Enzyme Inhibition and Antibiotics Properties of Six (6) Weeks Stable Chrysophyllum albidum Leaf Silver Nano-Particles †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and Preparation
2.2. Synthesis of Silver Nanoparticles
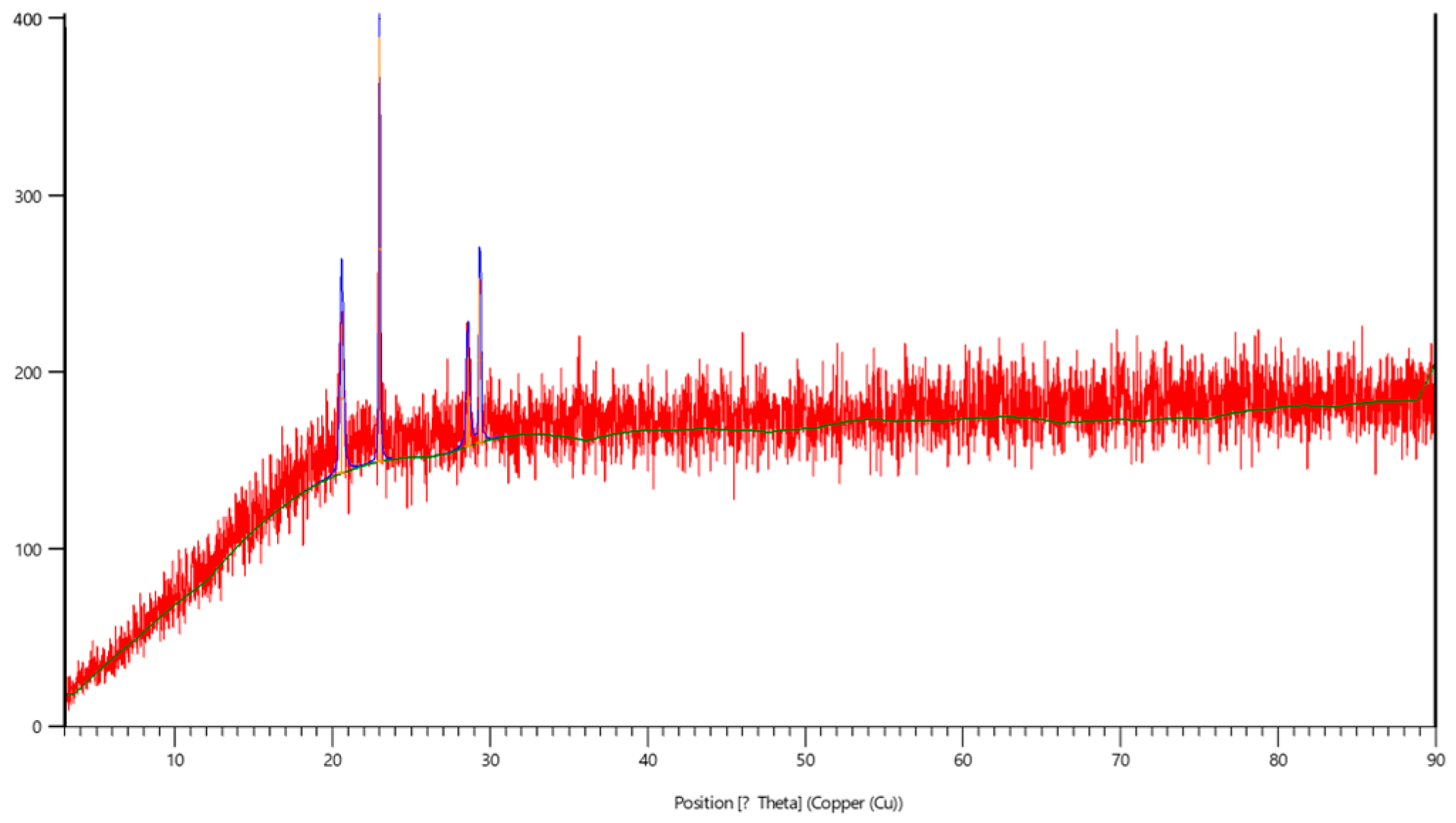
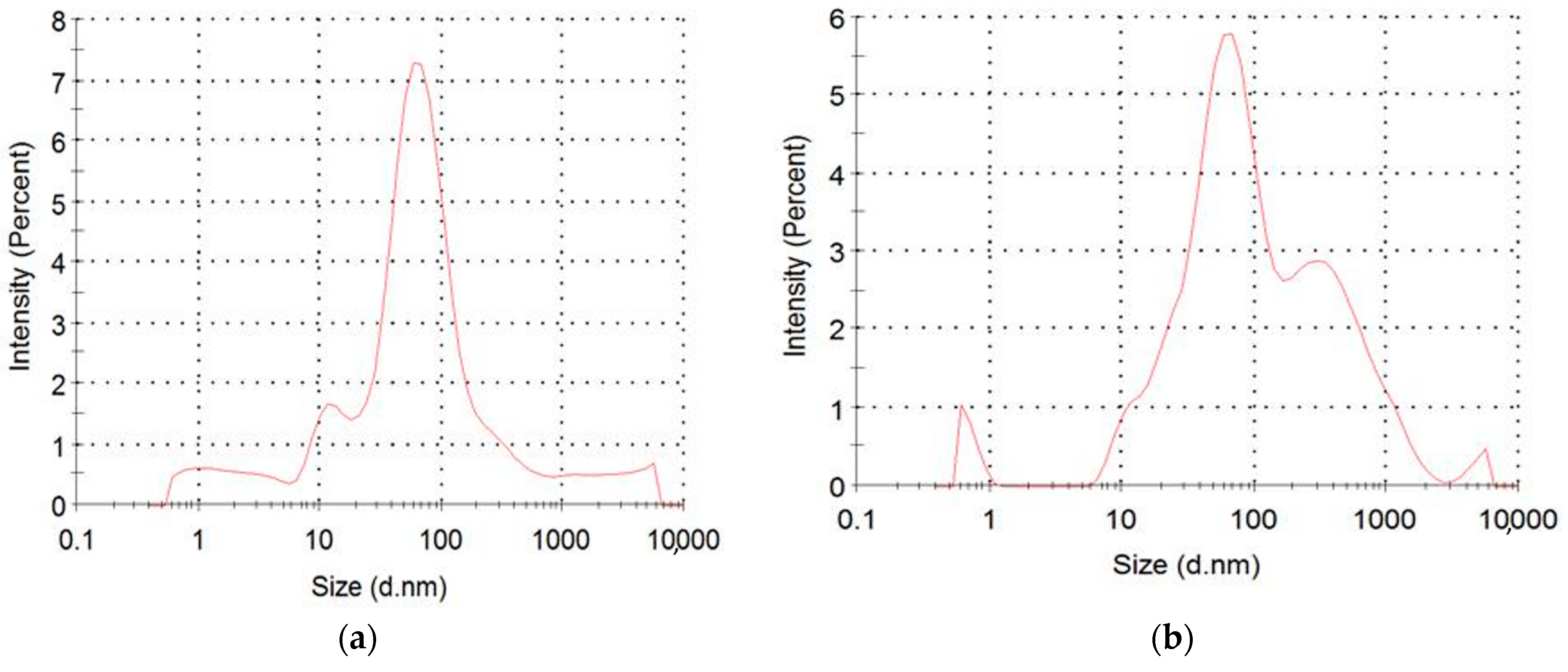
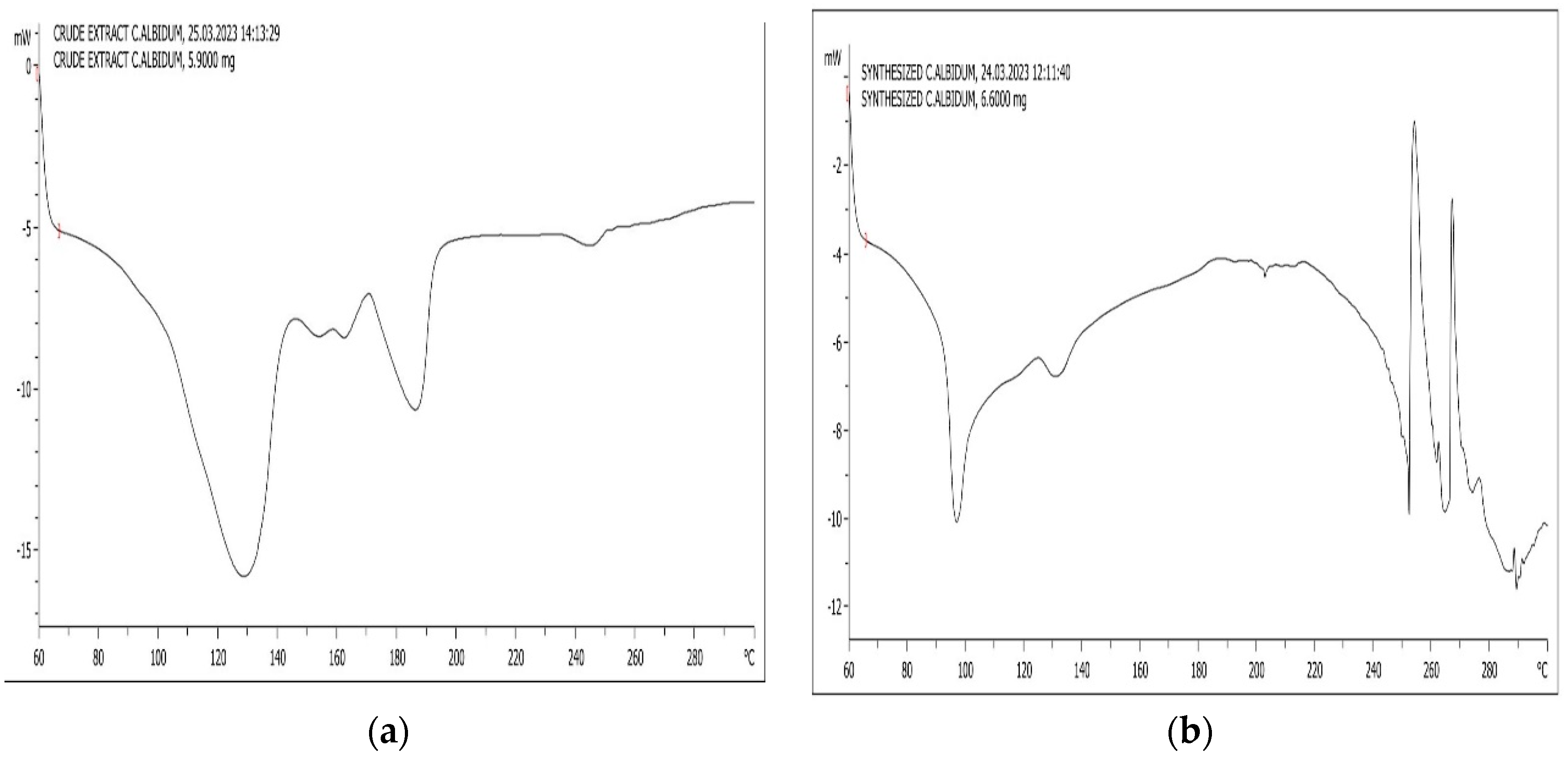
2.3. Characterization of Nanoparticles
2.4. In Vitro Antioxidant Activity
2.4.1. 1,1-Diphenyl-2-picryl Hydrazyl (DPPH) Scavenging Potential
2.4.2. Determination of Hydroxyl Radical Scavenging Activity
2.4.3. Nitric Oxide Radical Scavenging
2.4.4. Ferric Cyanide (Fe3+) Reducing Antioxidant Power Assay
2.4.5. Acetylcholinesterase and Butrylcholinesterase Inhibitory Activity Assay
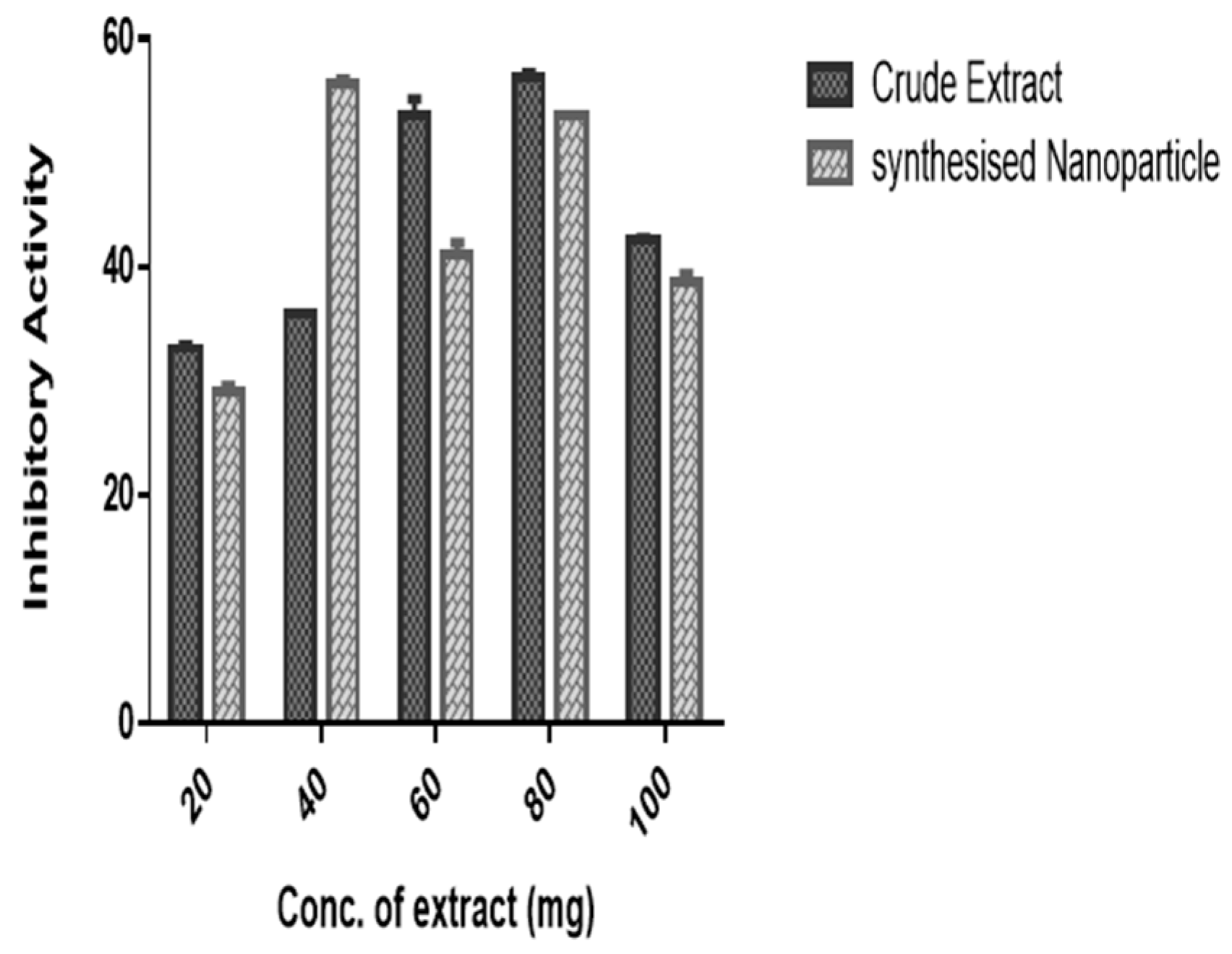
2.4.6. α-Amylase and α-Glucosidase Inhibition Assay
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vishwasrao, C.; Momin, B.; Ananthanarayan, L. Green synthesis of silver nanoparticles using sapota fruit waste and evaluation of their antimicrobial activity. Waste Biomass Valor. 2019, 10, 2353–2363. [Google Scholar] [CrossRef]
- Bharathi, D.; Josebin, M.D.; Vasantharaj, S.; Bhuvaneshwari, V. Biosynthesis of silver nanoparticles using stem bark extracts of Diospyros montana and their antioxidant and antibacterial activities. J. Nanostruct. Chem. 2018, 8, 83–92. [Google Scholar] [CrossRef]
- Nayagam, V.; Gabriel, M.; Palanisamy, K. Green synthesis of silver nanoparticles mediated by Coccinia grandis and Phyllanthus emblica: A comparative comprehension. Appl. Nanosci. 2018, 8, 205–219. [Google Scholar] [CrossRef]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Ezema, B.O.; Omeje, K.O.; Ozioko, J.N.; Urama, D.C.; Omeje, H.C.; Nnawelezi, A.; Ejim, A. Cholinesterase inhibition, biological activity, and characterization of Chrysophyllum albidum leaf and stem-bark chloroform extract using GC-MS: An in vitro study. Trop. J. Nat. Prod. Res. 2021, 5, 130–134. [Google Scholar] [CrossRef]
- Ajayi, A.M.; Chidebe, E.O.; Ben-Azu, B. Chrysophyllum albidum (African star apple) fruit-supplemented diet enhances cognitive functions and attenuates lipopolysaccharide-induced memory impairment, oxidative stress, and release of proinflammatory cytokines. Nutrire 2020, 45, 20. [Google Scholar] [CrossRef]
- Droepenu, E.K.; Jackson, N.A.; Kyene, M.O.; Ayertey, F.; Brew-Daniels, H.; Mensah, T.; Adomako, C.; Nartey, A.P.; Gyampoh, A.O.; Boamah, L.A. Synthesis and characterization of Chrysophyllum albidum (African Star Apple) plant-mediated Zinc oxide nanostructures and evaluation of their antioxidant, anti-inflammatory, and antimicrobial activity. J. Nanomater. 2022, 5508224, 14. [Google Scholar] [CrossRef]
- Adebayo, A.H.; Abolaji, A.O.; Kela, R.; Ayepola, O.O.; Olorunfemi, T.B.; Taiwo, O.S. Antioxidant activities of the leaves of Chrysophyllum albidum G. Pak. J. Pharm. Sci. 2011, 24, 545–551. [Google Scholar]
- Puja, A.M.; Rupa, E.J.; Kim, Y.J.; Yang, D.-C. Medicinal Plant Enriched Metal Nanoparticles and Nanoemulsion for Inflammation Treatment: A Narrative Review on Current Status and Future Perspective. Immuno 2023, 3, 182–194. [Google Scholar] [CrossRef]
- Osibe, D.A.; Chiejina, N.V.; Ogawaa, K.; Aoyagi, H. Stable antibacterial silver nanoparticles produced with seed-derived callus extract of Catharanthus roseus. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1266–1273. [Google Scholar] [CrossRef]
- Shen, X.; Chen, W.; Zheng, Y.; Lei, X.; Tang, M.; Wang, H.; Song, F. Chemical composition, antibacterial and antioxidant activities of hydrosols from different parts of Areca catechu L. and Cocos nucifera L. Ind. Crops Prod. 2017, 96, 110–119. [Google Scholar] [CrossRef]
- Jin, H.; Cia, M.; Li, Y.; Zhou, J. 1,10-phenanthroline-Fe2+ oxidative assay of hydroxyl radical produced by H2O2/Fe. Prog. Biochem. Biophys. 1996, 23, 553–555. [Google Scholar]
- Sangameswaran, B.B.; Balakrishnan, B.; Deshraj, B.R.; Jayakar, C. In vitro antioxidant activity of roots of Thespesia lampas Dalz and Gibs. Pak. J. Pharm. Sci. 2009, 22, 368–372. [Google Scholar] [PubMed]
- Oyaizu, M. Studies on products of browning reaction. Antioxidative activities of products of browning reaction prepared from glucosamine. Jpn. J. Nutr. Dietet. 1986, 44, 307–315. [Google Scholar] [CrossRef]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of AchE activity. Biochem. Pharmacol. 1961, 7, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Gulati, V.; Harding, I.H.; Palombo, E.A. Enzyme inhibitory and antioxidant activities of traditional medicinal plants: Potential application in the management of hyperglycemia. BMC Complement. Altern. Med. 2012, 12, 77. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Yu, Z.; Zhu, H.; Zhang, W.; Chen, Y. In vitro α-glucosidase inhibitory activity of isolated fractions from water extract of Qingzhuan dark tea. BMC Complement. Altern. Med. 2016, 16, 378. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, J.; Kim, S.-K.; Shim, M.S. Antimicrobial, antioxidant, and anticancer activities of biosynthesized silver nanoparticles using marine algae Ecklonia cava. Nanomaterials 2016, 6, 235. [Google Scholar] [CrossRef]
- Srikar, S.K.; Giri, D.D.; Pal, D.B.; Mishra, P.K.; Upadhyay, S.N. Green synthesis of silver nanoparticles: A review. Green Sustain. Chem. 2016, 6, 34. [Google Scholar] [CrossRef]
- González-Pedroza, M.G.; Benítez, A.R.T.; Navarro-Marchal, S.A.; Martínez-Martínez, E.; Juan, A.; Marchal, J.A.; Boulaiz, H.; Morales-Luckie, R.A. Biogeneration of silver nanoparticles from Cuphea procumbens for biomedical and environmental applications. Sci. Rep. 2023, 13, 790. [Google Scholar] [CrossRef]
- Omeje, K.O.; Magbo, C.; Ossai, E.C.; Ozioko, J.N.; Ezema, B.O.; Nnolim, N.E.; Eze, S.O.O. Immobilization of fungal peroxidase on paramagnetic nanoparticles for synthetic dye decolorization. Mater. Proc. 2022, 9, 24. [Google Scholar] [CrossRef]
- Tavan, M.; Hanachi, P.; Mirjalili, M.H.; Dashtbani-Roozbehani, A. Comparative assessment of the biological activity of the green synthesized silver nanoparticles and aqueous leaf extract of Perilla frutescens (L.). Sci. Rep. 2023, 13, 6391. [Google Scholar] [CrossRef] [PubMed]
- Omeje, K.O.; Ezema, B.O.; Onaebi, C.N.; Onoyima, S.C.; Ezeorba, T.P.C.; Eze, S.O.O. HPLC fngerprint of favonoids, enzyme inhibition and antioxidant activity of Newbouldia laevis stem-bark: An in vitro and in silico study. Fut. J. Pharma. Sci. 2023, 9, 36–52. [Google Scholar] [CrossRef]
- Shah, M.Z.; Guan, Z.H.; Din, A.U.; Ali, A.; Rehman, A.U.; Jan, K.; Faisal, S.; Saud, S.; Adnan, M.; Wahid, F.; et al. Synthesis of silver nanoparticles using Plantago lanceolata extract and assessing their antibacterial and antioxidant activities. Sci. Rep. 2021, 11, 20754. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Kumar, V.; Rana, M.; Kumar, D. Enzymes inhibitors from plants: An alternate approach to treat diabetes. Pharmacogn. Commun. 2012, 2, 18–33. [Google Scholar] [CrossRef]
- Deng, L.; Qi, Y.; Liu, Z.; Xi, Y.; Xue, W. Effect of tannic acid on blood components and functions. Colloids Surf. B Biointerfaces 2019, 184, 110505. [Google Scholar] [CrossRef]
- Patel, K.; Gadewar, M.; Tripathi, R.; Prasad, S.K.; Patel, D.K. A review on medicinal importance, pharmacological activity and bioanalytical aspects of beta-carboline alkaloid Harmine. Asian Pac. J. Trop. Biomed. 2012, 2, 660. [Google Scholar] [CrossRef]
- Srivastava, J.; Kumar, S.; Vankar, P.S. Correlation of antioxidant activity and phytochemical profile in native plants. Nutr. Food Sci. 2012, 42, 71–79. [Google Scholar] [CrossRef]




| Conc. (mg) | Klebsiella pneumoniae | Escherichia coli | Staphylococcus aureus |
|---|---|---|---|
| 6.5 | 0.83 | - | 1.09 |
| 12.5 | 1.21 | 3.06 | 3.27 |
| 25 | 6.92 | 19.41 | 21.75 |
| 50 | 9.88 | 11.73 | 19.03 |
| Streptomycin | 27.97 | 37.30 | 38.58 |
| Antioxidant Potentials | 20 mg/mL | 40 mg/mL | 60 mg/mL | 80 mg/mL | 100 mg/mL | Vit. C mg/mL) |
|---|---|---|---|---|---|---|
| DPPH (%) | 20.11 * a ± 0.01 | 30.10 * b ± 1.00 | 42.71 * c ± 1.99 | 42.06 * c ± 2.60 | 39.88 * c ± 0.10 | 73.20 * ± 0.93 |
| OH* Scavenging ability (%) | 9.84 * a ± 0.20 | 22.97 * b ± 0.09 | 55.83 * c ± 0.19 | 50.91 * c ± 0.26 | 46.15 * d ± 0.21 | 81.53 * ± 0.86 |
| NO* Scavenging ability (%) | 19.93 * a ± 1.30 | 27.08 * b ± 0.77 | 48.22 * c ± 1.57 | 47.80 * c ± 0.90 | 43.83 * c ± 0.11 | 86.82 * ± 0.41 |
| FRAP (%) | 22.84 * a ± 1.49 | 35.20 * b ± 1.07 | 50.48 * c ± 0.32 | 39.10 * b ± 0.19 | 47.94 * d ± 0.50 | 78.90 * ± 0.88 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omeje, K.O.; Nworah, F.N.; Ezema, B.O.; Eze, S.O.O. Enzyme Inhibition and Antibiotics Properties of Six (6) Weeks Stable Chrysophyllum albidum Leaf Silver Nano-Particles. Eng. Proc. 2023, 56, 196. https://doi.org/10.3390/ASEC2023-16578
Omeje KO, Nworah FN, Ezema BO, Eze SOO. Enzyme Inhibition and Antibiotics Properties of Six (6) Weeks Stable Chrysophyllum albidum Leaf Silver Nano-Particles. Engineering Proceedings. 2023; 56(1):196. https://doi.org/10.3390/ASEC2023-16578
Chicago/Turabian StyleOmeje, Kingsley Ozioma, Florence N. Nworah, Benjamin O. Ezema, and Sabinus O. O. Eze. 2023. "Enzyme Inhibition and Antibiotics Properties of Six (6) Weeks Stable Chrysophyllum albidum Leaf Silver Nano-Particles" Engineering Proceedings 56, no. 1: 196. https://doi.org/10.3390/ASEC2023-16578
APA StyleOmeje, K. O., Nworah, F. N., Ezema, B. O., & Eze, S. O. O. (2023). Enzyme Inhibition and Antibiotics Properties of Six (6) Weeks Stable Chrysophyllum albidum Leaf Silver Nano-Particles. Engineering Proceedings, 56(1), 196. https://doi.org/10.3390/ASEC2023-16578






