Abstract
Diagnosing brain tumors is a time-consuming process requiring radiologist expertise. With the growing patient population and increased data volume, conventional procedures have become expensive and ineffective. Scholars have explored algorithms for detecting and classifying brain tumors, focusing on precision and efficiency. Deep learning methodologies are being used to create automated systems that can diagnose or segment brain tumors with precision and efficiency, particularly in brain cancer classification. This approach facilitates transfer learning models in medical imaging. The present study undertakes an evaluation of three foundational models in the domain of computer vision, namely AlexNet, VGG16, and ResNet-50. The VGG16 and ResNet-50 models demonstrated praiseworthy performance, thereby instigating the amalgamation of these models into a groundbreaking hybrid VGG16–ResNet-50 model. The amalgamated model was subsequently implemented on the dataset, yielding a remarkable accuracy of 99.98%, sensitivity of 99.98%, and specificity of 99.98% with an F1 score of 99.98%. Based on a comparative analysis with alternative models, it can be deduced that the suggested framework exhibits a commendable level of dependability in facilitating the timely identification of diverse cerebral neoplasms.
1. Introduction
The brain and spinal cord make up the central nervous system of Homo sapiens [1]. The brain controls most biological activities, such as analyzing, integrating, organizing, determining, and issuing directions to the body. The structural complexity of the human brain is remarkable [2]. Certain CNC problems, including stroke, infection, brain tumors, and headaches, are difficult to diagnose, analyze, and cure [3].
Brain tumors are abnormal cell growths in the rigid skull that encloses the brain [4,5]. Any growth in a small space might cause problems. Any skull tumor may cause brain damage, posing a significant danger to the brain [6,7]. Brain tumors are the tenth leading cause of mortality in both adults and children [8]. Various tumor kinds have poor survival rates dependent on texture, location, and shape [9,10]. Brain tumors affect 700,000 people, with 80% benign and 20% malignant [11]. According to 2021 American Cancer Society projections, 78,980 persons were diagnosed with brain tumors, including 55,150 noncancerous and 24,530 malignant tumors (13,840 men and 10,690 females) [12]. Research indicates that brain tumors are the primary cause of cancer mortality in both children and adults globally [13].
Many disorders need digital medical images for diagnosis, which are also useful for training and research. The need for digitised medical images is rising. In 2002, the University Hospital of Geneva Radiology Department produced 12,000–15,000 pictures daily [14]. Medical reports and image studies need a reliable computer-aided diagnostic system. Manual medical ageing assessment is time-consuming, inaccurate, and error-prone [15]. Machine and deep learning are essential for medical diagnosis and treatment. Multiple algorithms for brain tumor identification and classification have shown strong performance and low error [16]. DL provides brain tumor classification using pre-trained CNN models for medical pictures such as GoogLeNet, AlexNet, and ResNet-34 [15,16]. DL employs multi-layered deep neural networks.
2. Related Work
This section discusses advanced deep learning (DL)-based brain tumor classification methods, including deep learning, machine learning, and hybrid approaches.
2.1. Deep Learning Methods
Mahmud et al. [15] compared a CNN architecture to ResNet-50, VGG16, and Inception V3 models, finding it performs better with 93.3% accuracy, 98.43% AUC, 91.19% recall, and 0.25 loss on 3264 MR images. ZainEldin et al. [16] used an adaptive dynamic sine–cosine fitness grey wolf optimizer technique to optimize CNN hyperparameters. The ADSCFGWO algorithm combines sine–cosine and grey wolf algorithms, achieving 99.98% accuracy on the BRaTS 2021 Task 1 dataset. Srikanth et al. [17] developed a 16-layer VGG-16 deep NN for brain tumor MR image multi-classification, achieving 98% accuracy after 20 training cycles. Musallam et al. [18] developed a DCNN brain tumor detection model using an MRI dataset, achieving 97.72% accuracy in glioma diagnosis, 98.26% in meningioma detection, 95.95% in pituitary identification, and 97.14% in normal image detection. Wozniak et al. [19] developed a new correlation learning technique (CLM) for deep neural networks, achieving 96% accuracy, precision, and recall in a study on 3064 brain malignancies.
2.2. Machine Learning Methods
Garg et al. [20] tested various machine learning models for brain tumor detection using 2556 photos. Their technique showed 97.305% accuracy, 97.73% precision, 97.60% specificity, 97.04% sensitivity, and 97.41% dependability. Pareek et al. [21] developed a technique for identifying and classifying brain tumors using KSVM, which accurately classifies them with 97% accuracy on 150 T1-weighted MRI brain images. The suggested approach in [22] improves MRI quality by using normalization, densely accelerated features, and gradient methods. Tested on a large dataset, it achieved a 90% higher accuracy than current methods. The Quantum Fully Self-Supervised Neural Network (QFS-Net) uses qubits/three-quantum states for brain lesion segmentation, replacing supervised QINN networks with a qutrit-based counter-propagating technique [23].
2.3. Hybrid Methods
Stadlbauer et al. [24] detected brain tumors early using physiological MRI and nine machine learning models. An automated technique employing MR images by Aamir et al. enhanced classification accuracy to 98.95%. For better brain tumor detection in BRATS MR images, Sajid et al. [25] created a hybrid CNN model with two- and three-path networks. The model has 86% dice, 86% sensitivity, and 91% specificity. Lotlikar et al. [26] presented a KNN classifier for early foetal brain abnormality identification with 95.6% accuracy and 99% AUC. A deep learning-based architecture for early embryonic neurodevelopmental disease identification by Attallah et al. [27] showed promising results. Khairandish et al. [20] used CNN, SVM, and threshold-based segmentation to create a hybrid classification approach with 98.4959 % accuracy. The research extracts tumor and tissue properties using pre-trained AlexNet, GoogLeNet, ShuffleNet, and ResNet-18 [28]. The article proposes a deep learning-based multimodal [29] brain tumor classification technique with 97.8%, 96.9%, and 92.5% accuracy on three datasets. Using ISLES2015 and BRATS2015 datasets, VGG19 with SVM-Cubic identifies brain tumors 96% more accurately than previous approaches [30]. For brain tumor identification, Irmak et al. [25] offer three CNN designs with 99.33, 92.66, and 98.14 percent accuracy. The suggested CNN models determine parameters via grid search optimization.
3. Materials and Methods
The method includes multiple stages. We pre-processed the dataset from Kaggle data. For validation, we used holdout validation. We trained our images using machine learning. The dataset was split by 80% training, 10% testing, and 10% validation. We checked four brain images: glioma, meningioma, no tumor, and pituitary tumors. To verify our findings, we checked accuracy, specificity, and sensitivity.
3.1. Experimental Setup
The proposed architectures are implemented using Python 3.12 software on a computer with an Intel Core i7-2.8 GHz CPU and 16 GB of RAM.
3.2. Dataset
The brain tumor detection dataset, sourced from kaggle.com, was analyzed using 3264 MRI images, as in Table 1, from four types of brain tumors: meningioma, no tumor, pituitary tumor, and gliomas (as shown in Figure 1).

Table 1.
A comprehensive overview of the dataset’s structure.
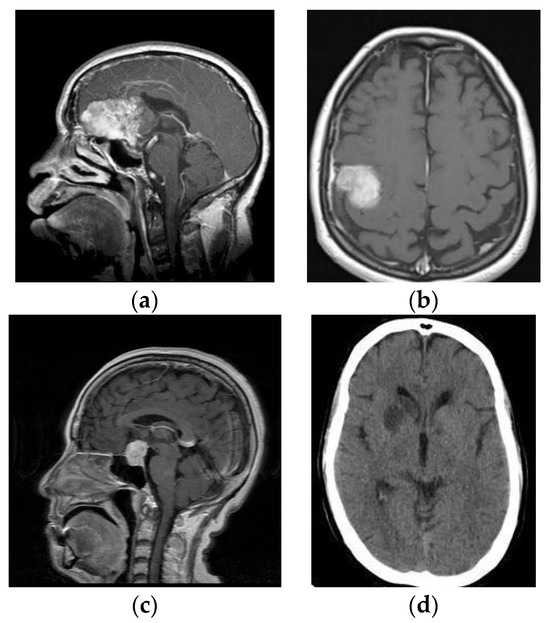
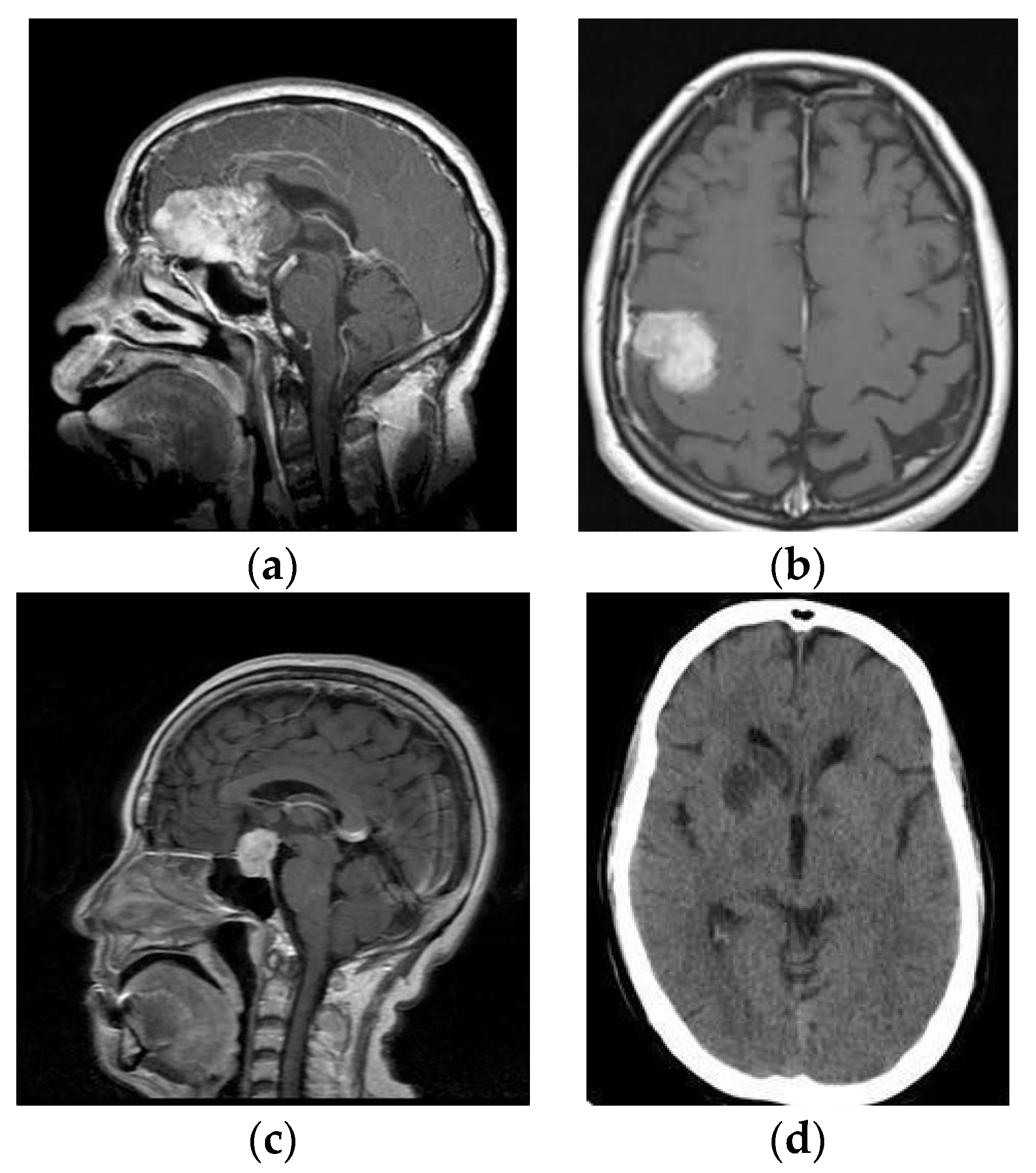
Figure 1.
MR images (a) Glioma, (b) Meningioma, (c) Pituitary and (d) No Tumor.
3.3. Propose Methodology
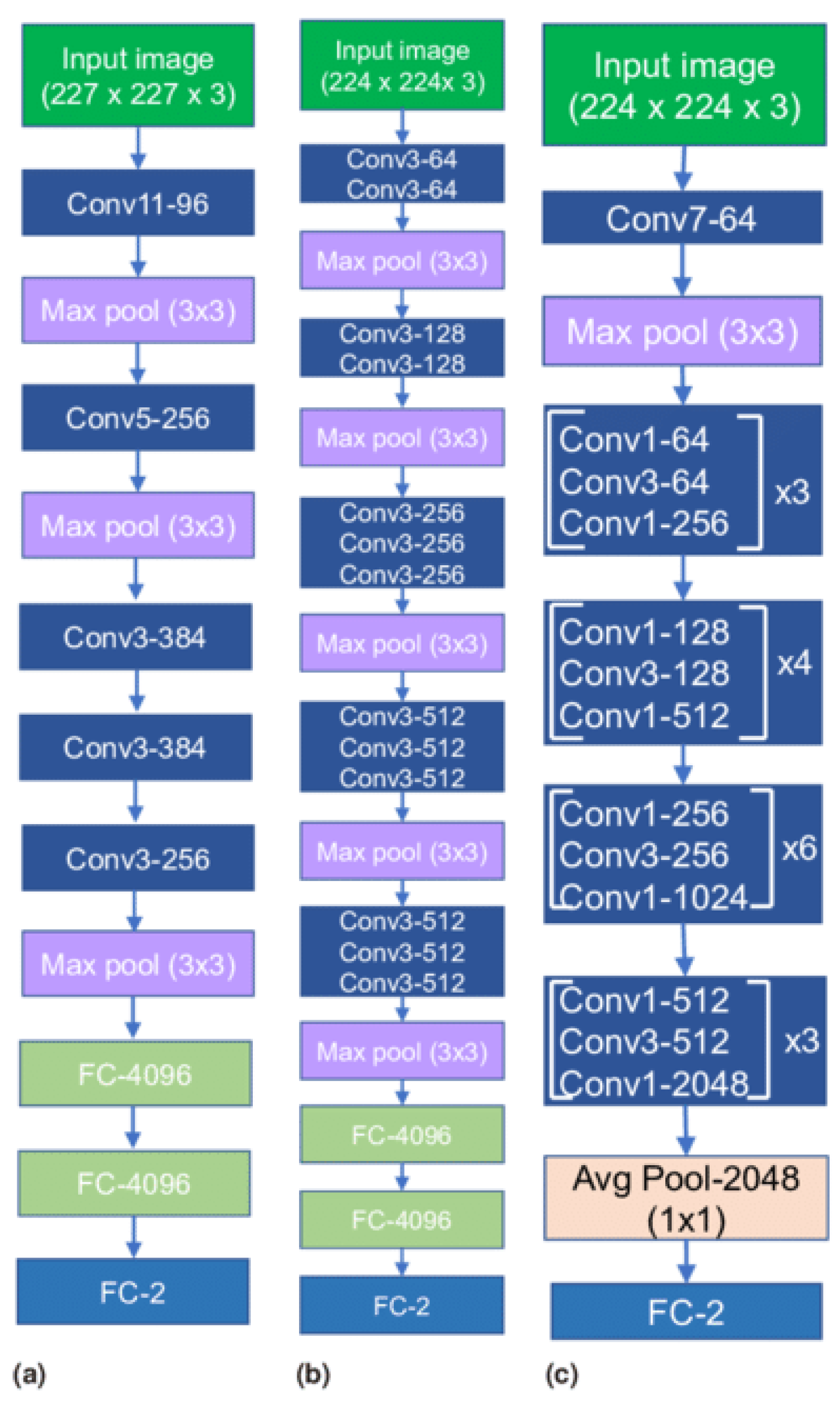
Recent advancements in deep learning, especially in medical image classification, offer promising opportunities for various CNN frameworks. Transfer learning methodologies accelerate data training and reduce sample quantity, allowing newly trained models to effectively utilize pre-existing information. The study evaluates three baseline models in computer vision, including AlexNet, VGG16, and ResNet-50 using transfer learning models which are shown in Figure 2. The models’ efficacy was assessed through adjustments to the output layer, ensuring alignment with the number of classes used.
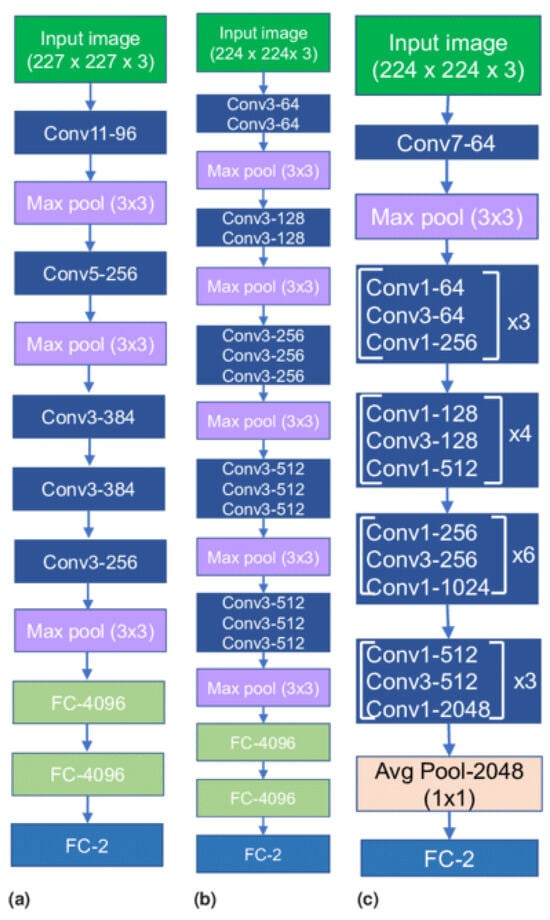
Figure 2.
Transfer Learning Architectures (a) AlexNet, (b) VGG16 and (c) ResNet-50.
3.3.1. AlexNet
The AlexNet architecture is a groundbreaking deep convolutional neural network model known for its efficacy in image classification and recognition tasks. It overcomes hardware constraints by using two NVIDIA GTX 580 GPUs for training. The model has five convolutional layers, three pooling layers, and three fully connected layers, with approximately 60 million trainable parameters.
3.3.2. VGG16
The VGG Net, a deep convolutional neural network architecture from the University of Oxford, demonstrated exceptional performance in the ILSVRC 2014 object localization and classification competitions. Its design aims to enhance the depth of convolutional neural network architectures by using multiple diminutive kernels, potentially improving precision. The VGG Net is widely used in computer vision applications, particularly in medical imaging.
3.3.3. ResNet-50
ResNet frameworks mitigate network performance degradation by stacking convolutional and pooling layers and using identity shortcut connections. These connections bypass layers, maintaining identity relationships within residual blocks and reducing training errors in deep architectures.
3.4. Confusion Metrics
The system model’s effectiveness was evaluated using confusion metrics, which categorize accurate and erroneous prognostications into four distinct classifications (Figure 3):
- True positive (TP) occurs when both the predicted and actual outcomes are positive;
- False positive (FP) occurs when a forecast predicts a positive outcome, but the actual outcome is negative;
- True negative (TN) occurs when both the observed outcome and prognostications are negative;
- False negative (FN) occurs when a prediction incorrectly predicts a negative outcome, despite the actual result being positive.
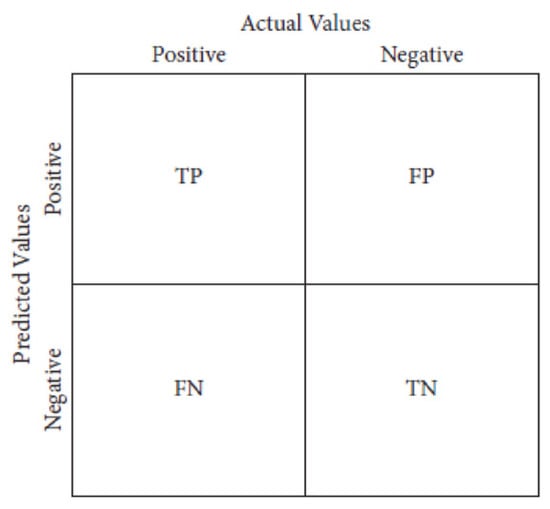
Figure 3.
Confusion Metrics.
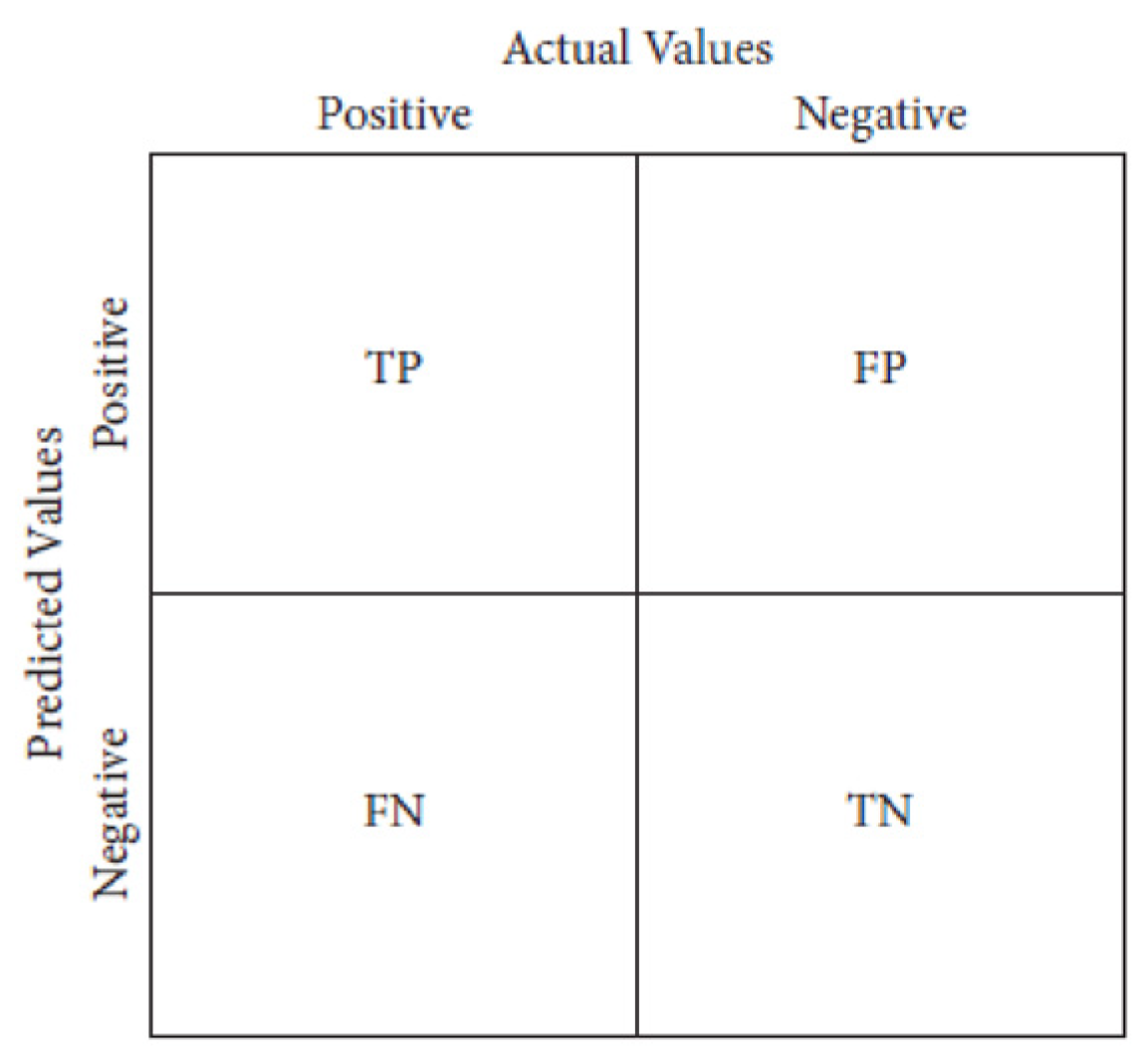
Figure 3.
Confusion Metrics.
3.5. Classification Metrics
The performance of the model was assessed through the utilization of three distinct metrics, as enumerated below:
- Accuracy is the ratio of accurate predictions to the total number of predictions made;
- Specificity refers to a model’s inherent capacity to accurately identify and classify negative samples within a specific dataset;
- Sensitivity is a model’s ability to identify positive samples.
4. Results and Discussion
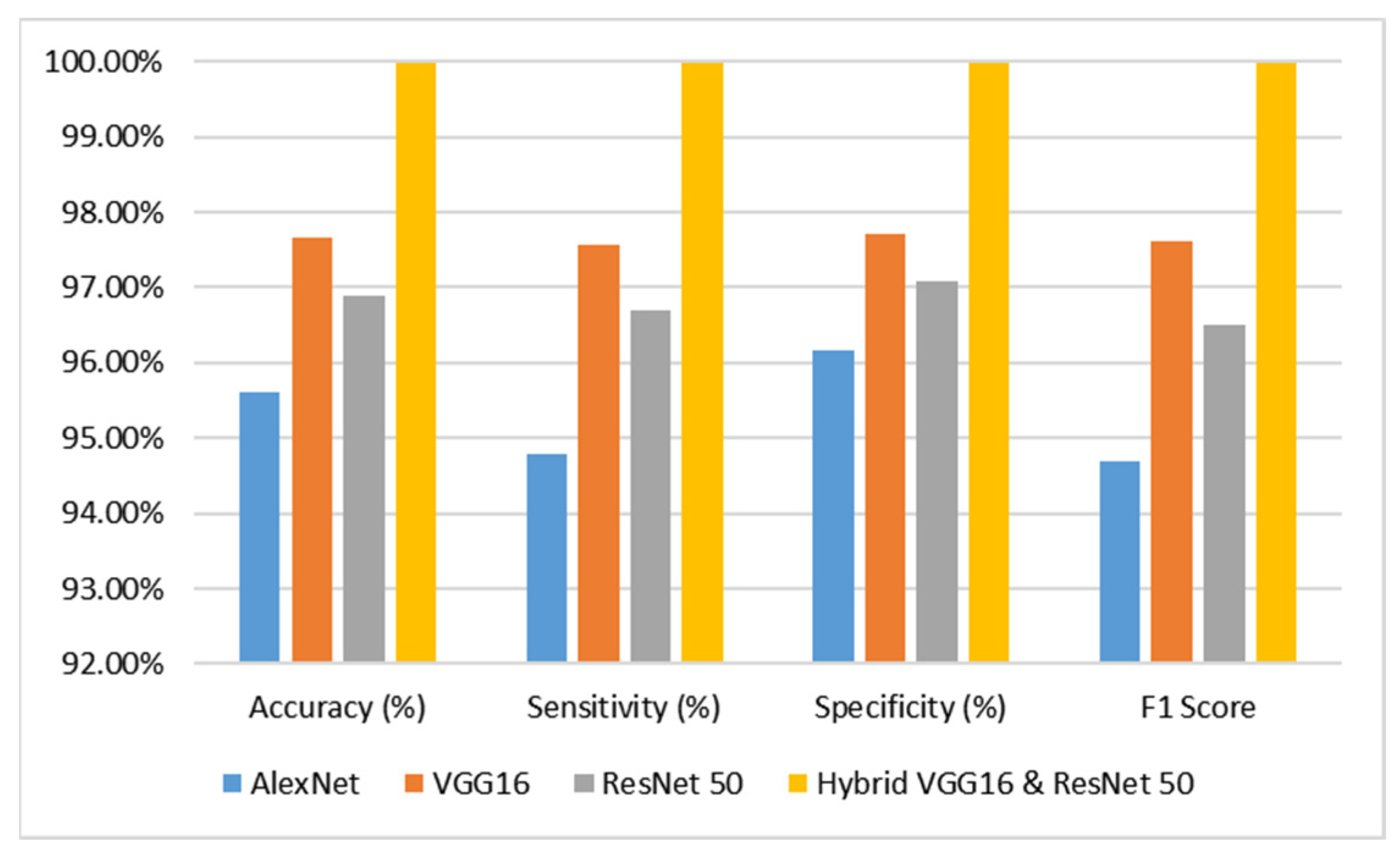
The analysis pertains to the outcomes derived from diverse categories of sophisticated deep learning models, namely AlexNet, VGG16, ResNet-50, and hybrid VGG16–ResNet-50 classification algorithms, when applied to the dataset comprising brain tumor MR images. The evaluation is conducted by considering performance metrics such as accuracy, specificity, and sensitivity, which are presented in Table 2 and juxtaposed in Figure 4 for comparative purposes. Based on the analysis conducted, it was duly noted that the AlexNet model demonstrated a commendable level of performance. Specifically, it achieved an impressive accuracy rate of 95.60%, indicating its ability to correctly classify instances with a high degree of precision. Furthermore, the model exhibited a sensitivity rate of 94.79%, signifying its proficiency in accurately identifying positive instances. Additionally, the model showcased a specificity rate of 96.15%, highlighting its capability to correctly classify negative instances with an F1 score of 94.68%. These findings underscore the effectiveness and reliability of the AlexNet model in the context of the analyzed task. In contrast, the VGG16 model demonstrated a remarkable level of performance, attaining an accuracy rate of 97.66%. Additionally, it exhibited a sensitivity rate of 97.56% and a specificity rate of 97.72% with an F1 score of 97.62%. The ResNet-50 model has demonstrated a remarkable level of performance, attaining an accuracy rate of 96.90%. Additionally, it has exhibited a sensitivity of 96.69%, indicating its ability to correctly identify positive instances, and a specificity of 97.06%, highlighting its proficiency in accurately recognizing negative instances with an F1 score of 96.51%.

Table 2.
Performance Evaluation of Various Transfer Learning Models.
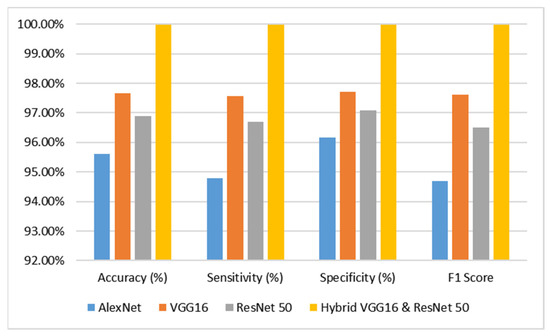
Figure 4.
Performance Analysis Graph of the Proposed Model.
The VGG16 and ResNet-50 models exhibited commendable performance, prompting the integration of both models into a novel hybrid VGG16–ResNet50 model. This amalgamated model was subsequently deployed on the dataset, resulting in an impressive accuracy of 99.98%, sensitivity of 99.98%, and specificity of 99.98% with an F1 score of 99.98%. Based on the meticulous examination of the accuracy graph analysis, it has been discerned that the hybrid VGG16–ResNet50 models have exhibited superior performance in comparison to the remaining models.
5. Conclusions and Future Works
The study aims to improve the early detection of brain tumors using magnetic resonance imaging (MRI) images and deep learning models. The researchers developed a hybrid model that uses a large number of MRI images for timely identification. The study used various indicators to evaluate the effectiveness of the machine learning models, and considered several other models to evaluate the results. The goal is to reduce global fatality rates and improve clinical diagnosis and therapeutic decision-making for brain tumor patients. This study aimed to scrutinize transfer learning models but did not conduct empirical investigations. Future research will focus on refining these models and understanding their inner workings. The absence of elucidation tools hindered the visualization of crucial brain tumor regions.
Author Contributions
Conceptualization, V.K.D.; methodology, M.D.N.; software, M.G.; validation, V.K.D. and M.D.N.; formal analysis, K.S.; investigation, M.G. and K.S.; resources, A.T.; data curation, M.D.N.; writing—original draft preparation, V.K.D.; writing—review and editing, V.K.D. and M.D.N.; visualization, V.K.D.; supervision, V.K.D.; project administration, V.K.D.; funding acquisition, V.K.D. and M.G. All authors have read and agreed to the published version of the manuscript.
Funding
Vinayaka Mission’s Kirupananda Variyar Engineering College, Vinayaka Mission’s Research Foundation Deemed to be University, Salem, Tamil Nadu, India. VMRF/Research/Seed Money/2022-23/VMKVEC-1.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors would like to thank the Vinayaka Mission’s Kirupananda Variyar Engineering College, Vinayaka Mission’s Research Foundation Deemed to be University, Salem, Tamilnadu, India for supporting this work.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Qureshi, S.A.; Raza, S.E.A.; Hussain, L.; Malibari, A.A.; Nour, M.K.; Rehman, A.U.; Al-Wesabi, F.N.; Hilal, A.M. Intelligent Ultra-Light Deep Learning Model for Multi-Class Brain Tumor Detection. Appl. Sci. 2022, 12, 3715. [Google Scholar] [CrossRef]
- Zahoor, M.M.; Qureshi, S.A.; Bibi, S.; Khan, S.H.; Khan, A.; Ghafoor, U.; Bhutta, M.R. A New Deep Hybrid Boosted and Ensemble Learning-Based Brain Tumor Analysis Using MRI. Sensors 2022, 22, 2726. [Google Scholar] [CrossRef]
- Arabahmadi, M.; Farahbakhsh, R.; Rezazadeh, J. Deep Learning for Smart Healthcare—A Survey on Brain Tumor Detection from Medical Imaging. Sensors 2022, 22, 1960. [Google Scholar] [CrossRef] [PubMed]
- Gore, D.V.; Deshpande, V. Comparative study of various techniques using deep Learning for brain tumor detection. In Proceedings of the 2020 IEEE International Conference for Emerging Technology (INCET), Belgaum, India, 5–7 June 2020; pp. 1–4. [Google Scholar]
- DeAngelis, L.M. Brain tumors. N. Engl. J. Med. 2001, 344, 114–123. [Google Scholar] [CrossRef]
- Borole, V.Y.; Nimbhore, S.S.; Kawthekar, D.S.S. Image processing techniques for brain tumor detection: A review. Int. J. Emerg. Trends Technol. Comput. Sci. (IJETTCS) 2015, 4, 2. [Google Scholar]
- Amin, J.; Sharif, M.; Yasmin, M.; Fernandes, S.L. Big data analysis for brain tumor detection: Deep convolutional neural networks. Future Gener. Comput. Syst. 2018, 87, 290–297. [Google Scholar] [CrossRef]
- Iorgulescu, J.B.; Sun, C.; Neff, C.; Cioffi, G.; Gutierrez, C.; Kruchko, C.; Ruhl, J.; Waite, K.A.; Negoita, S.; Hofferkamp, J.; et al. Molecular biomarker-defined brain tumors: Epidemiology, validity, and completeness in the United States. Neuro-Oncology 2022, 24, 1989–2000. [Google Scholar] [CrossRef]
- Cha, S. Update on brain tumor imaging: From anatomy to physiology. Am. J. Neuroradiol. 2006, 27, 475–487. [Google Scholar]
- Ranjbarzadeh, R.; Bagherian Kasgari, A.; Jafarzadeh Ghoushchi, S.; Anari, S.; Naseri, M.; Bendechache, M. Brain tumor segmentation based on deep learning and an attention mechanism using MRI multi-modalities brain images. Sci. Rep. 2021, 11, 10930. [Google Scholar] [CrossRef]
- Rahman, M.L.; Reza, A.W.; Shabuj, S.I. An internet of things-based automatic brain tumor detection system. Indones. J. Electr. Eng. Comput. Sci. 2022, 25, 214–222. [Google Scholar] [CrossRef]
- Key Statistics for Brain and Spinal Cord Tumors. Available online: https://www.cancer.org/cancer/brain-spinal-cord-tumorsadults/about/key-statistics.html (accessed on 12 February 2023).
- Ayadi, W.; Elhamzi, W.; Charfi, I.; Atri, M. Deep CNN for Brain Tumor Classification. Neural Process. Lett. 2021, 53, 671–700. [Google Scholar] [CrossRef]
- Al-Galal, S.A.Y.; Alshaikhli, I.F.T.; Abdulrazzaq, M.M. MRI brain tumor medical images analysis using deep learning techniques: A systematic review. Health Technol. 2021, 11, 267–282. [Google Scholar] [CrossRef]
- Mahmud, M.I.; Mamun, M.; Abdelgawad, A. A Deep Analysis of Brain Tumor Detection from MRImages Using Deep Learning Networks. Algorithms 2023, 16, 176. [Google Scholar] [CrossRef]
- ZainEldin, H.; Gamel, S.A.; El-Kenawy, E.-S.M.; Alharbi, A.H.; Khafaga, D.S.; Ibrahim, A.; Talaat, F.M. Brain Tumor Detection and Classification Using Deep Learning and Sine-Cosine Fitness GreyWolf Optimization. Bioengineering 2023, 10, 18. [Google Scholar]
- Srikanth, B.; Suryanarayana, S.V. Multi-Class classification of brain tumor images using data augmentation with deep neuralnetwork. Mater. Today Proc. 2021. [Google Scholar] [CrossRef]
- Musallam, A.S.; Sherif, A.S.; Hussein, M.K. A New Convolutional Neural Network Architecture for Automatic Detection of Brain Tumors in Magnetic Resonance Imaging Images. IEEE Access 2022, 10, 2775–2782. [Google Scholar] [CrossRef]
- Woźniak, M.; Siłka, J.; Wieczorek, M. Deep neural network correlation learning mechanism for CT brain tumor detection. Neural Comput. Appl. 2023, 35, 14611–14626. [Google Scholar] [CrossRef]
- Amin, J.; Sharif, M.; Haldorai, A.; Yasmin, M.; Nayak, R.S. Brain tumor detection and classification using machine learning: A comprehensive survey. Complex Intell. Syst. 2022, 8, 3161–3183. [Google Scholar] [CrossRef]
- Pareek, M.; Jha, C.K.; Mukherjee, S. Brain Tumor Classification from MRI Images and Calculation of Tumor Area. In Advances in Intelligent Systems and Computing; Springer: Singapore, 2020; pp. 73–83. [Google Scholar]
- Ayadi, W.; Charfi, I.; Elhamzi, W.; Atri, M. Brain tumor classification based on hybrid approach. Vis. Comput. 2020, 38, 107–117. [Google Scholar] [CrossRef]
- Konar, D.; Bhattacharyya, S.; Panigrahi, B.K.; Behrman, E.C. Qutrit-Inspired Fully Self-Supervised Shallow Quantum Learning Network for Brain Tumor Segmentation. IEEE Trans. Neural Netw. Learn. Syst. 2022, 33, 6331–6345. [Google Scholar] [CrossRef]
- Stadlbauer, A.; Marhold, F.; Oberndorfer, S.; Heinz, G.; Buchfelder, M.; Kinfe, T.M.; Meyer-Bäse, A. Radiophysiomics: Brain Tumors Classification by Machine Learning and Physiological MRI Data. Cancers 2022, 14, 2363. [Google Scholar] [CrossRef]
- Sajid, S.; Hussain, S.; Sarwar, A. Brain tumor detection and segmentation in MR images using deep learning. Arab. J. Sci. Eng. 2019, 44, 9249–9261. [Google Scholar] [CrossRef]
- Lotlikar, V.S.; Satpute, N.; Gupta, A. Brain Tumor Detection Using Machine Learning and Deep Learning: A Review. Curr. Med. Imaging 2022, 18, 604–622. [Google Scholar] [CrossRef] [PubMed]
- Attallah, O.; Sharkas, M.A.; Gadelkarim, H. Deep learning techniques for automatic detection of embryonic neuro developmental disorders. Diagnostics 2020, 10, 27. [Google Scholar] [CrossRef] [PubMed]
- Nayak, D.R.; Padhy, N.; Mallick, P.K.; Zymbler, M.; Kumar, S. Brain Tumor Classification Using Dense Efficient-Net. Axioms 2022, 11, 34. [Google Scholar] [CrossRef]
- Obeidavi, M.R.; Maghooli, K. Tumor Detection in Brain MRI using Residual Convolutional Neural Networks. In Proceedings of the 2022 IEEE International Conference on Machine Vision and Image Processing (MVIP), Ahvaz, Iran, 23–24 February 2022; pp. 1–5. [Google Scholar]
- Khalil, H.A.; Darwish, S.; Ibrahim, Y.M.; Hassan, O.F. 3D-MRI brain tumor detection model using modified version of level set segmentation based on dragonfly algorithm. Symmetry 2020, 12, 1256. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).