Abstract
Electrochemical studies of the drug and micellar aggregates of surfactants have gained interest in recent years. The aggregation of micelles aims to mimic the structures of biological membranes. It also helps to regulate the pharmacokinetic properties of medicines as they offer a path for formulations with controlled release abilities. Propranolol (PPL) is a beta blocker drug which is used as a medication in treatments for hypertension, cardiac arrhythmias, and atrial fibrillation, and it is also used to prevent migraines. The electro oxidation of Propranolol was observed using a glassy carbon electrode in cationic surfactants and ionic liquid surfactants with the same chain length using cyclic voltammetry. A well-defined single irreversible peak was found in the potential range of 0.6 to 1.6 V at room temperature. In this paper, Propranolol, in the absence and presence of both the surfactants, is discussed in terms of the pre- and postmicellar phases. The scan rate and effects of both concentrations were evaluated in the presence and absence of surfactants in biphasic surfactant conditions. Diffusion-controlled and irreversible processes involving an adsorption effect were observed in both the surfactants. The interactions of PPL in the presence of different cationic micelles provide an effective approach for estimating the stability of radicals in biological mimetic systems.
1. Introduction
The electrochemical investigation of drug–surfactants has garnered significant attention among researchers for over five decades. Surfactants, known as surface-active agents [1], possess the unique ability to adsorb onto surfaces, thereby influencing surface and interfacial phenomena. This characteristic makes them highly applicable in various fields [2]. Another characteristic of surfactants is their amphiphilic nature, which leads to their aggregation at certain concentrations, forming structures resembling membranes called micelles [3]. These self-assembled aggregations of amphiphiles find extensive applications in fields such as pharmacokinetics and those involving drug delivery systems.
Propranolol (Figure 1) is a beta blocker drug widely used to treat cardiac arrhythmias, high blood pressure, angina, and many more conditions [4]. Propranolol exhibits varying effects at different doses [5]. Previously, various analytical methods have been employed to investigate Propranolol, including electrophoresis, spectrophotometry, spectrofluorimetry, potentiometry, and atomic absorption spectrometry [6,7,8,9]. While these methods are accurate and selective, they are also complex and costly to utilize.
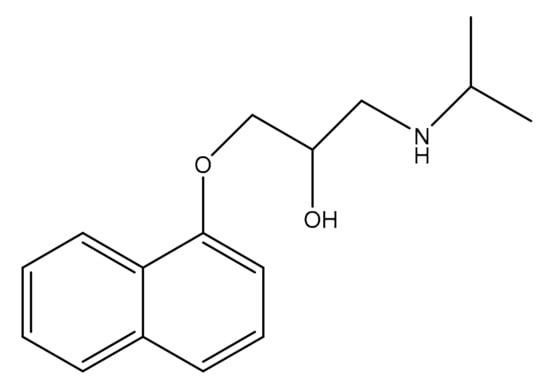
Figure 1.
Structure of Propranolol.
To overcome these limitations and achieve a more convenient, selective, and sensitive determination of Propranolol (PPL), there is a need for an electrochemical method. Electrochemical studies of Propranolol in the presence of surfactant micelles can be most sensitively and selectively conducted using cyclic voltammetry (CV). Cyclic voltammetry is preferred over other spectroscopic techniques as it is cheap, reliable, and easy to use (simple), and through using it, thermodynamics and kinetics mechanisms can also be determined [10]. Electrochemical studies involving cyclic voltammetry and the determination of Propranolol have been reported by different groups using different types of electrodes, such as carbon paste electrodes [11], gold electrodes [12], platinum nanoparticle-doped electrodes [13], and boron-doped diamond electrodes [14].
In this study, an investigation of the electroactive species Propranolol was conducted on a bare glassy carbon electrode (GCE). This study focused on investigating Propranolol in the presence and absence of surfactants. Two surfactants with the same chain length (C16) but different head groups were considered: cationic head group Cetyltrimethylammonium bromide (CTAB) and ionic liquid surfactant 1-Hexadecyl-3 methylimidazolium chloride monohydrate (HDMIC).
2. Materials and Methods/Methodology
Propranolol hydrochloride was obtained from TCI Chemicals, Tokyo, Japan. Cetyltrimethylammonium bromide (CTAB) was procured from LOBA Chemicals (Mumbai, India), and ionic liquid 1-Hexadecyl-3 methylimidiazolium chloride monohydrate (HDMIC) was obtained from Acros Organics (Brussels, Belgium). The obtained chemicals were used without any further purification.
The electrochemical studies were carried out using CH Instruments electrochemical workstation, Austin, TX, USA (Model CHI 600D). Three electrodes were used throughout the study: a glassy carbon electrode (GCE), a platinum wire, and a Ag/AgCl electrode, serving as the working electrode (3 mm), counter electrode, and reference electrode, respectively. All experiments were performed at room temperature (298.15 K).
The working electrode was washed with double-distilled water and polished using alumina powders of different sizes (measured in microns). A large-sized alumina powder (1.0 micron) was used to remove larger imperfections, and a small-sized alumina powder (0.3 and 0.05 microns) was used to smooth the electrode’s surface until mirror shining. Additionally, 1 M H2SO4 was used as a supporting electrolyte.
3. Results and Discussion
3.1. Effect of Scan Rate in the Absence and Presence of Premicellar Surfactants
The electrochemical behavior of Propranolol hydrochloride (PPL) in the absence or presence of surfactants was investigated using cyclic voltammetry on a bare glassy carbon electrode (GCE). Due to the redox activity of PPL, cyclic voltammetry was used to investigate the PPL’s interaction with the surfactants. Studying 1 mM pure PPL in the presence of 1 M H2SO4 as a supporting electrolyte, at a scan rate of 50 mVs−1, showed two oxidation peaks, with the first one being observed at 0.99 V and the other being observed at 1.19 V (shown in Figure 2a), which is in accordance with what has been observed by other groups [15]. After the second scan, one of the peaks vanished, indicating the unstable nature of one of the peaks. In a reverse scan, no peak was observed, which shows that the process is irreversible within the potential range of investigation.
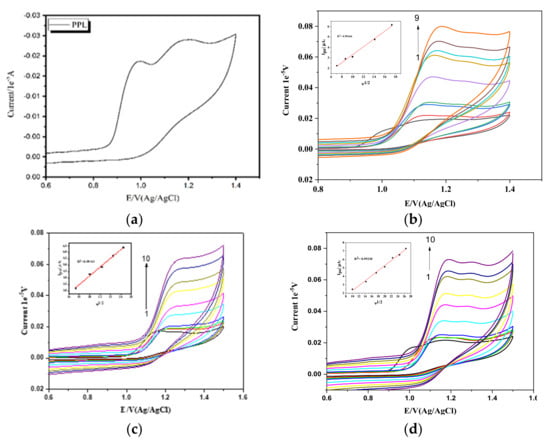
Figure 2.
(a) PPL in the presence of H2SO4. (b) Effect of scan rates in 1 × 10−3 M PPL: (1) 0.025 Vs−1, (2) 0.05 Vs−1, (3) 0.075 Vs−1, (4) 0.1 VS−1, (5) 0.2 Vs−1, (6) 0.3 Vs−1, (7) 0.4 Vs−1, (8) 0.5 Vs−1, (9) 0.6 Vs−1, and (10) 0.7 Vs−1. (b) Inset: linear plots of Ipa and v1/2. (c) PPl with 0.1 mM CTAB at the same scan rates as in (b). (d) PPL with 0.1 mM HDMIC at the same scan rates as in (b).
In cyclic voltammetry, the behavior of a drug on a glassy carbon electrode can be broadly categorized into two types: adsorption-controlled and diffusion-controlled behavior. These terms refer to the dominant factors that influence the drug’s electrochemical response during the cyclic voltammetry experiment. Adsorption-controlled behavior occurs when the drug molecules strongly adhere to the surface of the glassy carbon electrode. Diffusion-controlled behavior occurs when the rate of mass transport of the drug molecules in the solution to and from the electrode surface is the primary determinant of the observed electrochemical response. To check whether the behavior of the PPL on the electrode was adsorption controlled or diffusion controlled, experiments at different scan rates (25 mVS−1 to 700 mVs−1) using a supporting electrolyte of 1 M H2SO4 were carried out. From Figure 2b, we can see that the anodic peak increased with increasing scan rate. Also, there is a shift in the peak potential toward the higher potential range, and these shifts toward the higher potential may be due to the adsorption of a small amount of hydrophobic PPL on the Glassy carbon electrode. At higher scan rates, the increase in the peak current is due to the rate of diffusion being higher than the rate of reaction. Most of the ions and the electrolyte reached the electrode surface, whereas only a few ions participated in the charge transfer reaction.
This also may be due to the double-layer capacitance or uncompensated resistance [16]. The linear regression plot plotting the relationship between the scan rate (v) and peak current (Ipa) (figure not shown) shows that Ipa/µA = 0.01537 (mVs−1) + 1.600 with a correlation coefficient of R2 = 0.98938. Similarly, the Ipa/µA of the square rate of scan rate(v1/2) with peak current (Ipa) was found to be Ipa/µA = 0.37682 v1/2 (mVs−1) − 0.47632 with R2 = 0.99266. The relationship between the scan rate (v1/2) and the peak current (Ipa) is typically linear, showing that the system is diffusion controlled. The transfer of the electrolyte from the bulk to the electrode system is more rapid compared to the diffusion of the electrolyte into the bulk of a solution. The linear relationship of log Ipa versus log v (figure not shown) was found to be log Ipa/µA = 0.55295 log v (mVs−1) − 0.5900, with R2 = 0.99182. The slope value obtained is nearly equal or similar to the theoretical value; hence, the process is diffusion controlled [17] in the absence of surfactants.
We subsequently investigated the effect of the scan rate in the presence of premicellar surfactants. Both the 1 mM PPL and the 0.1 × 103 M CTAB were kept constant in the presence of 1 M H2SO4 (supporting electrolyte), and the scan rate, ranging from 25 mVs−1 to 700 mVs−1, was varied accordingly. The peak current drop was found to be linearly correlated with the scan rate from the following:
The correlation coefficient was found to be (R2) = 0.99181. These dependence results indicate that the process is diffusion controlled. For the PPL with a premicellar concentration, the square root of the scan rate (v) and peak current drop (Ipa) was plotted, and the linear relationship was given by the following relation:
Similarly, log v was plotted against log Ipa, and linear plot was obtained with a regression coefficient of R2 = 0.99631 was obtained and can be expressed as follows:
Likewise, 1 mM PPL in the presence of 0.1 mM HDMIC was also investigated within the same varying scan rate as in 0.1 mM CTAB. The variation in the relationship between the peak current drop (Ipa) and scan rate (v) was similar in the presence of 0.1 mM CTAB, though both the surfactants differ in terms of their structure, as well as in terms of their head group. Regarding the effect of scan rate (v) in PPL–HDMIC, a linear relationship was observed (as shown in Figure 2d): an increase in the scan rate also increases the peak current drop. The linear dependance of the peak current (Ipa) on the scan rate (v) was given by the following relation:
The regression coefficient was found to be R2 = 0.998. The behavior of PPL, in the presence of the minimum-concentration surfactant (0.1 mM), is diffusion controlled. The relationship between the peak current drop (Ipa) and the square root of the scan rate (v) can be expressed as follows:
The regression coefficient (R2) was found to be 0.99218. Analogously, in PPL–HDMIC the relationship between log (v) and log (Ipa) was examined and expressed using the following equation:
The regression coefficient was found to be (R2) = 0.99318. The slope value confirms that the process is also diffusion controlled in the presence of 0.1 mM HDMIC.
3.2. Effect of Scan Rate in Postmicellar Surfactants
The process of electron transfer is more rapid compared the diffusion of an analyte to the bulk of the solution at premicellar concentrations of CTAB as well as in HDMIC. To understand the process at postmicellar concentrations, 1 mM PPL with postmicellar (above CMC) concentrations of CTAB and HDMIC of 1 mM was used at different scan rates (v) ranging from 25 mV s−1 to 700 mV s−1. From Figure 3a, for PPL–CTAB, it can be observed that increasing the scan rate (v) enhances the peak current drop (Ipa) in the presence of CTAB. This increase is linear, as the peak current (Ipa) increases with the scan rate (v). The linearity plot plotting the relationship between the scan rate (v) and peak current (Ipa) shows that (µA) = 4.156 + 0.01026 ν (Vs−1), with R2 = 0.9868. Similarly, the linear regression equation for expressing the relationship between the scan rate (v) and peak current (Ipa) can be expressed as follows: Ipa (µA) = 1.66673 + 0.33208 ν (Vs−1), and R2 = 0.99109. The linear regression for log ν and log Ipa is given by the following: log Ipa (µA) = 0.44386 − 0.23447 log ν (Vs−1), and the regression coefficient is R2 = 0.99787. The slope from the linear plot of log (v) with potential (Ep) determines the process to be diffusion controlled.
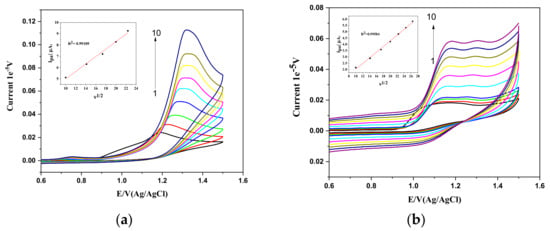
Figure 3.
Cyclic voltammograms of the effect of the scan rate in 1 mM PPl with (a) 1 mM CTAB at the same scan rates as in (a). Inset: linear plots of Ipa and ν1/2. (b) HDMIC (1 mM) at the same scan rates as in (a). Inset: linear plots of Ipa and ν1/2.
The behavior of PPL, in the presence of CTAB at pre and postmicellar concentrations is said to be diffusion controlled. A linear relationship between the scan rate (v) and the peak current (Ipa) was observed. The linear relationship between the scan rate and peak current is given by the following: Ipa (µA) = 1.62955 + 0.00619 ν1/2 (Vs−1), and R2 = 0.99717. This suggests that the PPL with HDMIC at a premicellar concentration was also controlled by diffusion. Likewise, the square of the scan rate (v) with peak current (Ipa) (shown Figure 2b inset) increases linearly and can be expressed by the following equation: Ipa (µA) = −0.243 + 0.22632 ν1/2 (Vs−1), and the regression coefficient is R2 = 0.99584. Meanwhile, a good linear relationship between the log v and log Ipa was observed, and this relation can be determined using the following: Ipa (µA) = −0.71935 + 0.51866 ν1/2 (Vs−1), and R2 = 0.99536. The obtained slope shows that the process is diffusion controlled for both pre- and postmicellar concentrations of HDMIC with PPL.
The standard rate constant values for the irreversible process were also calculated using the Laviron equation [18]:
where E0 is the formal potential, K0 is the standard rate constant, n is the number of electrons transferred. α is the charge transfer coefficient, R is the universal gas constant (8.314 Jmol−1k−1), T is the absolute temperature (298 K), and F refers to Faraday’s constant (96,487 C mol−1). The value of ‘α’ and ‘n’ was determined based on plotting Ep with v for n = 2. Likewise, the formal potential was calculated based on plotting Ep vs. v with an extrapolation of v = 0.
The calculated standard rate constant (K0) values, along with the charge transfer coefficient (α) and the value of the slope, are shown in Table 1.

Table 1.
Estimated slope, charge transfer coefficient (α), and rate constant (K0) values for the PPL−surfactant systems (values obtained from cyclic voltammograms).
Based on the observed data, the rate constant K0 of PPL in the absence of a surfactant is 4.9 s−1, whereas the value for PPL in the presence of cationic surfactants decreased to 1.196 s−1 and 2.446 s−1 for the pre and postmicellar concentrations of CTAB, respectively. Also, for the ionic liquid surfactant, the value of K0 was less than that of PPL when used in isolation. Based on these rate constant values, the rate of reaction decreases with the addition of a monomer. In the premicellar surfactant phase, in CTAB, the rate is slow compared to the postmicellar phase, and the same also likely applies in the context of HDMIC. The rate of reaction depends upon the increase in the peak current and shift in potential. In the postmicellar phase with PPL, HDMIC has higher values of K0 compared to those of CTAB, which indicates that the rate of the reaction taking place on the surface of the electrode is fast. This is the reason for the broad peak obtained in the presence of HDMIC. The rate of reaction at postmicellar surfactant concentrations is higher in HDMIC than in CTAB. The reason for this may be due to the fact that the process of micellization in HDMIC is faster than that in CTAB. The obtained values for the charge transfer coefficient are almost similar in the biphasic phase of CTAB, but in HDMIC, the increase in the postmicellar HDMIC is due to the head group size. The head group of HDMIC consists of a large imidazolium ring, where the transfer to the electrode is slow.
3.3. Effect of Concentration
3.3.1. PPL–Premicellar Concentrations of Surfactants
The electrochemical behavior of PPL in the presence of surfactants in the premicellization phase was also examined. Figure 4a,b present the interactions between 1 mM PPL and CTAB and HDMIC, respectively. Two oxidation peaks at 0.99 V and 1.19 V, respectively, can be observed with no peak at the reverse scan, which means the process is irreversible.
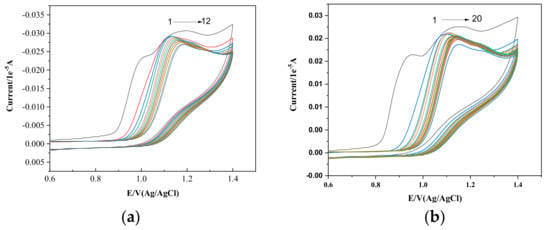
Figure 4.
(a) Cyclic voltammogram of (a) 1 mM PPL in varying concentrations of CTAB (1) 0.9 µM, (2) 1.0 µM, (3) 2.0 µM, (4) 3.0 µM, (5) 4.0 µM, (6) 5.0 µM, (7) 6.0 µM, (8) 7.0 µM, (9) 8.0 µM, (10) 9 µM, (11) 10 µM, and (12) 11 µM. (b) Cyclic voltammogram of 1 mM PPL in the presence of the same concentrations of HDMIC as those listed above.
The plots showing the relationship between concentration and peak current, given in Figure 4 for both CTAB and HDMIC, clearly show that the peak current drop (decay) decreases with the concentration of surfactant with the peak potential shift to the higher values. PPL, being the most hydrophobic beta blocker drug, is adsorbed in the working electrode (GCE). With the addition of surfactant monomers (both CTAB and HDMIC), the PPL molecules in the electrode become dislodged or replaced by the surfactant monomers, which causes the oxidation peak current drop to decrease with increasing surfactant concentrations. The drop in peak current continues with the addition of surfactants and the shift in potential towards higher values. The alteration in the oxidation peak current and shift in the peak potential are due to the hydrophobic interactions between the PPL molecules and the CTAB.
In the presence of CTAB, the variation is also due to the cation–π interaction between the naphthalene ring of PPL and the cationic head of CTAB. Likewise, in the presence of the monomers of HDMIC, the increase in the peak current drop occurs, and the shift in peak potential towards higher values is due to π–π interaction between the naphthalene ring of PPL and the π electrons of the imidazolium ring present in the head group of HDMIC. Hence, these effects allow for electroactive PPL molecules to bind strongly with the surfactants.
3.3.2. PPL–Postmicellar Concentrations of Surfactants
Figure 5 shows cyclic voltammograms of 1 mM PPL in the presence of postmicellar concentrations of CTAB and HDMIC. Changes in the peak current drop (Ipa) and in the peak potential (Ep) can be clearly observed. Though both the surfactants have the same chain length, their complex formations or interactions with PPL are not similar. This may be due to surfactant adsorption, micellar formations, and the kinetics involved between the Propranolol, surfactants, and the electrode surface. AS shown in Figure 4b, which refers to 1 mM PPL in the presence of HDMIC, with an increase in the concentration of HDMIC from 0.47 mM to 1.50 mM, there is decrease in the peak current drop. Also, the peak potential does not change or vary with increasing concentrations.
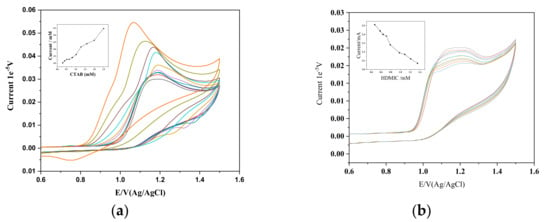
Figure 5.
(a) Cyclic voltammogram for PPL (1 mM) at a scan rate of 50 mVs−1 with different concentrations of CTAB. Inset: plot showing the relationship between the concentration of CTAB and current (μA). (b) Cyclic voltammogram for PPL (1 mM) at a scan rate of 50 mVs−1 with different concentrations of HDMIC. Inset: plot showing the relationship between the concentration of HDMIC and current (μA).
The increase in the peak current of PPL in CTAB and the decrease in the peak potential is due to the adsorption of CTAB micelles on the bare glassy carbon electrode. This process indicates that the oxidation is favored due to the presence of CTAB present in the system. CTAB, being hydrophobic, is mainly attracted to the electrode, which favors the dislodging of the PPL molecules (amphiphilic molecules).
To better understand the interactions between the electroactive PPL molecules and CTAB and HDMIC, the binding constant (Kb) was determined using cyclic voltammetry using the Langmuir equation [19]:
where Kb is the binding constant between the PPL and surfactants, C is the surfactant’s concentration, and ΔIp and ΔIp,max denote the current drop and maximum current drop, respectively. The values of Kb for the complexes involved in this study were calculated based on an intercept/slope ratio of 1/ΔIp vs. 1/C (figure not shown). The values for both the complexes are given in Table 2, along with regression coefficient values (R2).

Table 2.
Estimates of the Binding Constant (Kb), Free Energy Change (ΔG), and Correlation Coefficient (R2) values for the PPL–postmicellar surfactant (estimated using cyclic voltammetry).
The obtained binding constant values suggest that the PPL binds stronger in the presence of the cationic surfactant (CTAB) than with the ionic liquid surfactant (HDMIC). This may be due to the presence of the strong cation–π interaction between CTAB and PPL [20]. This also suggests that apart from the cation–π interaction, there could also exist many other interactions that play a role in the strong binding with the PPL. The low binding constant value may be due to the absence of cation–π interactions in PPL–HDMIC. The Gibbs free energy change (ΔG) for this system (PPL with surfactants) was calculated using the following relation: ΔG = −RT lnKb. The ΔG values for the PPL with both the surfactants are negative, which indicate that the binding of PPL with both the surfactants is spontaneous. The more negative values of PPL in CTAB signifies more enhanced binding and stable complex formation.
4. Conclusions
The electro oxidation of Propranolol was investigated via cyclic voltammetry using a glassy carbon electrode and a cationic surfactant and ionic liquid surfactant with the same chain length. The results show that PPL, in the presence of surfactants, shows an increase in charge transfer activities, and the process involved is irreversible. The plotting of the different concentrations of the surfactants, either at pre- or postmicellar concentrations, suggested that this process is a diffusion-controlled process. With a shift in the concentration of the surfactant from a pre- to a postmicellar concentration, the rate constant values (K0) increase for both the surfactants in PPL. In the absence of the cationic surfactant, the value of rate constant K0 for PPL was 4.9 s−1, whereas the values for PPL in the presence of the cationic surfactant were 1.196 s−1 and 2.446 s−1 for pre- and postmicellar concentrations of CTAB, respectively, and for HDMIC, the values are 3.946 s−1 and 3.233 s−1 for the pre- and postmicellar phases, respectively. In the premicellar phase of both surfactants, the rate is slower compared to the postmicellar phase of PPL only. HDMIC has higher values of K0 than CTAB, which indicates that the rate of reaction taking place on the surface of electrode is fast.
Our CTAB-mediated electrochemical studies showed that the oxidation peak at lower values increased with the scan rate due to the involvement of a hydrophobic interaction, and the HDMIC-mediated studies involved a π–π interaction between the naphthalene ring of PPL and imidazolium ring of HDMIC. It was also observed that PPL binds more strongly with CTAB due to the Cation–π interaction between the head group of surfactants and the π electrons of PPL. The increase in the peak current, as well as the shift in the potential to higher values, is due to the fact that PPL is a surface-active drug. Hence, it can be concluded that cationic surfactants are better carriers of PPL drugs compared to ionic liquid surfactants. By considering the advantages of using cationic surfactants in relation to these types of drugs, this study provides a reference for the analytical determination of PPL in pharmaceutical formulations.
Author Contributions
Conceptualization, N.C. and M.A.; methodology, M.A. and N.C.; software, N.C.; validation, M.A.; formal analysis N.C.; investigation, N.C. and M.A.; resources, N.C.; data curation, N.C.; writing—original draft preparation, N.C.; writing—review and editing, M.A.; visualization, M.A.; supervision, M.A.; project administration, M.A.; funding acquisition, M.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding from the TMA Pai University Research Fund.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors would like to thank TMA Pai University Research Fund for providing a minor grant for the carrying out of the research work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rosen, M.J.; Kunjappu, J.T. Surfactants and Interfacial Phenomena; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Cui, Z.G.; Canselier, J.P. Interfacial and micellar properties of some anionic/cationic binary surfactant ystems. 1. Surface properties and prediction of surface tension. Colloid Polym. Sci. 2000, 278, 22–29. [Google Scholar]
- Cui, X.; Mao, S.; Liu, M.; Yuan, H.; Du, Y. Mechanism of surfactant micelle formation. Langmuir 2008, 24, 10771–10775. [Google Scholar] [CrossRef] [PubMed]
- Seebauer, C.T.; Graus, M.S.; Huang, L.; McCann, A.; Wylie-Sears, J.; Fontaine, F.; Francois, M. Non–beta blocker enantiomers of propranolol and atenolol inhibit vasculogenesis in infantile hemangioma. J. Clin. Investig. 2022, 132, e151109. [Google Scholar] [CrossRef] [PubMed]
- Purushothama, H. Electrochemical determination of propranolol using reduced graphene oxide modified carbon paste electrode. Anal. Bioanal. Electrochem. 2019, 11, 1575–1589. [Google Scholar]
- Gowda, B.G.; Seetharamappa, J.; Melwanki, M.B. Indirect spectrophotometric determination of propranolol hydrochloride and piroxicam in pure and pharmaceutical formulations. Anal. Sci. 2002, 18, 671–674. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Micke, G.A.; Costa, A.C.O.; Heller, M.; Barcellos, M.; Piovezan, M.; Caon, T.; de Oliveira, M.A.L. Development of a fast capillary electrophoresis method for the determination of propranolol—Total analysis time reduction strategies. J. Chromatogr. A 2009, 1216, 7957–7961. [Google Scholar] [CrossRef] [PubMed]
- Silva, L.C.; Trevisan, M.G.; Poppi, R.J.; Sena, M.M. Direct determination of propranolol in urine by spectrofluorimetry with the aid of second order advantage. Anal. Chim. Acta 2007, 595, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Sartori, E.R.; Barbosa, N.V.; Faria, R.C.; Fatibello-Filho, O. Conductometric determination of propranolol hydrochloride in pharmaceuticals. Eclética Química 2011, 36, 110–122. [Google Scholar] [CrossRef]
- Savéant, J. Elements of Molecular and Biomolecular Electrochemistry: An Electrochemical Approach to Electron Transfer Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Gaichore, R.R.; Srivastava, A.K. Electrocatalytic determination of propranolol hydrochloride at carbon paste electrode based on multiwalled carbon-nanotubes and γ-cyclodextrin. J. Incl. Phenom. Macrocycl. Chem. 2014, 78, 195–206. [Google Scholar] [CrossRef]
- Casella, I.G.; Bonito, R.; Contursi, M. Determination of some β-blockers by electrochemical detection on polycrstalline gold electrode after solid phase extraction (SPE). Electroanalysis 2016, 28, 1060–1067. [Google Scholar] [CrossRef]
- Kun, Z.; Hongtao, C.; Yue, Y.; Zhihong, B.; Fangzheng, L.; Sanming, L. Platinum nanoparticle-doped multiwalled carbon-nanotube-modified glassy carbon electrode as a sensor for simultaneous determination of atenolol and propranolol in neutral solution. Ionics 2015, 21, 1129–1140. [Google Scholar] [CrossRef]
- Sartori, E.R.; Medeiros, R.A.; Rocha-Filho, R.C.; Fatibello-Filho, O. Square-wave voltammetric determination of propranolol and atenolol in pharmaceuticals using a boron-doped diamond electrode. Talanta 2010, 81, 1418–1424. [Google Scholar] [CrossRef] [PubMed]
- Chhetri, N.; Ali, M. Effect of hydrophilic atenolol and lipophilic propranolol β-blockers on the surface and bulk aggregation of quaternary ammonium bromide surfactants: A comparative study. J. Mol. Liq. 2023, 382, 121858. [Google Scholar] [CrossRef]
- Bard, A.J.; Faulkner, L.R.; White, H.S. Electrochemical Methods: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2022. [Google Scholar]
- Santos, A.M.; Wong, A.; Fatibello-Filho, O. Simultaneous determination of salbutamol and propranolol in biological fluid samples using an electrochemical sensor based on functionalized-graphene, ionic liquid and silver nanoparticles. J. Electroanal. Chem. 2018, 824, 1–8. [Google Scholar] [CrossRef]
- Li, K.; Li, Y.; Wang, L.; Yang, L.; Ye, B. Study the voltammetric behavior of 10-hydroxycamptothecin and its sensitive determination at electrochemically reduced graphene oxide modified glassy carbon electrode. Arab. J. Chem. 2019, 12, 2732–2739. [Google Scholar] [CrossRef]
- Akram, M.; Anwar, S.; Bhat, I.A.; Kabir-ud-Din. Exploration of ibuprofen binding with micellar assemblies of the efficiently-engineered gemini surfactants: Insights from spectroscopic and voltammetric studies. Colloids Surf. A Physicochem. Eng. Asp. 2018, 555, 121–132. [Google Scholar] [CrossRef]
- Ma, J.C.; Dougherty, D.A. The cation-π interaction. Chem. Rev. 1997, 97, 1303–1324. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).