Abstract
In this study, a comparative study was conducted on the two reactor types (the plug-flow and continuous stirred tank reactor) employed for the traditional esterification process to investigate their potential applications to the esterification reaction with the ethanol-rich feed. Aspen Plus software was used to conduct a sensitivity analysis on the temperature profiles in the axial and radial directions, focusing in particular on the reactor and feed stream temperatures, operating parameters, and ethyl acetate yields for the reactors. The energy analysis for esterification processes with the different reactor types has also been evaluated. Compared with the continuous stirred tank reactor, the plug-flow reactor process with the ethanol-rich feed exhibited reduced hotspot temperatures. The simulation results show that the hotspot temperatures in the continuous stirred tank reactor can be within the operating temperature range of 90–100 °C. Regarding the comparison of these reactor types for the esterification process, the plug-flow reactor shows advantages in terms of efficient hotspot temperature with the operating temperature range of 70–75 °C. On the other hand, the yield of ethyl acetate product from the continuous stirred tank reactor is slightly higher than from the alternative esterification process with excess ethanol feed.
1. Introduction
Chemical reactors are the main devices used in chemical engineering to carry out various chemical reactions. They provide a controlled environment for the reaction to occur, allowing for optimal conditions such as temperature, pressure, and mixing. For esterification reactions, which involve the formation of esters from the reaction between an alcohol and an acid, different types of chemical reactors can be used depending on the specific requirements of the reaction. In this study, an esterification reaction for obtaining ethyl acetate (EtAc) was chosen as an object of the research. EtAc can undergo various reactions depending on the conditions and reactants involved. Due to the various applications for the produced esters, esterification is one of the most attractive reactions in industries. For esterification reactions, which involve the formation of esters from the reaction between an alcohol and an acid, different types of chemical reactors, such as batch reactors [1], continuous stirred tank reactors (CSTR) [2], packed bed reactors [3], plug-flow reactors (PFR), fixed bed reactors [1,4], microwave reactors [5,6] and membrane reactors [7], can be used depending on the specific requirements of the reaction. However, there are lack of comparative techno-economic data for simulated PFR and CSTR processes.
The current work aims to simulate and compare an industrial scale of different reactors for the formation of EtAc from the esterification reaction between ethanol and acetic acid. First, reaction kinetics was chosen from references [8]. Then, PFR and CSTR were simulated using Aspen Plus 12.0 software. Later, both of reactors were optimized using sensitivity analysis. Finally, the last results of reactors were compared by technical and economical methods.
2. Background
In esterification reaction, EtAc is formed in an exothermic, reversible, and highly selective reaction between acetic acid and ethanol in liquid phase, catalyzed by an ion exchange resin [8,9]. The overall scheme of conversion in the synthesis of EtAc and kinetic factors can be represented as [8]:
where Equation (1) represents the etherification reaction, and Equations (2) and (3) represent the dependence of the activity-based equilibrium constant with temperature. The UNIFAC estimates of activity coefficients were used to describe the liquid-phase nonideality.
In order to reduce the number of experiments, many scientists and engineers nowadays are creating models of physical, chemical, and biological systems using process simulators. Process models are essential for assessing performance, studying system behavior, and determining the effects of different operational parameters. CSTR and PFR comparison models are generated using simulation software, although Aspen Plus is also frequently used by researchers in the modeling of the various esterification processes. Aspen Plus is an equation-oriented program that uses a database of phase equilibrium and mass-energy balance to run in order to examine the effects of various process factors. It may be used to mimic the esterification processes and is a crucial piece of software for designing chemical processes.
3. Methodology and Description of Alternative Reactors for the Esterification Process
In this work, a comparative study between the CSTR and PFR processes in EtAc production is presented by means of process simulation in Aspen Plus. The reaction parameters reported by Calvar et al. [8] have been adopted as reference case. Based on reference work, experimental information on the process parameters of esterification reactions was used to simulate reactors. After validation, the material balance and the energy consumptions of the different alternatives were evaluated. In the initial case of alternatives, the feed stream content for both of alternatives have the same properties with following parameters (Table 1):

Table 1.
Initial feed stream characteristics.
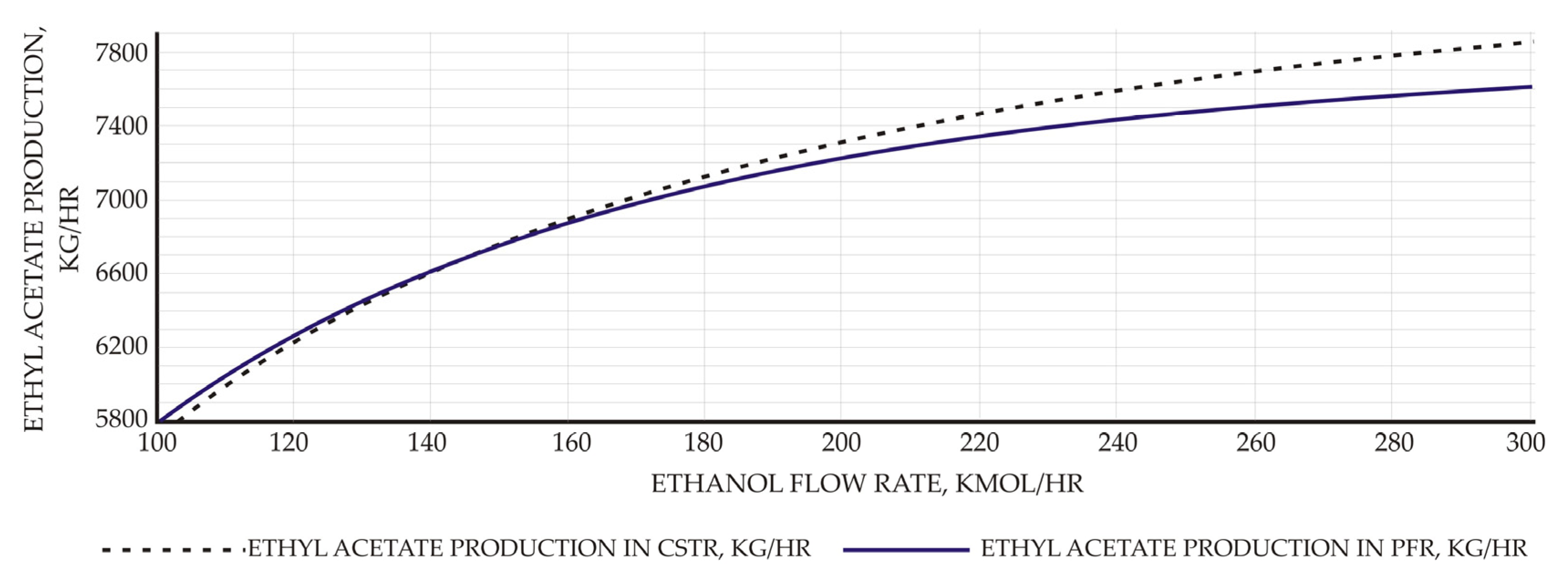
Both ACID and ETHANOL feed streams are in normal ambient conditions (1 atm, 25 °C). The analysis of ethanol to acetic acid molar flow rate to ethyl acetate yield has also been studied using sensitivity analysis. According to the results, the productivity of EtAc increases in proportion to the flow rate of ethanol, and this value is higher in the CSTR process than in the PFR process (Figure 1).
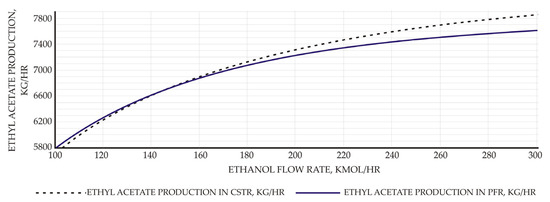
Figure 1.
Effect of ethanol molar flow rate to EtAc productivity.
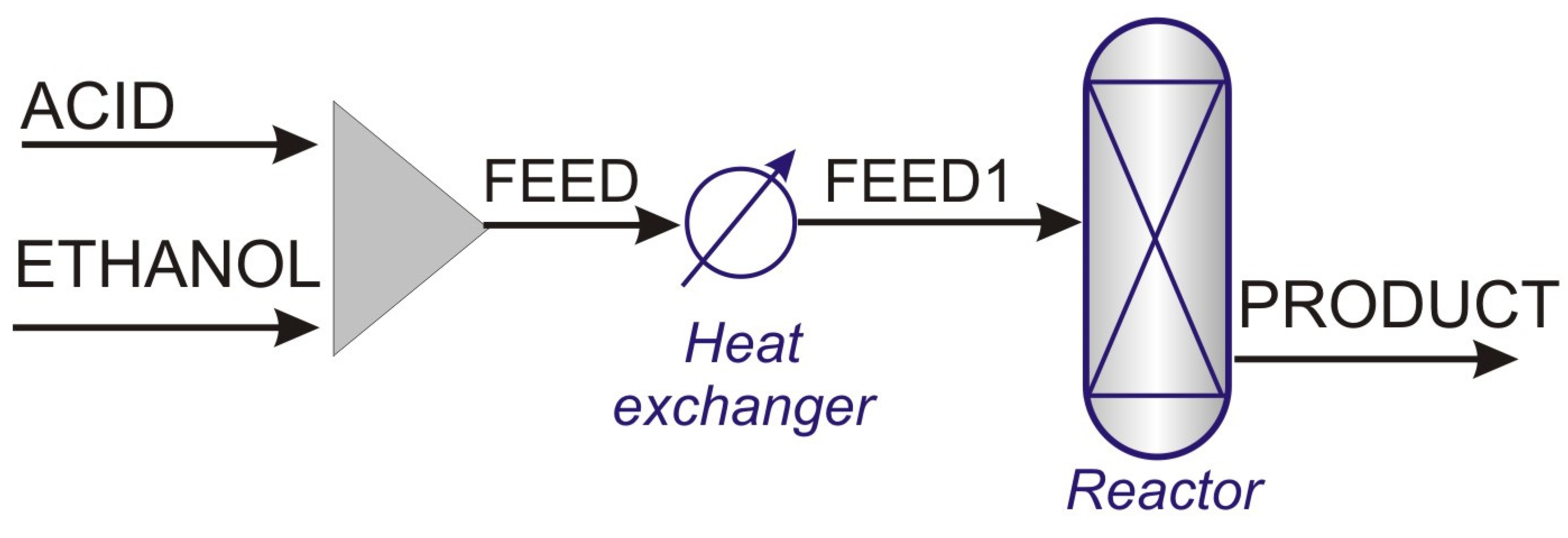
A typical configuration of an esterification process is shown in Figure 2. In a PFR process, due to the exothermic characteristics of the EtAc-forming reaction, the use of a multi-tubular reactor is preferred, which allows the removal of part of the heat generated. This type of reactor consists of a set of small diameter tubes filled with a catalyst, arranged in a housing through which cooling water is circulated [10,11].
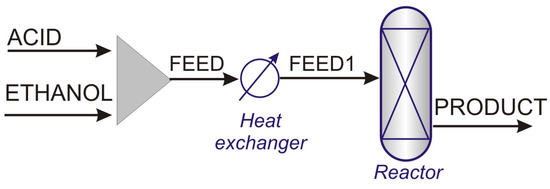
Figure 2.
Typical PFR process for ethyl acetate production.
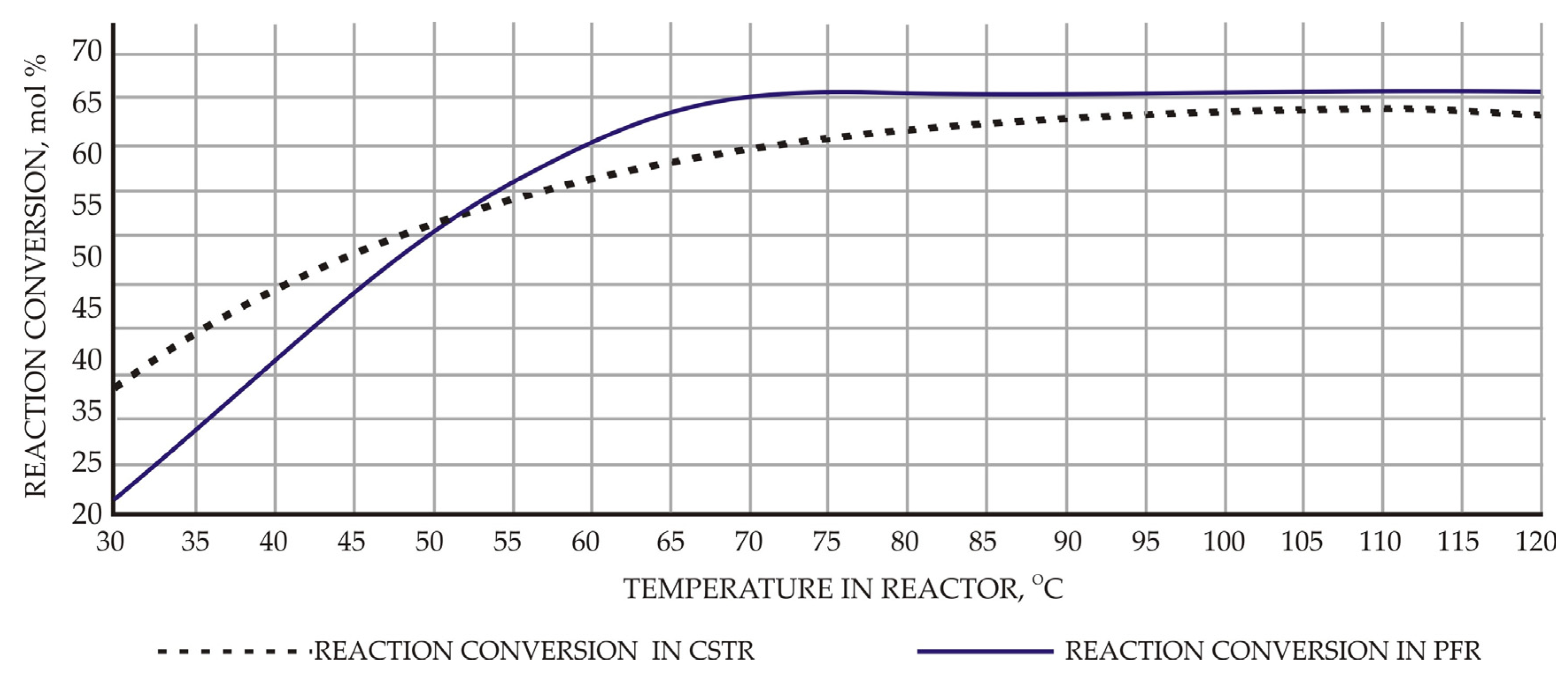
Experiments were conducted under a fixed feed rate of 100 mol/h for both acid and alcohol feeds. The effects of temperature on conversion for PFR and CSTR are shown in Figure 3. Maximum conversion of 65.9% was obtained at a temperature of about 70 °C for PFR when the volume of reactor was adjusted to 7.9 m3 and 110 °C for CSTR when the volume of reactor was adjusted to 7.9 m3 and residence time to 36 min. In general, reaction conversion decreases as ethanol feed rate is increased for both types of reactors. With increased feed rate, residence time decreases and this leads to decreased conversion values. For PFR with reactor volume of 7.9 m3, reaction conversion varies from 65.9% to 28.8% as ethanol feed rate varies from 100 mol/h to 300 mol/h, respectively. In the case of CSTR with an adjusted reactor volume of 7.9 m3, reaction conversion varies from 63.6% to 28.9% corresponding to ethanol flow rate values from 100 mol/h to 300 mol/h, respectively. Overall, PFR performed better under constant operating conditions compared with CSTR.
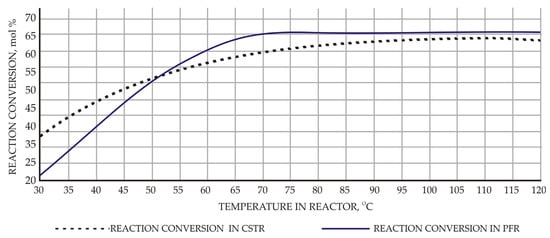
Figure 3.
Effect of reactor temperature on the reaction conversion.
Reaction conversion increases as temperature rises in the both cases under studied reaction kinetics and temperature range. Maximum conversion of 65.9% at 70–75 °C was obtained for PFR compared with reaction conversion of 63.6% at 110 °C for CSTR as temperature varies.
4. Conclusions
A comparative model was developed for the production of EtAc in atmospheric plug-flow and continuous stirred tank reactors using the Aspen Plus simulator. To provide the model, RPLUG and RCSTR operation blocks were used and, where necessary, kinetic expressions and hydrodynamic models were developed using data and models from the literature. The model was used to predict the results of esterification of ethanol with acetic acid. A reaction conversion of 65.9% and negative heat duty about 0.44 Gcal/h were obtained for PFR compared with a slightly lower value of 63.6% and 1.77 Gcal/h for CSTR under steady-state conditions. CSTR process required 110 °C for maximum conversion, whereas this value was approximately 70–75 °C in the PFR process. Maximum conversion of 65.9% was obtained for multi-tubular PFR compared with reaction conversion of 63.6% for CSTR as the temperature varies. For PFR and CSTR with reactor volume of 7.9 m3, corresponding to flow rates of 100 mol/h to 300 mol/h, reaction conversion varies from 65.9% to 28.8% and 63.6% to 28.9%, respectively. In both types of reactors, increasing reactant flow rate results in a reduction in residence time, which lowers the values of reaction conversion.
Author Contributions
Conceptualization, A.N. and A.B.; writing—original draft preparation, A.N. and A.B.; visualization, A.E., A.N. and A.B.; writing—review and editing, O.M. and A.B.; supervision, A.N.; A.B. and A.E. contributed equally to this paper. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the Agency of Innovative Development under the Ministry of Higher Education, Science, and Innovation of the Republic of Uzbekistan (contract no. 54, 65).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ni, J.; Meunier, F. Esterification of free fatty acids in sunflower oil over solid acid catalysts using batch and fixed bed-reactors. Appl. Catal. A Gen. 2007, 333, 122–130. [Google Scholar] [CrossRef]
- Tang, J.; Chen, G.; Wang, L.; Miao, M.; Jiang, B.; Feng, B. Immobilization of Y. lipolytica lipase and the continuous synthesis of geranyl propionate. J. Mol. Catal. B Enzym. 2016, 133, 311–316. [Google Scholar] [CrossRef]
- Rade, L.L.; Lemos, C.O.T.; de Souza Barrozo, M.A.; Ribas, R.M.; de Souza Monteiro, R.; Hori, C.E. Optimization of esterification reaction over niobium phosphate in a packed bed tubular reactor. Renew. Energy 2019, 131, 348–355. [Google Scholar] [CrossRef]
- Son, S.M.; Kimura, H.; Kusakabe, K. Esterification of oleic acid in a three-phase, fixed-bed reactor packed with a cation exchange resin catalyst. Bioresour. Technol. 2011, 102, 2130–2132. [Google Scholar] [CrossRef] [PubMed]
- Umrigar, V.R.; Chakraborty, M.; Parikh, P.A.; Kohli, H.P. Optimization of process parameters for oleic acid esterification using microwave reactor: Catalytic activity, product distribution and reactor energy model. Energy Nexus 2022, 7, 100127. [Google Scholar] [CrossRef]
- Baraka, F.; Robles, E.; Labidi, J. Microwave-assisted esterification of bleached and unbleached cellulose nanofibers. Ind. Crops Prod. 2023, 191 Pt A, 115970. [Google Scholar] [CrossRef]
- Ghahremani, M.; Ghasemzadeh, K.; Jalilnejad, E.; Basile, A.; Iulianelli, A. Vapor phase esterification of acetic acid with ethanol in a CHA zeolite membrane reactor: A CFD analysis. Chem. Eng. Sci. 2021, 236, 116536. [Google Scholar] [CrossRef]
- Calvar, N.; González, B.; Dominguez, A. Esterification of acetic acid with ethanol: Reaction kinetics and operation in a packed bed reactive distillation column. Chem. Eng. Process. Process Intensif. 2007, 46, 1317–1323. [Google Scholar] [CrossRef]
- Van de Steene, E.; Jeriffa, D.C.; Joris, W.T. Ion-exchange resin catalyzed transesterification of ethyl acetate with methanol: Gel versus macroporous resins. Chem. Eng. J. 2014, 242, 170–179. [Google Scholar] [CrossRef]
- Miracca, I.; Tagliabue, L.; Trotta, R. Multitubular reactors for etherifications. Chem. Eng. Sci. 1996, 51, 2349–2358. [Google Scholar] [CrossRef]
- Rusydi, F.; Aisyah, N.D.; Fadilla, R.N.; Dipojono, H.K.; Faozan Ahmad, M.; Puspitasari, I.; Rusydi, A. The transition state conformational effect on the activation energy of ethyl acetate neutral hydrolysis. Heliyon 2019, 5, e02409. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).