Performance of the Biosensor in Batch Mode
2.5. Analysis in Continuous Mode
3. Results and Discussion
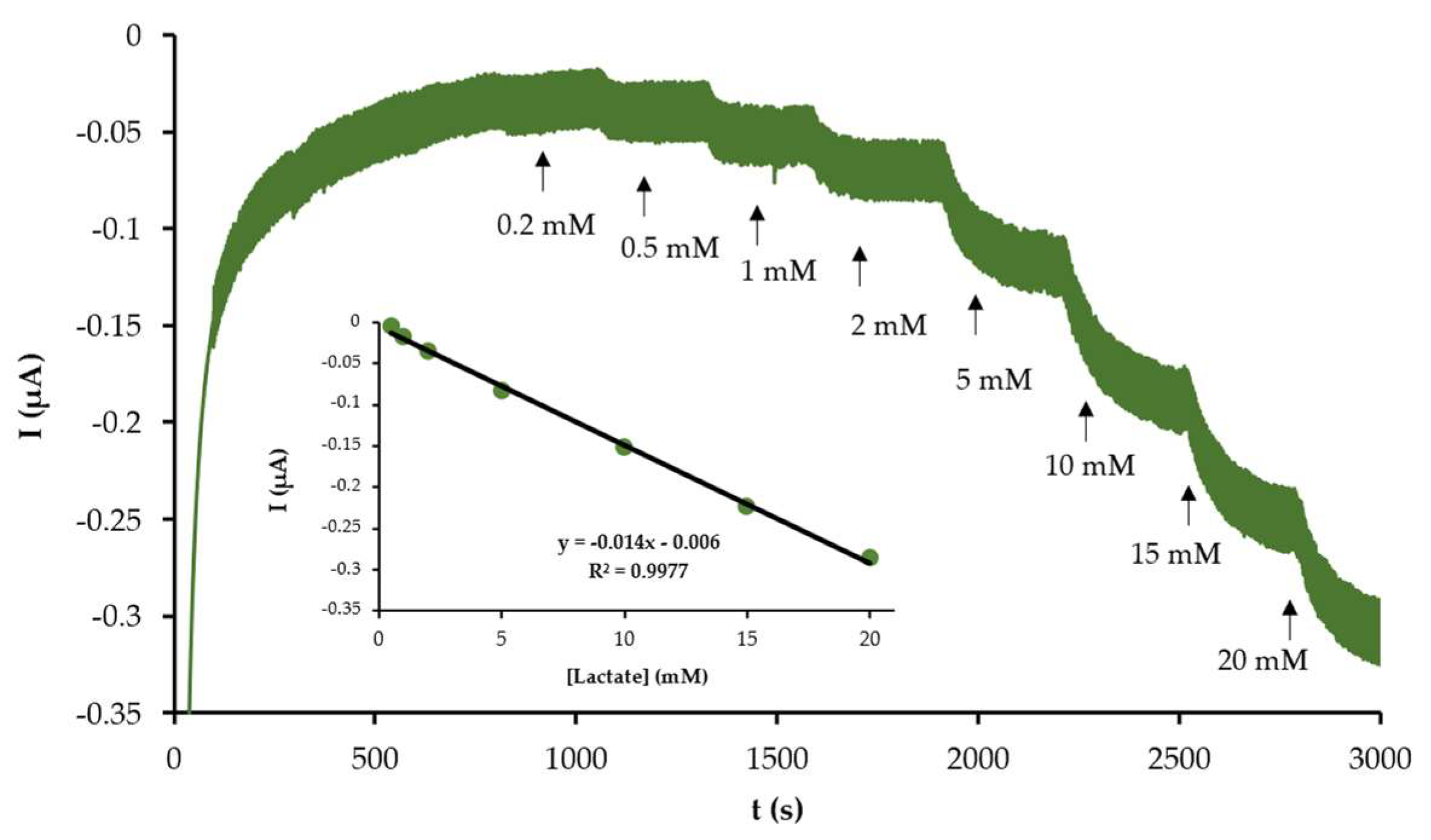
3.1. Assert the Electroanalytical Performance of the Biosensor
3.2. Applicability of the Biosensor in the Monitoring of Lactate
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rattu, G.; Khansili, N.; Maurya, V.K.; Krishna, P.M. Lactate detection sensors for food, clinical and biological applications: A review. Environ. Chem. Lett. 2021, 19, 1135–1152. [Google Scholar] [CrossRef]
- Nikolaus, N.; Strehlitz, B. Amperometric lactate biosensors and their application in (sports) medicine, for life quality and wellbeing. Microchim. Acta 2008, 160, 15–55. [Google Scholar] [CrossRef]
- Liu, M.; Yang, M.; Wang, M.; Wang, H.; Cheng, J. A Flexible Dual-Analyte Electrochemical Biosensor for Salivary Glucose and Lactate Detection. Biosensors 2022, 12, 210. [Google Scholar] [CrossRef] [PubMed]
- García-Guzmán, J.J.; Sierra-Padilla, A.; Palacios-Santander, J.M.; Fernández-Alba, J.J.; Macías, C.G.; Cubillana-Aguilera, L. What Is Left for Real-Life Lactate Monitoring? Current Advances in Electrochemical Lactate (Bio)Sensors for Agrifood and Biomedical Applications. Biosensors 2022, 12, 919. [Google Scholar] [CrossRef] [PubMed]
- Rathee, K.; Dhull, V.; Dhull, R.; Singh, S. Biosensors based on electrochemical lactate detection: A comprehensive review. Biochem. Biophys. Rep. 2016, 5, 35–54. [Google Scholar] [CrossRef] [PubMed]
- Alimi, O.A.; Meijboom, R. Current and future trends of additive manufacturing for chemistry applications: A review. J. Mater. Sci. 2021, 56, 16824–16850. [Google Scholar] [CrossRef] [PubMed]
- Cordero-rando, M.M.; De Cisneros, L.H.; Blanco, E.; Naranjo-Rodríguez, I. The Sonogel-Carbon Electrode As a Sol—Gel Graphite-Based Electrode. Anal. Chem. 2002, 74, 2423–2427. [Google Scholar] [CrossRef] [PubMed]
- Cubillana-Aguilera, L.M.; Franco-Romano, M.; Gil, M.L.A.; Naranjo-Rodríguez, I.; De Cisneros, J.L.H.-H.; Palacios-Santander, J.M. New, fast and green procedure for the synthesis of gold nanoparticles based on sonocatalysis. Ultrason. Sonochem. 2011, 18, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Baronas, R.; Ivanauskas, F.; Kulys, J. Modelling dynamics of amperometric biosensors in batch and flow injection analysis. J. Math. Chem. 2002, 32, 225–237. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sierra-Padilla, A.; García-Guzmán, J.J.; Blanco-Díaz, L.; Bellido-Milla, D.; Palacios-Santander, J.M.; Cubillana-Aguilera, L. Innovative Multipolymer-Based Electrochemical Biosensor Built on a Sonogel–Carbon Electrode Aiming for Continuous and Real-Time Lactate Determination in Physiological Samples: A New Scenario to Exploit Additive Printing. Eng. Proc. 2023, 48, 48. https://doi.org/10.3390/CSAC2023-14902
Sierra-Padilla A, García-Guzmán JJ, Blanco-Díaz L, Bellido-Milla D, Palacios-Santander JM, Cubillana-Aguilera L. Innovative Multipolymer-Based Electrochemical Biosensor Built on a Sonogel–Carbon Electrode Aiming for Continuous and Real-Time Lactate Determination in Physiological Samples: A New Scenario to Exploit Additive Printing. Engineering Proceedings. 2023; 48(1):48. https://doi.org/10.3390/CSAC2023-14902
Chicago/Turabian StyleSierra-Padilla, Alfonso, Juan José García-Guzmán, Lorena Blanco-Díaz, Dolores Bellido-Milla, José María Palacios-Santander, and Laura Cubillana-Aguilera. 2023. "Innovative Multipolymer-Based Electrochemical Biosensor Built on a Sonogel–Carbon Electrode Aiming for Continuous and Real-Time Lactate Determination in Physiological Samples: A New Scenario to Exploit Additive Printing" Engineering Proceedings 48, no. 1: 48. https://doi.org/10.3390/CSAC2023-14902
APA StyleSierra-Padilla, A., García-Guzmán, J. J., Blanco-Díaz, L., Bellido-Milla, D., Palacios-Santander, J. M., & Cubillana-Aguilera, L. (2023). Innovative Multipolymer-Based Electrochemical Biosensor Built on a Sonogel–Carbon Electrode Aiming for Continuous and Real-Time Lactate Determination in Physiological Samples: A New Scenario to Exploit Additive Printing. Engineering Proceedings, 48(1), 48. https://doi.org/10.3390/CSAC2023-14902








